Abstract
Background:
Dense granules are immunodominant proteins for the standardization of immunodiagnostic procedures to detect neosporosis. In the presented study different fragment of a dense-granule protein was evaluated for serodiagnosis of Neospora caninum in cattle and water buffalo.
Methods:
NcGRA7, from N. caninum tachyzoites was amplified. PCR product and pMAL-c2X plasmid were digested with EcoR1 restriction enzyme and expressed in Escherichia coli to evaluate its competence for detection of anti- N. caninum antibodies with ELISA in comparison with commercial IDEXX ELISA. Furthermore, 230 sera of presumably healthy cattle and water buffaloes (108 cattle and 122 water buffaloes) were analyzed by both tests to determine the agreement of these two procedures.
Results:
Sensitivities and specificities of NcGRA7-based ELISA were 94.64% and 90.38% respectively using sera of cattle, but were 98.57% and 86.54% in the case of buffaloes respectively. A good correlation between the results of IDEXX ELISA and ELISA based on recombinant NcGRA7 for detecting N. caninum antibodies was appeared. Analyzing by Mc Nemar′s showed that NcGRA7-based ELISA has acceptable capability to differentiate the positive results in comparison with IDEXX ELISA.
Conclusion:
NcGRA7-based ELISA considering utilized new fragment of genomic DNA is a good tool for serodiagnosis of anti- N. caninum antibodies for screening and epidemiological purposes on cattle herd and water buffaloes as well.
Keywords: Neospora caninum, ELISA, NcGRA7, Recombinant antigen, Cattle, Water buffalo, Iran
Introduction
Cyst-forming coccidian Neospora caninum (Apicomplexa: Sarcocystidae) is an important intracellular protozoan parasite and causes neosporosis that related to congenital birth defects in cattle and water buffalo (Bubalus bubalis) worldwide (1, 2). The parasite was first recognized in 1984 from Norwegian dogs when Bjerkas et al. reported unidentified protozoan parasites belong to sporozoa (3).
Confirmed definitive hosts of N. caninum are dogs and coyotes, but other wild canids may play a role as definitive hosts. Multitude of mammalian species including cattle, water buffalo, sheep, goat, deer, horse and canids are defined as intermediate hosts of the N. caninum, however researches revealed that the rage of these hosts is actually numerous (4–7). This infection can cause serious problems especially when occur simultaneously with other infectious diseases. For instance, 9.4% of dogs in Meshkin-Shahr, which infected with N. caninum had afflicted with Leishmania infantum infection (8). Transmission of N. caninum to intermediate hosts occurs either by ingestion of sporoulated oocysts via contaminated food and water or vertically from mother to fetus through the placenta (5–9). Abortion and other reproductive failures are the main features of the infection in adult cows and water buffaloes. Abortions originated from N. caninum can occur at any time of gestation but the majority of abortions are at 5–6 months of gestation and this situation always leads to serious economic losses (2, 4, 9, 10).
Serologic procedures are routinely and widely used among the available diagnostic tests for detection of N. caninum infection. Currently, serologic assays have been developed to determine the anti-N. caninum antibodies in cattle including immunoblotting, the indirect fluorescent antibody test ( IFAT), direct modified agglutination test (MAT) and ELISA (4,11–14).
For these purposes, using the recombinant immunodominant proteins, for instance surface NcSAG1, NcSRS2 and NcSAG4 bradyzoite stage-specific proteins and the dense-granule protein NcGRA7 of N. caninum have been reported as helpful immunogenic targets in the literature (15–20).
Most serodiagnostic tools such as N. caninum commercial kits utilize antigens from whole and or sonicated tachyzoites, which always burden production costs. On the other hand, cross-reactions with prevalent and related parasite, Toxoplsama gondii, and even existence of host materials affect the specificity of the tests (13, 14, 21). Recombinant antigen based procedures could overcome these drawbacks and it seems that dense-granule antigen, GRA7, which present by both tachyzoite and bradyzoite stages of N. caninum has a good competence to detect antibodies in early phase of infection (18, 22, 23).
Water buffaloes (Bubalus bubalis) as well as cattle are economically important animals in tropical areas and little is known about the applied epidemiological tools to investigate or to screen of neosporosis in these animals. Hence, we aimed to establish an ELISA based upon different fragments of recombinant NcGRA7 protein in an efficient prokaryotic expression system for diagnosis of N. caninum infection in cattle and water buffaloes as well.
Materials and Methods
Parasite
N. caninum tachyzoites, Nc-1 strain, were grown in monolayers of Vero cells cultured in RPMI 1640 medium (Sigma Co., USA) fortified by 2% of fetal calf serum, penicillin (10,000 U/ml), streptomycin (100 mg/ml) and amphotericin B (25 mg/ml) at 37 ºC with 5% CO2 in tissue culture flasks. Tachyzoites were harvested by scraping the monolayer when 80% of the cells were infected and then purified by successive passage of the cell monolayer through a 27-gauge needle and centrifuged to remove host cell debris. The supernatant was collected, centrifuged and washed twice in phosphate-buffered saline afterward (PBS, pH 7.2) (15).
DNA extraction and PCR
Tachyzoites were prepared at a concentration of 1×108/ml and extraction of genomic DNA was carried out by commercial kit (Qiagen) according to the kit protocol. The DNA sequences were obtained from the GenBank database and the accession number—U82229 used to design the required primers. Designed and applied forward and reverse primers were 5′- GCGAGAATTCGCTGGAGACTTGG-3′ and 5′-GCGAGAATTCCTATTCGGTGT-3′ respectively. PCR was carried out in a total volume of 50 μl, containing 5 μl of DNA template, 1 μl of each primer at 20 pmol, 1 μl of 10 mM dNTPs mix, 5 μl of 109 PFU PCR buffer, 1.5 μl of 50 mM MgCl2 and 5u PFU DNA polymerase (BioNeer, Korea). PCR condition was as follow: pre denaturation at 94°C for 5 min, denaturation at 94 °C for 1 min, annealing at 51°C for 1 min, extension at 72 °C for 90s (35 repeats) and final extension at 72°C for 7 min in a thermal cycler. The amplified DNA was visualized on 1.0% agarose gel stained with cyber safe then sequence analysis of PCR product was followed up.
Cloning and expression of NcGRA7
PCR product was purified from gel with the gel extraction kit (Vivantis, Malaysia). The purified PCR product and pMAL-c2X plasmid (New England Biolabs, USA) were digested with EcoR1 restriction enzymes (Fermentase, Latvian) at 37 °C for 2.5 h and were extracted from agarose gel using the gel extraction kit. Ligation process was applied on digested DNA fragment and plasmid vector at 22 °C for 2 h by T4 DNA ligase (Fermentas, Latvian), followed by overnight incubation at 4 °C. Escherichia coli host strain TG1 was transformed by the ligation product using Chung transforming method (24). Transformed colonies appeared on the selective Luria–Bertani (LB) agar, were grown in LB broth containing ampicillin. Extraction of plasmid was performed by Vivantis plasmid extraction kit and presence of GRA7 inserts were confirmed by restriction digestion of recombinant plasmids with EcoR1.
Western blotting
After SDS-PAGE, the protein bands were transferred onto nitrocellulose membrane. The membrane was blocked with 0.2% Tween 20 in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2PO4, 1.4 mM KH2PO4 plus 0.05% Tween) at ambient temperature for 2 h. The blots were washed three times with PBST (0.05% Tween 20 in PBS) and incubated with 1/100 dilution of positive bovine serum for 1 h. The membranes washed three times with PBST and incubated with 1/1000 dilution of peroxidase conjugated with rabbit anti-bovine IgG (Sigma, USA) for 1 h. The membranes were washed with PBST then by PBS and soaked in 4-chloro-1-naphthol and hydrogen peroxide afterward (H2O2).
Serum samples
Negative and positive sera of cattle and water buffaloes confirmed by two commercial ELISA kits (IDEXX and ID VET, France) and N. caninum agglutination test (NAT) as well. Blood samples were also collected utilizing venoject tubes from jugular vein of 230 apparently healthy cattle and water buffaloes from Ahvaz district (108 cattle and 122 water buffaloes). Collected blood samples were centrifuged at 1000×g and the supernatants were frozen at −20°C until the examinations were performed.
ELISA
Checkerboard procedure was conducted to set up the NcGRA7-based ELISA. The cutoff point calculated as average of optical density of negatives plus two standard deviations. Finally, polystyrene microtitre plates (kariz Mehr, Iran) were coated with 0.1 μg/μl of recombinant NcGRA7 diluted in 100 μl of 0.1 M carbonate buffer (pH=9.6) as coating buffer and incubated overnight at 4°C. After three times washing with PBS-T (PBS containing 0.05% Tween 20), wells were blocked for 2.5 hours at 37°C using PBS containing 0.2% Tween 20 and washed as mentioned. The sera, negative and positive controls were diluted 1:400 in blocking buffer, added to each well at a volume of 100 μl and incubated at ambient temperature for 30 minutes and washed subsequently. Thereafter, 100 μl/well of horse-radish anti-bovine peroxidase conjugate (Sigma, USA) diluted 1:10000 in blocking buffer was added at room temperature for 1 hour. Plates were washed and enzyme activities were revealed by 10 minutes incubation of 100 μl/well with tetramethylbenzidine (TMB) (Sigma, USA) and 3% H2O2 as substrate and chromogen. Reactions were stopped with 0.1 M of HCL and at last the plates were read with microtiter plate reader (Tadjhiz Afzar Teb, Iran) at wavelength of 450 nm. For each test a well was coated with purified MBP to cover false reactions of positive sera with this protein.
Due to appearing the existence of cross-reaction with closely related parasite, T. gondii, all sera were tested for the presence of T. gondii antibodies utilizing the agglutination tests based on the direct agglutination of fixed parasites with sera pre-treated with 2-mercaptoethanol to prevent non-specific IgM agglutination, as described by Dubey and Desmonts (21).
Statistical analyzes
The Kappa statistic test (ϰ) was used to test the level of agreement between the IDEXX ELISA and ELISA based on recombinant NcGRA7 for detection of N. caninum infection in cattle and water buffaloes. Kappa and its 95% Confidence Interval (CI), was used further to measure the degree of agreement between the procedures after taking into account the probability of agreement by chance alone. Strength of agreement based on ϰ was judged according to the following guidelines: <0.2=slight; 0.2–0.4=fair; 0.4–0.6=moderate; 0.6–0.8=good; >0.8=very good. Mc nemar's test was also utilized for comparing the percentage of positive reactions (25).
Results
Cloning, expression and purification of the recombinant NcGRA7
Utilizing mentioned primers, expected PCR product of 600 bp was amplified (Fig. 1) and its integrity was assured by sequencing.
Fig. 1:
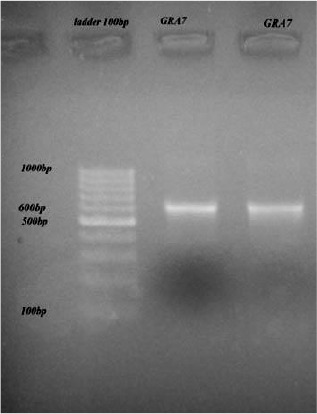
The PCR products revealing 600 bp bands
Sixty clones appeared on LB agar after expression and subsequent transformation. Screening was done with digesting plasmids by EcoR1 enzyme. Finally, three clones carried the recombinant plasmid and expressed the MBPNcGRA7 protein. Expression of a protein of about 66 kDa, corresponding to predicted molecular weight of MBPNcGRA7 (GRA7:24 kDa and MBP: 42 kDa) was recognized by SDS-PAGE (Fig. 2).
Fig. 2:
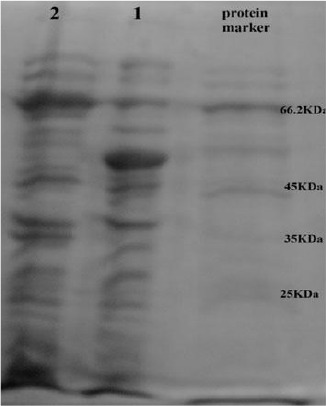
SDS-PAGE electrophoresis on 10% polyacrylamide gel. 1 E. coli strain TG1 containing pMAL-c2X as control after induction. 2 E. coli extracts expression of pMAL-c2X-GRA7
Cloning and expression of NcGRA7 in E. coli confirmed with reaction of expressed protein in Western blotting (Fig. 3) with a N. caninum positive serum.
Fig. 3:

Western blotting analysis. Lane 1 reveals marker protein and lane 2 reveals the reactivity positive serum with bacteria expressing MBP and MBP-GRA7
Serology
The mean value of ELISA OD of the negative sera plus 2 standard deviations gave a cutoff point of 0.355 and 0.384 for determination of existing N. caninum specific antibodies in cattle and water buffaloes respectively. Compared with IDEXX ELISA, relative sensitivities and specificities of NcGRA7-based ELISA were 94.64% and 90.38% respectively using sera of cattle, but were 98.57% and 86.54 in the case of buffaloes respectively (Table 1). There was no cross-reaction with related apicomplexan parasite, T. gondii.
Table 1:
The sensitivity and specificity of recombinant NcGRA7 ELISA in comparison with IDEXX ELISA
| IDEXX ELISA | ELISA(Buffalo) | ELISA(Cattle) | |||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Positive | 69 (a) | 7 (b) | Positive | 53 (a) | 5 (b) |
| Negative | 1 (c) | 45(d) | Negative | 3 (c) | 47 (d) |
| Total | 70 (a + c) | 52 (b + d) | Total | 56 (a + c) | 52 (b + d) |
| Sensitivity% | 98.57 | 94.64 | |||
| Specificity% | 86.54 | 90.38 | |||
Sensitivity = a/ (a + c); Specificity = d/ (b + d)
Kappa statistical test showed a good correlation between the results of IDEXX ELISA and ELISA based on recombinant NcGRA7 for detecting N. caninum antibodies in cattle (k= 0.85), and also water buffaloes (k= 0.83).
Furthermore, Mc Nemar′s analyzing revealed that NcGRA7-based ELISA has acceptable competence to discriminate the positive results in comparison with IDEXX ELISA (P >0.05).
Discussion
Neosporosis is one of the serious economic problems in dairy and beef industry worldwide. To date, many researchers have studied on different aspects of N. caninum and many studies were reported the prevalence of bovine infection with N. caninum. The precise and fast diagnosis of N. caninum infections has always a key role on interpreting the prevalence and distribution patterns and subsequently prediction from transmission especially in its vertical form. Since, there is no practical effective vaccine or treatment for neosporosis (26), this ideal goal will lead us to preclude the abortions because of this disease.
On the other point of view, it is also necessary to finding definitive markers detecting specific antibodies of N. caninum and decrease cross-reaction with closely related parasite, Toxoplasma gondii (4, 27).
Succeeding the evaluation of the first serological test, the IFAT, for detection of antibodies to N. caninum (28), numerous ELISA procedures have been defined by many authors applying whole or recombinant antigens (29, 30). In the present study, potential of one recombinantly produced antigen was evaluated for the first time in water buffalo.
Recombinant antigens of N. caninum revealed appropriate for improving diagnostic purposes that can be used instead of native antigens, which often have inconstant and unreliable quality (31). Secretary proteins like micronemes, rhoptries, and dense granules released in early stages of infection with N. caninum, just as adhesion onto the host cell surface directly depends on the secretion of these substances from the sporozoites of the parasite. Moreover, dense granules also discharge their content into the parasitophorous vacuole throughout the intracellular stage (32). We predict that these purified and high quality proteins can alternatively detect the early infections when they utilize as antigen in ELISA.
NcGRA7 is an immunodominant antigen in both of tachyzoite and bradyzoite stages (33). Therefore, it sounds applying the recombinant NcGRA7 is a suited and helpful marker for both active and chronic diagnosis of N. caninum infection and can serve as a potential vaccine candidate (34). This issue is more considerable when the competence of NcGRA7 for demonstration of infection with N. caninum in early stages of parasite life cycle has been reported in bovines (34, 35).
In the present study, we intended to clone and express the NcGRA7 using the suitable and novel plasmid and vector for this purpose. At our knowledge, cloning and expression of NcGRA7 has been performed in different expression systems such as pCMVi-luc1 eukaryotic expression vector or bacterial expression vector, pGEX-4T-3 (31, 36). Here, we successfully cloned and expressed NcGRA7 for the first time with new and different primers which have sites for EcoR1 restriction enzyme. The pMALc2x used as a vector for gene expression and protein production. This vector has a strong promoter and its protein expression will be high compared to the other vectors. The rate of gene cloning into this vector and subsequent transformation is high and noticeable, which enables work with this vector to be done more easily than with the other vectors. The presence of lac promoter in this vector increases the protein expression and under IPTG induction, this will be enhanced considerably. In addition, the presence of maltose in recombinant expressed protein will facilitate its purification. The NcGRA7 (24 KDa) protein showed a very similar molecular mass for protein expressed by N. caninum.
Western blotting revealed that performed cloning and expression with different primers can produce protein that able to bind successfully with N. caninum antibodies.
Serological results of current study were compared with widely used commercially available ELISA by IDEXX. Shown by kappa statistical and Mc Nemar's analysis, there was good agreement between NcGRA7-based ELISA and frequently used commercial ELISA to diagnose anti- N. caninum antibodies either for cattle or water buffaloes samples. On the other hand, sensitivity and specificity of presented ELISA were nearly comparable with previous reports (34, 37). N. caninum has huge antigenic analogy to those of T. gondii. The sensitivity of our developed ELISA is more acceptable since there was no cross reactivity with T. gondii, by inference sera that did not react with commercial ELISA but identified positive by NcGRA7-based ELISA was not due to cross reactivity with this jointly related and very common protozoa.
Conclusion
We believe that NcGRA7-based ELISA under considered circumstances and high sensitivity and specificity is an appropriate achievement to discriminate between seropositive and seronegative bovines and water buffaloes and is cost-effective simple procedure, do not need skilled technicians for screening and epidemiological purposes.
Acknowledgments
This study was supported by Shahid Chamran University of Ahvaz, Iran, and the authors wish to thank the Vice- Chancellor for Research of Shahid Chamran University for the research opportunity. The authors also declare that there is no conflict of interests.
References
- 1.Dubey JP, Schares G.Diagnosis of bovine neosporosis. Vet Parasitol. 2006; 140: 1– 34. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M, Barr B, Rowe J, Conrad P.Neosporosis in dairy cattle. Jpn J Vet Res. 2012; 60: 51– 4. [PubMed] [Google Scholar]
- 3.Bjerkas I, Mohn SF, Presthus J.Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkunde 1984; 70: 271– 274. [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP, Lindsay DS.A review of Neospora caninum and neosporosis. Vet Parasitol. 1996; 67: 1– 59. [DOI] [PubMed] [Google Scholar]
- 5.McAllister MM, Dubey JP, Lindsay DS, et al. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998; 28: 1473– 1478. [PubMed] [Google Scholar]
- 6.Gondim LFP, McAllister MM, Pitt WC, Zemlicka DE.Coyotes ( Canis latrans) are definitive hosts of Neospora caninum. Int J Parasitol. 2004; 34: 159– 161. [DOI] [PubMed] [Google Scholar]
- 7.Klevar S.Tissue cyst forming coccidian; Toxoplasma gondii and Neospora caninum as a cause of disease in farm animals. Acta Vet Scand. 2007. 49, ( Suppl1): S1.18221573 [Google Scholar]
- 8.Sharifdini M, Mohebali M, Keshavarz H, Hosseininejad M, Hajjaran H, Akhoundi B, Rahimi Foroushani A, Zarei Z, Charehdar S. Neospora caninum and Leishmania infantum co-infection in domestic dogs ( Canis familiaris) in Meshkin-Shahr district, Northwestern Iran. Iran J Arthropod-Borne Dis. 2011; 1( 3): 60– 68. [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey JP.Recent advances in Neospora and neosporosis. Vet Parasitol. 1999; 84: 349– 367. [DOI] [PubMed] [Google Scholar]
- 10.Hall CA, Reichel MP, Ellis JT. Neospora abortions in dairy cattle: diagnosis, mode of transmission and control. Vet Parasitol. 2005; 128: 231– 241. [DOI] [PubMed] [Google Scholar]
- 11.Osawa T, Wastling J, Maley S, et al. A multiple antigen ELISA to detect Neospora-specific antibodies in bovine sera, bovine foetal fluids, ovine and caprine sera. Vet Parasitol. 1998; 79: 19– 34. [DOI] [PubMed] [Google Scholar]
- 12.Packham AE, Sverlow KW, Conrad PA, et al. A modified agglutination test for Neospora caninum: Development, optimization, and comparison to the indirect fluorescent-antibody test, and comparison to the indirect fluorescent-antibody test and enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1998; 5: 467– 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkinson R, Harper PAW, Reichel MP, Ellis JT.Progress in the serodiagnosis of Neospora caninum infections of cattle. Parasitol Today. 2000; 3: 110– 114. [DOI] [PubMed] [Google Scholar]
- 14.Silva DAO, Lobato J, Mineo TWP, Mineo JR.Evaluation of serological tests for the diagnosis of Neospora caninum infection in dogs: optimization of cut-off titers and inhibition studies of cross reactivity with Toxoplasma gondii. Vet Parasitol. 2007; 143: 234– 244. [DOI] [PubMed] [Google Scholar]
- 15.Howe DK, Crawford AC, Lindsay D, Sibley LD.The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect Immun. 1998; 66: 5322– 5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguado-Martínez A, lvarez-García Á, Fernández-García G, et al. Usefulness of rNcGRA7- and rNcSAG4-based ELISA tests for distinguishing primo-infection, recrudescence, and chronic bovine neosporosis. Vet Parasitol. 2008; 157: 182– 195. [DOI] [PubMed] [Google Scholar]
- 17.Romano MI.An applied printing immunoassay with recombinant Nc-SAG1 for detection of antibodies to Neospora caninum in cattle. J Vet Diagn Inves. 2011; 23: 971– 976. [DOI] [PubMed] [Google Scholar]
- 18.Hiasa J, Nishimura M, Itamoto K, et al. Enzyme-linked immunosorbent assays based on Neospora caninum dense granule protein 7 and profilin for estimating the stage of neosporosis. Clin Vaccine Immunol. 2012; 19: 411– 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiménez-Ruiz E, Bech-Sàbat G, Alvarez-García G, et al. Specific antibody responses against Neospora caninum recombinant rNcGRA7, rNcSAG4, rNcBSR4 and rNcSRS9 proteins are correlated with virulence in mice. Parasitology. 2013; 140: 569– 79. [DOI] [PubMed] [Google Scholar]
- 20.Terkawi MA, Kameyama K, Rasul NH, et al. Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clin Vaccine Immunol. 2013; 20: 596– 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubey JP, Desmonts G.Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet J. 1987; 19: 337– 9. [DOI] [PubMed] [Google Scholar]
- 22.Nigro M, Gutierrez A, Hoffer AM, et al. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn Micr Infect Dis. 2003; 47: 609– 613. [DOI] [PubMed] [Google Scholar]
- 23.Pietkiewicz H, Hiszczyńska-Sawicka E, Kur J, et al. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J Clin Microbiol. 2004; 42: 1779– 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung CT, Niemela SL, Miller RH.One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Biochemistry. 1989; 86: 2172– 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohoo IR, Martin SW, Stryhn H. In: Dohoo IR. (Ed.). Vet Epidemiol Res. AVC Inc., Charlottetown, 2003; p: 706. [Google Scholar]
- 26.Dubey JP, Schares G, Ortega-Mora LM.Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007; 20: 323– 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chahan B, Gaturaga I, Huang X, et al. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet Parasitol. 2003; 118: 177– 185. [DOI] [PubMed] [Google Scholar]
- 28.Dubey JP, Hattel AL, Lindsay DS, Topper MJ.Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988; 193: 1259– 1263. [PubMed] [Google Scholar]
- 29.Paré J, Hietala SK, Thurmond MC.An enzyme-linked immunosorbent assay (ELISA) for serological diagnosis of Neospora sp. infection in cattle. J Vet Diagn Invest. 1995; 7: 352– 599. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkman C, Alvarez-Garcia G, Conraths FJ, et al. Neospora caninum IgG avidity tests: an interlaboratory comparison. Vet Parasitol. 2006; 140: 273– 280. [DOI] [PubMed] [Google Scholar]
- 31.Hara AO, Liao M, Baticados W, Bannai H, et al. Expression of recombinant dense granule protein 7 of Neospora caninum and evaluation of its diagnostic potential for canine neosporosis. J Protozool Res. 2006; 16: 34– 41. [Google Scholar]
- 32.Sonda S, Fuchs N, Gottstein B, Hemphill A.Molecular characterization of a novel micro-neme antigen in Neospora caninum. Mol Biochem Parasitol. 2000. 108: 39– 51. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Garcia G, Pitarch A, Zaballos A, et al. The NcGRA7 gene encodes the immunodominant 17 kDa antigen of Neospora caninum. Parasitology. 2007; 134: 41– 50. [DOI] [PubMed] [Google Scholar]
- 34.Huang P, Liao M, Zhang H, Lee G, et al. Dense-granule protein NcGRA7, a new marker for the serodiagnosis of Neospora caninum infection in aborting cows DI196. Clin Vaccine Immunol. 2007; 14: 1640– 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Garcia G, Lopez-Perez I, Collantes-Fernandez Innes E, et al. Use of an immunodominant P17 antigenic fraction of Neospora caninum in detection of antibody response in cattle. Mem Inst Oswaldo Cruz. 2006; 101: 529– 534. [DOI] [PubMed] [Google Scholar]
- 36.Liddell S, Parker C, Vinyard B, et al. Immunization of mice with plasmid DNA coding for NcGRA7 or NcsHSP33 confers partial protection against vertical transmission of Neospora caninum. J Parasitol. 2003; 89: 496– 500. [DOI] [PubMed] [Google Scholar]
- 37.Wilkowsky SE, Bareiro GG, Mon ML, et al. An applied printing immunoassay with recombinant Nc-SAG1 for detection of antibodies to Neospora caninum in cattle. J Vet Diagn Invest. 2011; 23: 971– 6. [DOI] [PubMed] [Google Scholar]


