Abstract
Background:
Cystic echinococcosis is an important zoonosis caused by the Echinococcus granulosus with a substantial impact in human and animal health in endemic areas. The purpose of the present study was serodiagnosis optimizing of dog echinococcosis in order to achieve a rapid diagnostic method.
Methods:
Eight dogs were challenged with protoscoleces in order to have positive echinococcosis serum and 2 two-month old puppies were used as uninfected controls. Colloidal gold was prepared by controlled reduction of a boiling solution of chloroauric acid (H [AuCl4]) with sodium citrate and labeled with recombinant EPC1. Dot immunogold filtration assay (DIGFA) was developed by coating rEPC1 labeled colloidal gold on nitrocellulose membrane. The canine sera, taken three times including, 15, 28 and 35 days post infection were tested. A total of 30 serum samples including 24 sera from 8 infected dogs and 6 sera from 2 puppies were comparatively detected with both DIGFA and ELISA.
Results:
Gold labeled antigen, showed a dark purple dot with agglutination particles in positive sera and light purple dot without agglutination in negative sera. Among 30 serum samples, 23 were positive and 7 were negative with DIGFA and 24 were positive, 6 were negative with ELISA.
Conclusion:
DIGFA as a rapid and simple procedure could be utilized in quickly diagnosis of echinococcosis.
Keywords: Dot immunogold filtration assay, Serodiagnosis, Recombinant EPC1, Echinococcus granulosus
Introduction
Cystic echinococcosis (CE) is a zoonosis caused by the metacestode of Echinococcus granulosus. In Iran, this cestode is commonly found in all provinces, while it is usually maintained through a domestic life cycle involving dogs and sheep (1, 2).
CE is still one of the health and economic problems in developing countries, where it causes mortality both in humans and animals (3, 4). It remains one of the most important zoonoses in Iran yet (5).
Moreover, direct and indirect losses in veterinary industry are reported annually. Direct losses are calculated on the base of condemned organs, while indirect losses are expected based on live weight loss caused by hydatidosis (6).
In Iran hydatidosis (both in human and animal) is due to G1 (sheep strain), G6 (camel strain) and G3 (buffalo) strains of Echinococcus granulosus (7). CE diagnosis is mostly based on imaging techniques (8).
. While CE is often diagnosed by imaging methods, that most notably included CT scan and Sonography but serological test is also very considerable.
Therefore, there is a need for standardized and approachable diagnostic tools, which may complement imaging data. An immunoassay can be used as a confirmatory test, usually consisted of E. granulosus recombinants antigens. Several recombinant antigens have shown potential for CE serodiagnosis (9). Reliable test with accepted sensitivity and specificity for the serodiagnosis of human cystic echinococcosis (CE) and dog echinococcosis is still needed, because of the low specificity and sensitivity of the currently available commercial tools. Developing a rapid, simple, sensitive, specific and convenient immmunological assay is a crucial step.
The dot immunogold filtration assay which has been in use since 1989 is a technique with the property of simple and rapid immunological detection, by using the red colloidal gold particles to label the antibodies/antigens as indicator, and a nitrocellulose membrane coated with antigen as the carrier. Affected by filtration and condensation, the antigen antibody reaction occurs rapidly. When the reaction is positive, red to purple dots appear on the membrane. It takes about 2 min to 4 min for the whole reaction to be carried out. With the above technique, we have optimized the dot immunogold filtration assay (DIGFA) for rapid detection of specific IgGs in dogs infected with Echinococcus granulosus. DIGFA is a highly sensitive and specific assay when it is used to detect anti-parasite antibodies (10–13). In the present study, the capacity of labeled recombinant EPC1 with nano gold particles to diagnose CE was assessed based on DIGFA method and the diagnostic performance was compared with ELISA.
Serodiagnosis optimizing of dog echoinococcosis was the aim of the present article in order to achieve a rapid and accessible diagnosis method in the field.
Although recombinant antigens with potential to replace native antigens have been proposed in the literature, none has been systematically tested for diagnostic performance. Thus, in this study, we produced a recombinant antigen EPC1 from protoscoleces for the diagnosis and monitoring of echinococcosis.
Materials and Methods
Dogs experimental infection
This work was performed as a cross- sectional study on ten dogs (2–3 months old). Dogs were labeled in two groups. The first group (8 dogs) was inoculated with 90000–100000 (viability more than 85%) protoscoleces. Each dog was fed about 100000 protoscoleces. The second group, as negative controls (2 puppies), was kept in the same condition as the first group but not fed any protoscolex.
Blood samples were collected three times including, 15, 28 and 35days post infection. The blood was clotted at room temperature for 30 minutes and then at 4 °C for 4 hours. The clot was separated from the serum by centrifugation at 3,000× g for 10 minutes and the serum stored in 1ml aliquots at −20°C, until use
On day 35 after challenged, each dog was euthanized by intravenous injection of an overdose of Sodium Pentobarbital for necropsy. The presence of adult Echinococcus granulosus was confirmed in the intestines contents of the first group.
Recombinant protein EPC1
Recombinant EPC1 (rEPC1) was produced according to method previously reported (14). Briefly Liver and lung hydatid cysts from naturally infected sheep were collected from slaughterhouses in the vicinity of Tehran city, Iran. Protoscoleces were aspirated from cysts, pooled, and washed in phosphate buffered saline (PBS- 1%). Total RNA was extracted immediately from protoscoleces using a RNeasy minikit (Qiagen, Germany) according to the manufacturer instruction. Then cDNA was synthesized using Reverse Transcriptase (Fermentas, Lithuania). The EPC1 gene of E. granulosus was amplified by PCR. PCR product, a band of 228 bp, was digested with BamHI and EcoRI and consequently the expression plasmid vector pET28a was double digested with the same restriction endonucleases as mentioned above. The EPC1-cDNA product was cloned in the multicloning site (BamHI and EcoRI site) of pET28a plasmid using rapid DNA ligation kit (Roche, Germany).
In order to express rEPC1, recombinant plasmid pET28a containing the EPC1 was transformed into competent E. coli BL21 cells. Then the recombinant plasmid extracted from transformed E. coli BL21 was digested by the restriction enzymes EcoRI and BamHI, which confirmed the successful construction of the recombinant expression plasmid EPC1/ pET28a.
After that, Protein expression was induced at 33°C for 6 h in the presence of isopropyl-b-D-thiogalactoside (IPTG) at a final concentration of 0.5 mM.
The recombinant His6-tagged EPC1 was purified from the extract of transformed E. coli BL21 by Ni-NTA agarose Kit (Qiagen, Germany) according to the manufacturer’s instructions. The purified His6-tagged protein was analyzed on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel. Western blot analysis was done with HRP-conjugated mouse anti-T7 tag at a 1:5000 dilution. Finally, the positive reaction was developed using DAB (3, 3′-diaminobenzidine) (Sigma_Aldrich, USA) as substrate under visual observation within 5 min.
Indirect ELISA
ELISA was conducted as reported earlier (15). Briefly, 96-well polystyrenes microtiter plates were coated with rEPC1 (5 μg/well). Horseradish peroxidase (HRP)-conjugated rabbit anti-dog IgG (Sigma_Aldrich) at a 1:10000 dilution was used as secondary antibody. Collected dog sera were evaluated and consequently it was shown that rEPC1 was able to distinguish positive echinococcosis sera (first dogs group) from negative control.
Preparation of Colloidal gold
Colloidal gold solutions were prepared according to the methods by Frens (16) Gold nano particles were produced by reduction of chloroauricacid (H [AuCl4]). After dissolving of 0.01gr H [AuCl4] in 100 ml H2O, a yellow color appeared; 50 ml of the solution was rapidly stirred and heated to the boiling point with 1 ml of 10 g/L sodium citrate as a reducing agent. Sodium citrate causes Au3+ ions to be reduced to neutral gold atoms. As more and more of these gold atoms formed, the solution became supersaturated, and gold gradually started to precipitate in the form of sub-nanometer particles. The boiling lasted 5 min. While the solution was stirred vigorously, the particles became fairly uniform in size. The preparation was finished when the solution became dark red in color.
Wavelength Spectrum of Colloidal gold
According to Rance, the nano gold particles of 20–22 nanometer size show the maximum absorption in the wavelength of 525 nanometer (17). In the present study the absorption of nano gold particles was measured. Since the particles showed the maximum optical density in the wavelength of 520 nanometer, those probably were 20–22 nanometers in size which indicating that the colloid gold met the experimental requirements.
Recombinant EPC1colloidal gold labeling
The protein was labeled with colloidal gold as follows: 250 μl of recombinant EPC1 was added to 12 ml of liquid contains Colloidal gold with the help of magnetic stirring. The pH of 1 ml colloidal gold adjusted to a pH of 8.8 using 0.1 mol/L K2CO3. After 10 min, bovine serum albumin (BSA) was added to get the concentration of 10 g/L. After that, the mixture was centrifuged at 13000 × g for 15 min. The supernatant was discarded and the precipitate was dissolved by 5 g/L BSA-PBS, thus forming the colloidal gold labeling reagent (Fig. 1).
Fig. 1:
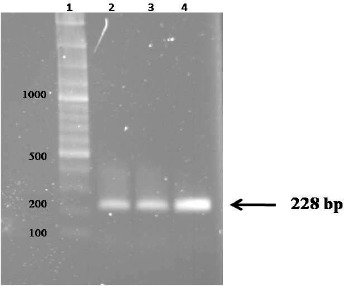
Lane 1: 100 bp DNA Ladder, Lane 2, 3 & 4 EPC1 gene PCR products
DIGFA procedure
The nitrocellulose membrane (which is used as basic membrane in DIGFA and can concentrate gold probes) was divided by pencil into 1 cm squares. A drop of the labeled rEPC1 (about 5μl) was spotted with a micropipette onto the center of each small square and the paper was dried at room temperature (RT) and stored at 4°C over night.
The paper was rinsed with the PBS-T twice for 10 min each time at RT.
After being dried, 10 μl of dog serum was dripped slowly to the center of the membrane and membrane was incubated at RT for 3 minutes without any washing after adding the sera. The canine sera were tested by different concentration 1:10, 1:50, 1:100 and 1:200.
Results
Recombinant EPC1
Figure 1 shows three bands of 228 bp representing the PCR products of EPC1. Enzyme digestion of recombinant plasmid confirmed that ligation was done successfully (Fig. 2).
Fig. 2:
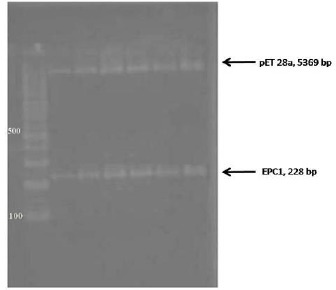
Digestion of recombinant plasmid pET28a/EPC1 by EcoRI and Bam
The SDS–PAGE staining results demonstrated that the His-tagged EPC1 protein was expressed in BL21 cells, a band of 12.8 kDa (Fig. 3). Also the result of purified EPC1 Western blot analysis using T7 tag antibody shows a specific band of 12.8 kDa (Fig. 4).
Fig. 3:
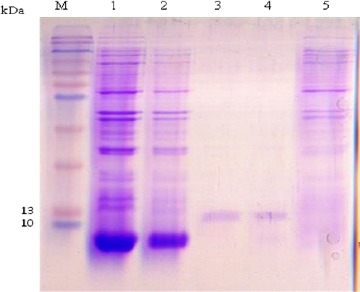
SDS–PAGE analysis of the rEPC1. The gel was Coomassie brilliant blue-stained., the expected molecular mass of the His6-tagged rEPC1protein was 12.8 kDa. M, protein marker; lane 1: Escherichia coli lysates with IPTG 0.5 mM after 6 h of induction, lane 2: Escherichia coli lysates with IPTG 1 mM after 6 h of induction, Lane 3 and 4: rEPC1 protein extracts, lane 5: Escherichia coli lysates without IPTG induction
Fig. 4:
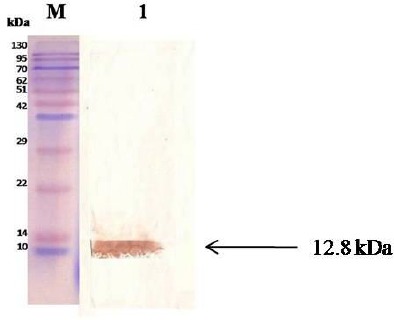
Western blot analysis of rEPC1. M: protein marker; Lane 1; the protein rEPC1was immunoblotted with pooled T7 tag antibody
Spectrophotometric analysis results
The influence of 20nm gold nanoparticle size on the absorbance density is illustrated in Fig. 5. It is revealed that absorption maximum increases to 520nm.
Fig. 5:
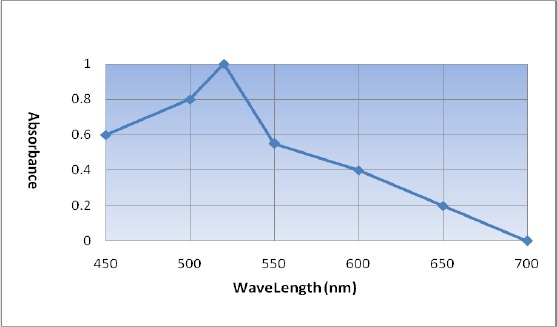
UV-vis absorption spectra of colloidal gold nano particle in different Wavelengths
Dot intensity of DIGFA.
The intensity of the dot color was judged with naked eye (Fig. 6). Positive results were decided by the appearance of regular, round, dark purple dot at the center of the square with agglutination particles whereas the negative squares appeared light purple as we expected since the rEPC1 became purple after nano gold labeling process.
Fig. 6:
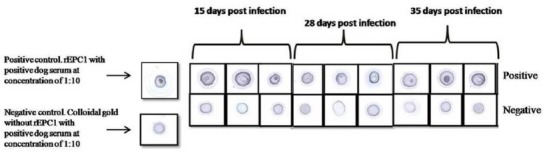
–Some of the positive and negative results of DIGFA with dogs positive and negative sera (dilution of 1:50) in different days post infection. Round, dark purple dot at the center of the square with agglutination particles can be seen in positive samples. In negative samples, a color of light purple is seen which is belong to the colloidal gold
Sensitivity and specificity of DIGFA and ELISA
The results of DIGFA and ELISA assays for dog sera are shown in Table 1.
Table 1.
Comparison of the results detected by ELISA and DIGFA in dogs serum samples
| ELISA* | DIGFA | ||||
|---|---|---|---|---|---|
| Cases | Serum samples | No. of positive | No. of Negative | No. of positive | No. of Negative |
| Infected | 24 | 24 | − | 23 | 1 |
| Non infected | 6 | − | 6 | − | 6 |
| Total | 30 | 24 | 6 | 23 | 7 |
ELISA results are based on the study outcome, which has been done recently (10). Sensitivity, specificity derived from analysis of rEPC1 in DIGFA against sera from dogs are reported respectively, 96% and 100% and indirect ELISA showed sensitivity, specificity of 100%, 100%.
Between 24 sera taken from 8 infected dogs, 23 exhibited dot reaction in DIGFA with optimal results obtained from 1:50 serum dilution and one showed no reaction. Furthermore, two uninfected dogs did not indicate any reaction.
All infected dogs demonstrated anti-rEPC1 antibody response in ELISA test and the 2 negative control dogs were negative. The sensitivity and specificity of the two assays are compared in Table 1. As can be seen the negative rate between the two assays was the same while there was a poor difference in the positive rate. Nevertheless, this difference between sensitivity in mentioned two methods was due to serum samples limitation. Subsequently large number of sera is needed to have a more trustworthy and constant comparable evaluation of these two techniques.
Discussion
Accurate diagnosis of the infection in the definitive host plays a central role in epidemiological studies and for surveillance of hydatid control programs (8).
Current serological methods for detecting dog echinococcosis are too limited and this inadequacy is considered as an important issue. EPC1 as a recombinant protein has been introduced as one of the admissible candidates in hydatidosis/echinococcosis detection (18).
The necessity of an uncomplicated, rapid, and non-instrumented test is obvious in many basic units, where laboratory facilities and trained personnel are limited. The dot immunogold test is a new immunological technique, which has been developed in recent years (13–19–20). Since the nitrocellulose membrane not only absorbs protein, but also affords rapid filtration and acts as capillaries, the antibody in serum is able to combine rapidly with the counterpart on the membrane. Moreover, as the colloidal gold labeling reagent is red in color, red dots appear after the combination takes place. Therefore, no color-developing reagent is needed (19).
In 1989, Chun developed DIGFA as (21) a rapid test, to detect HCG, C-reactive protein, immunoglobulin G antibody and others (22, 23).
In 1999, DIGFA was developed and evaluated for detection of anti-HAV IgM (24). In 1996, Huang et al. developed DIGFA as a simple, rapid, and reproducible test for the rapid detection of reaginic antibody in the serum of syphilitic patients. Subsequently the levels of agreement between DIGFA and the rapid regain test and between DIGFA and the fluorescent treponemal antibody-absorption test were 100 and 98%, respectively. Regarding the results of clinical application, they indicated that DIGFA could be used as a routine screening test for syphilis (25).
Ye et al. used DIGFA as a rapid and cost-effective screening tool for the determination of aflatoxin B (1) in foods in the field within 15min without complicated steps (26).
Wang et al. developed DIGFA to detect white spot syndrome virus of shrimp. They showed that the sensitivity of DIGFA is similar to dot-blot nitrocellulose enzyme immunoassay while DIGFA can be performed conveniently pond side providing a faster result without the production of false positive results (27).
This method, which has aroused our interest greatly, keeps not only with the sensitivity and specificity of the ELISA, but also with the advantage of affording prompt result. Actually, the disadvantages of ELISA lie on its requirement of prolonged operation time, complicated procedures and instruments. Our results showed that compared with ELISA, DIGFA had relatively similar results, but with significantly much shorter time. We considered that DIGFA assay was not only very fast and simple, but also considerably stable, sensitive, specific, and practical.
Overall, the advantages in using DIGFA to test the Anti-rEPC1 IgG are as follows: The operation period is shortened from a few hours to 5 min and the results are reliable and visible to the naked eye. The specificity and sensitivity are approximately equal to those of the ELISA. The rEPC1is coated on the nitro-cellulose millipore filtering membrane in a solid phase, with durable activity; the colloidal gold labeling reagent can be preserved beyond one year; the manipulations are simple and no sophisticated testing instrument required; the operator can be trained in a simple way. Finally, DIGFA is an ideal, rapid and simple procedure, with the visual interpretation of results that can be utilized in quickly diagnosis of hydatidosis/echinococcosis both in field situation and in the clinic.
Results obtained from used method (DIGFA) in this study are relatively similar to Dot-ELISA results, while blocking and washing steps, which should be done in dot-ELISA, are not needed in this method. Moreover, no secondary antibody is used in DIGFA. So DIGFA could be done in shorter time in compare to dot-ELISA.
It should be noticed that such method, which its results are, reported ocular could be affected by any kind of mistake. Since rEPC1 labeled with colloidal gold has a base color of purple and this color is seen always on the paper, then in order to distinguish negative from positives results, there should be always a negative and a positive control beside any test.
Nevertheless, in future supplementary studies, in order to standardization of this method cross-reactions should be studied with dog sera infected with other parasites and infections.
At the same time as infected dogs are usually herd dogs or guard dogs and this needs a rapid, simple and possible screening technique, hence mentioned method in this study could be a appropriate candidate.
Conclusion
Since dog has primary role in the transmission dynamics of CE, detection of E. granu-losus in the definitive host plays a significant role in implementing hydatid control programs. So a rapid and simple procedure, with the visual interpretation of results and reagent stability with the aid of nano particles could be particularly suitable for field testing and epidemiological surveys.
Acknowledgement
This study was supported by grant number: 88000408, from Iran National Science Foundation. The authors declare that they do not have any conflicts of interest.
References
- 1.Rajabloo M, Hosseini SH, Jalousian F.Morphological and molecular characterisation of Echinococcus granulosus from goat isolates in Iran. Acta Trop. 2012; 123: 67– 71. [DOI] [PubMed] [Google Scholar]
- 2.Rokni MB.Echinococcosis /hydatidosis in Iran. Iran J Parasitol. 2009; 4( 2): 1– 16. [Google Scholar]
- 3.Moro P, Schantz PM.Echinococcosis: a review. Int J Infect Dis. 2009; 13: 125– 33. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti E, Garcia HH, Junghanss T.International CE Workshop in Lima, Peru, Cystic echinococcosis: chronic, complex, and still neglected. PLoSNegl Trop Dis. 2011; 5: 11– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmadi NA, Badi F.Human hydatidosis in Tehran, Iran: A retrospective epidemiological study of surgical cases between 1999 and 2009 at two university medical centers. Trop Biomed. 2011; 28( 2): 450– 6. [PubMed] [Google Scholar]
- 6.Tavakoli H, Bayat M, Kousha A.Hydatidosis Infection Study in Human and Livestock Populations During 2002–2007. Am-Eurasian J Agri Environ Sci. 2008; 4: 473– 7. [Google Scholar]
- 7.Amin Pour A, Hosseini SH, Shayan P.Comparative genotyping of Echinococcus granulosus infecting buffalo in Iran using cox1 gene. Parasitol Res. 2011; 108: 1229– 34. [DOI] [PubMed] [Google Scholar]
- 8.WHO Informal Working Group International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003; 85: 253– 61. [DOI] [PubMed] [Google Scholar]
- 9.Carmena D, Benito A, Eraso E.Antigens for the immunodiagnosis of Echinococcus granulosus infection. An update. Acta Trop. 2006; 98: 74– 86. [DOI] [PubMed] [Google Scholar]
- 10.Chen XH, Wen H, Zhang ZX, Feng XH, Zhang JP, Zhang JH, Ma XD, Zheng SS.Field trial on rapid detection of echinococcosis by dot immunogold filtration assay (DIGFA) with whole blood sample. Chin J Parasitol Parasit Dis. 2005; 23( 2): 90– 92. [PubMed] [Google Scholar]
- 11.Liu DY, Hu WQ, Zhang HM.Application of dot immunogold filtration assay for detecting serum antibodies in clonorchiasis patients. Chin J Parasitol Parasit Dis . 2001a; 19( 2): 97– 99. [PubMed] [Google Scholar]
- 12.Liu ZP, Li H, Zhu LY.Application of dot immunogold filtration assay in antibody detection for cysticercosis. Chinese Journal of Parasitology & Parasitic Diseases. 2001b; 19( 6): 354– 6. [PubMed] [Google Scholar]
- 13.Wang Y, Shim XH, Gan XX.Dot immuno-gold filtration assay in the diagnosis of suspected paragonimiasis and evaluation of chemotherapeutic effect. Chin J Parasitol Parasit Dis. 2007; 25( 1): 65– 68. [PubMed] [Google Scholar]
- 14.Abdi J, Kazemi B, Haniloo A, Mohebali M, Mahmoudi M, Rezaei S, Bandehpour M, Maghen L, Rokni MB.Serological Evaluation of EgAgB16 kDa, a Recombinant Antigen from Echinococcus granulosus for Diagnosis of Human Hydatidosis. Iran J Parasitol. 2010; 5( 3): 1– 10. [PMC free article] [PubMed] [Google Scholar]
- 15.Bora U, Chugh L, Nahar P.Covalent immobilization of proteins onto photoactivated polystyrene microtiter plates for enzyme linked immunosorbent assay procedures. J Immunol Meth. 2002; 268: 171– 7. [DOI] [PubMed] [Google Scholar]
- 16.Frens G.Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat Phys Sci. 1973; 241: 20– 22. [Google Scholar]
- 17.Rance G, Marsh D, Khlobystov A.Extinction coefficient analysis of small alkanethiolatestabilised gold nanoparticles. Chem Phys Lett. 2008; 460: 230– 6. [Google Scholar]
- 18.Etebar F, Jalousian F, Hosseini SH, Kordafshari S, Najafi A.Immunoproteomics approach for EPC1 antigenic epitope prediction of G1 and G6 strains of Echinococcus granulosus. Parasitol Res. 2013; 112( 9): 3129– 35. [DOI] [PubMed] [Google Scholar]
- 19.Dar VS, Ghosh S, Broor S.Rapid detection of rotavirus by using colloidal gold particles labeled with monoclonal antibody. J Virol Meth. 1994; 47: 51– 58. [DOI] [PubMed] [Google Scholar]
- 20.Xiao LY, Yan XJ, Mi MR, Han FC, Hou Y.Preliminary study of a dot immunogold filtration assay for rapid detection of anti-HCV IgG. World J Gastroenterol. 1999; 5( 4): 349– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urdal P, Borch SM, Landaas S, Krutnes MB, Gogstad GO, Hjortdahl P.Rapid immunometric measurement of C-Reactive protein in whole blood. Clin Chem. 1992; 38: 580– 4. [PubMed] [Google Scholar]
- 22.Beristain CN, Rojkin LF, Lorenze LE.Evaluation of dipstick method for detection of human immunodeficiency virus infection. J Clin Lab Anal, 1995; 9: 347– 50. [DOI] [PubMed] [Google Scholar]
- 23.Spielberg F, Kabeya CM, Ryder RW, Kifuani NK, Harris J, Bender TR.Field testing and comparative evaluation of rapid visually read screening assays for antibody to human immunodeficiency virus. The Lancet. 1989; 1: 580– 4 [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Xu DZ, Yan Y-P, Zhang JX, Liu Y, Li RL.Evaluation of dot immunogold filtration assay foranti-HAV IgM antibody. World J Gastroenterol. 1999; 5( 2): 132– 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q, Lan X, Tong T, Wu X, Chen M, Feng X, Liu R, Tang Y, Zhu Z.Dot-Immunogold Filtration Assay as a Screening Test for Syphilis. J Clin Microbiol. 1996; 34( 8): 2011– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Y, Zhou Y, Mo Z, Cheng W, Yang S, Wang X, Chen F.Rapid detection of aflatoxin B(1) on membrane by dotimmunogold filtration assay. Talanta. 2010; 81( 3): 792– 8. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Zhan W, Xing J.Development of dot-immunogold filtration assay to detect white spot syndrome virus of shrimp. J Virol Meth. 2006; 132: 212– 5. [DOI] [PubMed] [Google Scholar]


