Abstract
Background
Multiple sclerosis (MS) is a demyelinating autoimmune disease of the central nervous system with possible involvement of vascular dysregulation secondary to endothelial dysfunction caused by destruction of the vessel wall. Vascular dysregulation leads to excessive vasoconstriction or insufficient vasodilatation, resulting in vasospasm mediated by endothelin-1 (ET-1), the most potent and long-lasting mediator. Vascular dysregulation can play an important role in the pathogenesis of some eye disorders and it has been hypothesized that it is a vascular risk factor for glaucomatous optic neuropathy. The aim of this study was to estimate endothelin-1 (ET-1) plasma levels in patients with MS.
Material/Methods
The MS group consisted of 39 patients (9 males, 30 females), mean age: 38.8±10.02 years, range: 22–62. The control group consisted of 27 healthy volunteers (3 males and 24 females), mean age: 37.4±10.88 years, range: 20–62; clinically, in a non-active stage of the disease. ET-1 plasma levels were measured using the Endothelin-1 ELISA Kit (Immuno-Biological Laboratories Co., Japan). Statistical analysis was performed with the nonparametric Mann-Whitney U test for independent groups.
Results
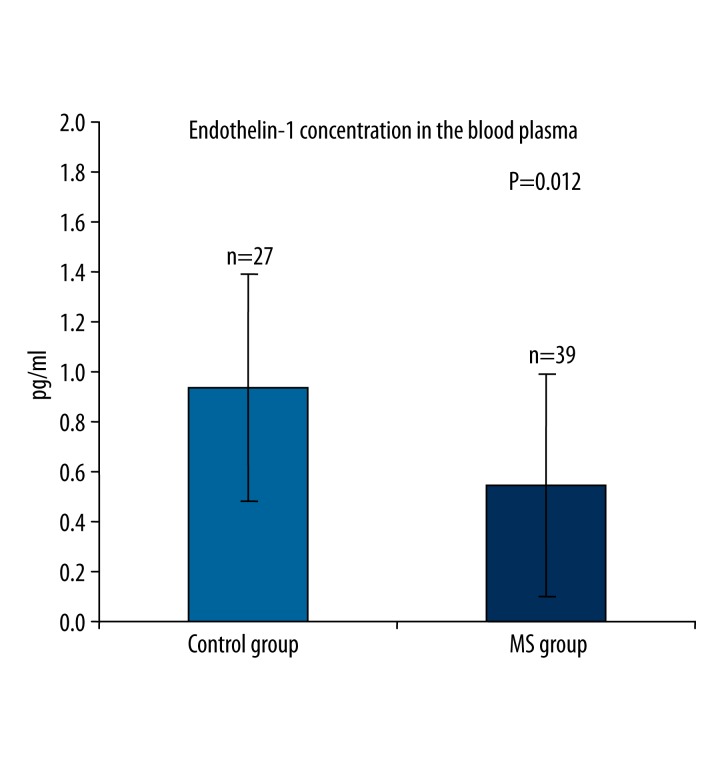
Endothelin-1 (ET-1) plasma levels were significantly lower in MS patients compared to healthy controls: mean value 0.55±0.44 pg/ml (146.05±118.27 fmol/ml) vs. 0.95±0.48 pg/ml (252.83±127.16 fmol/ml); P=0.012.
Conclusions
Significantly decreased ET-1 plasma levels in the MS patients could reflect the non-active disease at the time of ET-1 measurements or the effects of immunomodulatory treatment, but it cannot be excluded that decreased ET-1 plasma levels in these patients might result from vascular dysregulation.
Keywords: Endothelin-1, Glaucoma, Multiple Sclerosis
Background
In the pathogenesis of multiple sclerosis (MS), which is an autoimmune disease, pathologic remodelling of the blood-brain barrier precedes the formation of focal demyelination in the brain white matter [1–3]. The remodelling of the blood-brain barrier in the course of MS is controlled by the activated T CD4+ lymphocytes and cytokines they release [2]. In the initial phases, endotheliopathy caused by the adhesion of activated T lymphocytes is observed within the blood-brain barrier, and these lesions can lead to vascular dysregulation. Vascular dysregulation can be congenital, or primary (PVD) or secondary (SVD). The latter is often a result of autoimmune disease, including MS [4–6]. The vascular dysregulation syndrome, earlier referred to as the vasospastic syndrome, is characterized by increased spasticity of blood vessels manifested as excessive vasoconstriction or insufficient vasodilation [4]. Endothelial dysfunction is thought to play the main role in its pathogenesis as it results in the impairment of vasodilation and in increased blood levels of endothelin-1 (ET-1) [4,7]. ET-1 is one of the most potent and longest acting vasospastic substances [8–10] produced by the vascular endothelium. Apart from endothelial dysfunction, impaired humoral or neurogenic mechanisms, which additionally may be interrelated, can play a role in the pathogenesis of the vasospastic syndrome [7].
In the course of severe clinical attacks of MS increased concentrations of ET-1 were measured in the cerebrospinal fluid [11]. The few studies published to date found increased levels of ET-1 also in the blood of MS patients [12,13]. A transient increase of ET-1 plasma level has been reported in a patient with optic neuritis which very often is the first sign of MS [14]. MS-dependent optic neuropathy is characterized by a mild increase in the cup to disc ratio [15–17] and pallor of the optic disk suggesting ischaemia of the initial segment of the optic nerve which can result from a spasm of the supplying blood vessels. ET-1 has been shown to have a potent vasoconstrictive effect in the eye and hence to decrease the ocular blood flow [7,18–20], including blood supply to the optic disc [21,22]. Studies using color Doppler imaging have shown in MS patients a decreased blood flow velocity and increased vascular resistance index (RI) in the extraocular vessels [13,23–25], which indicates a reduction of the ocular blood flow in the course of MS.
The aim of this study was to determine the ET-1 levels in the plasma of MS patients in view of a potential role of ET-1 in the vascular dysregulation observed in the course of MS [4,6,26].
Material and Methods
The study protocol was approved by the Bioethics Committee at the Medical Centre for Postgraduate Education in Warsaw. The patients were requested to sign written informed consent to participate in the research study. The exclusion criteria were as follows: a history of cardiovascular disease, tobacco smoking, dyslipidemia, and current treatment with vasoconstrictors or vasodilators. Lipid fractions (total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol) in the blood were determined in the study group and controls to exclude subjects with dyslipidemia, i.e., those whose measurements were outside any of the following ranges: total cholesterol: 130–200 mg/ml; triglycerides: 35–131 mg/ml; LDL cholesterol: <135 mg/ml; HDL cholesterol: 42–80 mg/ml. Following that, 20.41% and 12.9% of subjects were excluded from the initial MS group of 49 and from among 31 prospective controls, respectively. Additionally, subjects with a history of diseases known to be associated with increased blood levels of ET-1 were also excluded [4]. Ultimately, the study group consisted of 39 MS patients (9 males, 30 females) aged 22–62 years, mean age: 38.8±10.02. The diagnosis of MS was established using the McDonald criteria [29] and the patients were recruited at the Department of Neurology and Epileptology, Medical Centre for Postgraduate Education in Warsaw. The control group consisted of 27 healthy volunteers, free of any systemic disease, recruited mainly from among the hospital staff: 3 males and 24 females aged 20–62 years, mean age 37.4±10.88. Of the study group, 12.8% of the patients were diagnosed with primary progressive multiple sclerosis (PPMS), 15.4% with secondary progressive multiple sclerosis (SPMS), 20.5% with progressive relapsing multiple sclerosis (PRMS), and 51.3% with relapsing remitting multiple sclerosis (RRMS). At the time of the study, none of the patients were in the clinically active stage of the disease. A history and/or medical records revealed a relapse in the previous 2 years in 12 patients, in the previous year in 9 patients, and in the previous 6 months in 2 patients. However, in none of the patients a clinical relapse occurred in the 3 months preceding ET-1 measurements within the study. The mean duration of MS (the medically documented diagnosis) was 4.4 years (range: 1 year to 22 years). Of the 46.2% of patients in the study group who received treatment, 13 were treated with interferon β-1b, 4 patients with glatiramer acetate and 1 patient with azathioprine. The remaining patients did not take any medication either because of low activity of their disease or because their disease was newly diagnosed and no treatment had yet been instituted. The levels of clinically observed disabilities in the study patients were measured with the Kurtzke Expanded Disability Status Scale (EDSS) (30), the mean score being 2.55 (range: 1.0–6.5).
Blood was drawn from the basilic vein after at least a 30-minute rest in a relaxed position (supine), at room temperature, in the morning hours (9 a.m.–10 a.m.). In patients on interferon therapy, blood was collected not earlier than 24 hours after the last interferon injection. ET-1 plasma level determinations were conducted at the Department of Neuroendocrinology, Medical Centre for Postgraduate Education. Blood samples from 27 controls and 39 study patients were placed in test-tubes containing EDTA and aprotinin (protease inhibitor). After centrifugation of the blood samples at 4°C, plasma samples were frozen and stored at −70°C until endothelin extraction. ET-1 was extracted from 1.5 ml of plasma. Prior to plasma sample injections, Sep-Pak C18 extraction columns (Phoenix Pharmaceuticals, INC.; USA) were rinsed with 1 ml 60% acetonitrile with 1% trifluoroacetic acid, followed by 9 ml 1% trifluoroacetic acid. The plasma solution was loaded onto the pre-treated C18 column. Each column was washed with 6ml 1% trifluoroacetic acid. ET-1 was collected with 3ml of 60% acetonitrile in 1% trifluoroacetic acid. The eluate was evaporated under a stream of nitrogen. The sample extracts were stored at −70°C until endothelin-1 determination. Immediately prior to the quantitative determination of ET-1, the extracts were dissolved in 0.15 ml of PBS buffer containing 1% BSA and 0.05% Tweed 20. The ET-1 concentration was determined using the Endothelin-1 ELISA Kit (Immuno-Biological Laboratories Co., Japan; Code No. 27165). The efficiency of the extraction procedure was tested by adding a specified amount of exogenous endothelin-1 standard (20 pg/ml and 10 pg/ml) to 1.5 ml of plasma with the endogenous endothelin content below the analytical sensitivity of the method. Thus prepared samples were assessed using the same procedure as the study samples. The extraction efficiency of the procedure was 75.9%. The final measurements of ET-1 concentrations in the study samples were adjusted by the extraction efficiency. The ELISA method sensitivity was 8 fmol/ml. The ET-1 concentrations were below the limit of detection in 29.0% of the control samples and in 36.7% of the samples from MS patients. It was assumed that in such samples, endothelin-1 concentrations were below 8 fmol/ml.
Statistical analysis
The differences between the measurements were analyzed with the nonparametric Mann-Whitney U test for 2 independent groups of observations. Correlation analysis was performed using Spearman rank correlation. Statistical significance was established at P<0.05. Additionally, to assess the homogeneity of the study group, the ET-1 plasma levels were statistically analyzed in patients receiving immunomodulatory treatment vs. untreated patients, and in patients with RRMS vs. progressive courses of multiple sclerosis (SPMS, PPMS, PRMS). Statistical analysis of potential differences in the ET-1 levels between the patients with progressive courses of multiple sclerosis (SPMS, PPMS, PRMS) could not be performed due to small numbers of patients in these subgroups.
Results
The mean ET-1 (±SD) plasma level in MS patients was significantly lower than in healthy subjects: 0.55±0.44 pg/ml (146.05±118.27 fmol/ml) vs. 0.95±0.48 pg/ml (252.83±127.16 fmol/ml) (P=0.012) (Figure 1). Patients receiving immunomodulatory treatment tended to have lower ET-1 plasma levels than untreated patients, but the difference was not statistically significant: 0.51±0.51 pg/ml (135.98±136.66 fmol/ml) vs. 0.58±0.39 pg/ml (155.20±104.75 fmol/ml) (P=0.44). Also, there were no statistically significant differences in the ET-1 plasma levels between the patients with RRMS and those with the other progressive courses of MS (PPMS, SPMS, PRMS): 0.64±0.45 pg/ml (170.85±118.67 fmol/ml) vs. 0.45±0.44 pg/ml (118.77±117.72 fmol/ml)(P=0.46). No correlation was established between the ET-1 plasma levels and age (control group P=0.69, MS group P=0.77, all P=0.50) or the EDSS scores (P=0.66). Weak correlation between the ET-1 plasma levels and the disease duration was found (P=0.044).
Figure 1.
Endothelin-1 (ET-1) plasma levels (mean ± standard error of mean) in healthy controls and patients with multiple sclerosis (MS).
Discussion
The pathogenetic changes in MS result from the remodelling of the blood-brain barrier associated with endotheliopathy due to the effect of activated CD4+ T-cells [2]. Vasospastic mediators, including ET-1, are produced. In MS, ET-1 not only regulates the vascular wall tension, but may also act as a proinflammatory mediator, including its effect on the proliferation of astrocytes with an associated increase in the production of metalloproteases, which are involved in the remodeling of the extracellular matrix and the blood-brain barrier [3].
In the present study we observed significantly lower plasma levels of ET-1 in MS patients compared to healthy subjects, unlike earlier studies [12,13] that found significantly higher ET-1 blood levels in MS patients than in controls. Interestingly, the groups of MS patients in those studies and in our study are comparable in terms of age and size (Haufschild et al.: 20 patients, 10 females and 10 males, mean age 43.8 years ±14.7, Pache et al.: 30 patients, 22 females and 8 males, mean age 38 years ±7.5 years, the present study: 39 patients, 30 females and 9 males, mean age 38.8 years ±10.02. The MS patients in our study, as compared to the study by Pache et al. had a slightly lower level of disease activity and almost twice as short disease duration (4.4 years vs. 8.4 years), which might account for the lower blood levels of ET-1. Moreover, 46.2% of the patients in our study group were treated with immunosuppressants, which decrease the activity of T lymphocytes [31] and the production of T cell-dependent mediators involved in the pathophysiologic process. The earlier studies also found that untreated MS patients [12] had higher blood levels of ET-1 compared to patients who received treatment [13], a tendency which we confirmed. However, in both our study and the study by Pache et al., the percentage of patients receiving treatment was similar (Pache et al. 56.7%.), which makes explanation of the differences between the studies difficult.
Since ET-1 blood levels are increased in cardiovascular disease, we excluded from our study not only patients with clinically diagnosed cardiovascular disease, as it had been done in earlier studies, but also subjects with dyslipidemia confirmed by laboratory investigations. With these criteria, 20.41% of the MS patients and 12.9% of the prospective controls were excluded at baseline, which could account for lower ET-1 levels in the MS patients. Additionally, the ET-1 plasma levels might be related to a particular clinical course of MS. However, we did not find any statistically significant differences in the ET-1 plasma levels between RRMS patients and those with the other forms of MS. It could be speculated that, compared to other studies, lower disease activity and shorter duration of MS, and exclusion of patients with cardiovascular disease associated with endothelial dysfunction, including clinically ‘silent’ dyslipidemia, may have accounted for the plasma levels of ET-1 found in this study. However, these factors seem unlikely to cause the decrease of
ET-1 plasma levels in the MS patients to values below the normal range. This finding may suggest another type of vascular dysregulation different from secondary vascular dysregulation, dependent on the autoimmune process in the course of MS, in which increased levels of ET-1 in blood were observed [12,13].
The finding of lower than normal, ET-1 plasma levels in the study group is in agreement with the results of studies in patients with optic neuropathy not associated with MS (glaucomatous optic neuropathy), which found lower blood levels of ET-1 in glaucoma patients compared to healthy subjects [32–35]. Increased spasticity of blood vessels in the course of primary vascular dysregulation (PVD) has been considered a vasogenic risk factor for glaucomatous optic neuropathy for over 20 years [4,26,36–46]. Endothelial dysfunction with increased ET-1 blood levels can play a role in the pathogenesis of PVD syndrome and normal tension glaucoma (NTG) [4,26]. A recent meta-analysis of studies concerning ET-1 levels in the blood of primary open-angle glaucoma (POAG) patients found that only NTG patients had significantly higher levels of ET-1 compared to healthy individuals [22]. However, the results of studies measuring the ET-1 levels in the serum of NTG patients are inconsistent as various authors have shown in NTG patients compared to healthy individuals either increased blood levels of ET-1 [47–50] or no difference at all [51,52] or decreased levels [32,34]. These discrepancies in measurements of ET-1 blood concentration in NTG patients may be due to many factors. Each study was carried out in a different age group, while ET-1 levels are known to increase with age [22,52] and the associated progression of endothelial dysfunction. Ethnic differences can also be of significance. Lower ET-1 levels in patients with glaucomatous optic neuropathy were observed mainly in the Japanese population [32,35]. NTG is more common in the Japanese population than in Caucasians [53]. Cerebral vasospasm was also reported more frequently in the Japanese patients [54,55]. These observations suggest that the decreased blood levels of ET-1 in NTG patients can be associated with the PVD syndrome. The lower blood levels of ET-1 in blood could be a result of interactions between neurogenic and endothelium-dependent vascular tone control mechanisms, associated with the already present dysregulation of the autonomous nervous system [34,45,47]. Yoshida et al. suggest that the low levels of ET-1 in the serum of glaucoma patients can be related to the up-regulation of endothelin receptors (ET-A, ET-B1, ETB2) [35]. Other researchers observed in POAG patients a significantly higher increase in the serum levels of ET-1 following exposure to cold [34,56], particularly in patients with NTG [34]. In this situation, a transient increase of ET-1 level in the blood serum due to cold can intensify the ET 1-induced vasospastic reaction. Such a response may indicate an imbalance between the release of ET-1 which is a vasoconstrictor and NO which is a vasodilator [57,58], and can be related to the impairment of the vasodilating function of endothelium in NTG patients [51,59,60]. The above observations may suggest that as a result of impaired neural-endothelial control of vascular tone in PVD syndrome blood levels of ET-1 can be low under normal conditions, while an increase in the ET-1 concentration occurs only on a cold challenge test, which would provoke a vasospastic reaction. It has been also noted that MS patients, similar to NTG patients, tend to develop PVD syndrome. Patients with MS experience problems typical of PVD, i.e., a tendency to peripheral vasospastic reactions due to exposure to cold or emotional stress which often precedes MS symptoms [4,26]. This is confirmed by our observations as 76.92% of the MS patients had a history of peripheral vasospastic reactions before the actual diagnosis of MS. It is possible that the lower levels of ET-1 in MS patients found in the present study are accounted for by the ET-1 measurements being performed at the time when the levels of ET-1 had not yet increased as such increases are a consequence of SVD, which results from the progression of the underlying autoimmune process.
Conclusions
The results showing significantly lower levels of ET-1 in the plasma of patients with multiple sclerosis compared to healthy subjects suggest that some form of endothelium-dependent vascular dysregulation could occur in these patients. This finding is in agreement with the reports of low blood levels of ET-1 from other studies in patients with primary open-angle glaucoma, especially normal tension glaucoma. This may suggest that similar pathophysiologic mechanisms may be involved in both, multiple sclerosis and primary open-angle glaucoma. The role of ET-1 in the pathogenesis of these 2 diseases, characterized by optic neuropathy, remains to be explained.
Footnotes
The study was presented at The European Association for Vision and Eye Research Conference, Crete 2011
Source of support: The study was funded by The National Centre for Research and Development, Warsaw, Poland, grant No. R13 033 03
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Minager A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–49. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 2.Minager A, Wenche Jy, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurological Res. 2006;28:230–35. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 3.Reijerkerk A, Lakeman KA, Drexhage JA, et al. Brain endothelial barrier passage by monocytes is controlled by the endothelin system. J Neurochem. 2012;121:730–37. doi: 10.1111/j.1471-4159.2011.07393.x. [DOI] [PubMed] [Google Scholar]
- 4.Flammer J, Pache M, Resink T. Vasospam, its role in the pathogenesis of diseases with particular reference to the eye. Prog Ret Eye Res. 2001;20:319–49. doi: 10.1016/s1350-9462(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 5.Moore D, Harris A, Wu Dunn D, et al. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin Ophthalmol. 2008;2:849–61. doi: 10.2147/opth.s2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolela M. Clinical clues of vascular dysregulation and its association with glaucoma. Can J Ophthalmol. 2008;43:337–41. doi: 10.3129/i08-063. [DOI] [PubMed] [Google Scholar]
- 7.Resch H, Garhofer G, Fuchsjager-Mayrl G, et al. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–15. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 9.De Meyer GRY, Herman AG. Vascular endothelial dysfanction. Prog Cardiovasc Dis. 1997;4:325–42. doi: 10.1016/s0033-0620(97)80031-x. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan BC. Endothelin and its potential role in glaucoma. Can J Ophthalmol. 2008;43:356–60. doi: 10.3129/i08-060. [DOI] [PubMed] [Google Scholar]
- 11.Speciale L, Sarasella M, Ruzzante S, et al. Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol. 2000;6(Suppl 2):S62–66. [PubMed] [Google Scholar]
- 12.Haufschild T, Shaw SG, Kesselring J, Flammer J. Increased endothelin-1 plasma levels in patients with multiple sclerosis. J Neuroophthalmol. 2001;21:37–38. doi: 10.1097/00041327-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Pache M, Kaiser HJ, Akhalbedashvili N, et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurology. 2003;49:164–68. doi: 10.1159/000069085. [DOI] [PubMed] [Google Scholar]
- 14.Haufschild T, Shaw SG, Kaiser HJ, Flammer J. Transient raise of endothelin-1 plasma level and reduction of ocular blood flow in patient with optic neuritis. Ophthalmologica. 2003;217:451–53. doi: 10.1159/000073079. [DOI] [PubMed] [Google Scholar]
- 15.Syc SB, Warner CV, Saidha S, et al. Cup to disc ratio by optical coherence tomography is abnormal in multiple sclerosis. J Neurol Sci. 2011;302:19–24. doi: 10.1016/j.jns.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noval S, Contreras I, Munoz S, et al. Optical Coherence Tomography In Multiple Sclerosis and Neuromyelitis Optica: An Update. Mult Scler Int. 2011;2011:472790. doi: 10.1155/2011/472790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebolleda G, Noval S, Contreras I, et al. Optic disc cupping after optic neuritis evaluated with optic coherence tomography. Eye. 2009;23:890–94. doi: 10.1038/eye.2008.117. [DOI] [PubMed] [Google Scholar]
- 18.Haefliger IO, Flammer J, Luscher T. Heterogeneity of endothelium-dependent regulation of ophthalmic artery and ciliary arteries. Invest Ophthalmol Vis Sci. 1993;34:1722–30. [PubMed] [Google Scholar]
- 19.Schmetterer L, Findi O, Strenn K, et al. Effects of endothelin-1 (ET-1) on ocular hemodynamics. Curr Eye Res. 1997;16:687–92. doi: 10.1076/ceyr.16.7.687.5065. [DOI] [PubMed] [Google Scholar]
- 20.Strenn K, Matulla B, Wolzt M, et al. Reversal of endothelin-1 hemodynamic effects by low- dose nifedipine in humans. Clin Pharmacol Ther. 1998;63(1):54–63. doi: 10.1016/S0009-9236(98)90121-7. [DOI] [PubMed] [Google Scholar]
- 21.Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9:34–36. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- 22.Shoshani YZ, Harris A, Shoja MM, et al. Endothelin and its suspected role in the pathogenesis and possible treatment of glaucoma. Curr Eye Res. 2012;37:1–11. doi: 10.3109/02713683.2011.622849. [DOI] [PubMed] [Google Scholar]
- 23.Akarsu C, Tan FU, Kendi T. Color Doppler imaging in optic neuritis with multiple sclerosis. Graefes Arch Clin Exp Ophthalmol. 2004;242:990–94. doi: 10.1007/s00417-004-0948-1. [DOI] [PubMed] [Google Scholar]
- 24.Modrzejewska M, Karczewicz D, Wilk G. Assesment of blood flow velocity in eyeball arteries in multiple sclerosis patients with past retrobulbar optic neuritis in color Doppler ultrasonography. Klin Oczna. 2007;109:183–86. [PubMed] [Google Scholar]
- 25.Hradílek P, Stourac P, Bar M, et al. Colour Doppler imaging evaluation of blood flow parameters in the ophthalmic artery in acute and chronic phases of optic neuritis in multiple sclerosis. Acta Ophthalmol. 2009;87:65–70. doi: 10.1111/j.1755-3768.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 26.Grieshaber M, Mozaffarieh M, Flammer J. What is the link between vascular dysregulation and glaucoma? Sur Ophthalmol. 2007;52:144–54. doi: 10.1016/j.survophthal.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Luscher T, Tanner FC, Yasuaki D. Age, hypertension, hypercholesterolemia alter endothelium dependent vascular regulation. Pharmacol Toxicol. 1992;70(Suppl):32–39. doi: 10.1111/j.1600-0773.1992.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 28.Feletou M, Vanhoutte PM. Endothelial dysfunction – a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 29.Polman CH, Reingold SC, Edan G, et al. Diagnosic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–46. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 31.Cendrowski W. Treatment modification in multiple sclerosis classification, alternatives and the outcomes. Neurol Neurochir Pol. 2007;41:365–68. [PubMed] [Google Scholar]
- 32.Ohguro I, Ohguro H, Ohkuro K, Nakazawa M. Study of contribution of low levels of plasma endothelin-1 concentrations to pathogenesis of glaucomatous optic neuropathy. Hirosaki Med J. 2006;57:59–64. [Google Scholar]
- 33.Hudson C, Venkataraman ST, Rachmiel R, et al. Plasma Levels of ET-1 and cGMP in Primary Open Angle Glaucoma (POAG) During Isoxic Hypercapnia. Invest Ophthalmol Vis Sci. 2008;49 E- Abstract 4609. [Google Scholar]
- 34.Terelak-Borys B, Czechowicz-Janicka K. Investigation into the vasospastic mechanisms in the pathogenesis of glaucomatous neuropathy. Klin Oczna. 2011;113:201–8. [PubMed] [Google Scholar]
- 35.Yoshida K, Ohguro I, Ohguro H. Black Currant Anthocyanins normalized abnormal levels of serum concentrations of endothelin-1 in patients with glaucoma. J Ocular Pharmacol Ther. 2013;29(5):480–87. doi: 10.1089/jop.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasser P, Flammer J. Influence of vasospasm on visual function. Doc Ophthalmol. 1987;66:3–18. doi: 10.1007/BF00144735. [DOI] [PubMed] [Google Scholar]
- 37.Flammer J, Guthauser U, Mahler F. Does ocular vasospasm help cause low tension glaucoma? Doc Ophthalmol Proc Ser. 1987;49:397–99. [Google Scholar]
- 38.Drance SM, Douglas GR, Wijsman K, et al. Response of blood flow to warm and cold in normal and low tension glaucoma patients. Am J Ophthalmol. 1988;105:35–39. doi: 10.1016/0002-9394(88)90118-3. [DOI] [PubMed] [Google Scholar]
- 39.Gasser P, Flammer J. Blood-flow velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol. 1991;111:585–88. doi: 10.1016/s0002-9394(14)73703-1. [DOI] [PubMed] [Google Scholar]
- 40.Drance SM. Possible implications of vasospasm as a risk factor in glaucoma “International symposium on glaucoma, ocular blood flow and drug treatment”. Williams & Wilkins; Baltimore: 1992. pp. 78–81. [Google Scholar]
- 41.Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Ret Eye Res. 1998;17:267–89. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien C, Butt Z. Blood flow velocity in the peripheral circulation of glaucoma patients. Ophthalmologica. 1999;213:150–53. doi: 10.1159/000027410. [DOI] [PubMed] [Google Scholar]
- 43.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 44.Pache M, Dubler B, Flammer J. Peripheral vasospasm and nocturnal blood pressure dipping – two distinct risk factors for glaucomatous damage? Eur J Ophthalmol. 2003;13:260–65. doi: 10.1177/112067210301300304. [DOI] [PubMed] [Google Scholar]
- 45.Gherghel D, Hosking SL, Cunliffe IA. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci. 2004;45:3546–54. doi: 10.1167/iovs.04-0290. [DOI] [PubMed] [Google Scholar]
- 46.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013;4(1):14. doi: 10.1186/1878-5085-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser HJ, Flammer J, Wenk M, Luscher T. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefe’s Arch Clin Exp Ophthalmol. 1995;233:484–88. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama T, Moriya S, Oku H, Azuma I. Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol. 1997;39:S49–56. doi: 10.1016/s0039-6257(05)80073-6. [DOI] [PubMed] [Google Scholar]
- 49.Cellini M, Possati GL, Profazio V, et al. Color Doppler imaging and levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol Scand. 1997;224(Suppl):11–13. doi: 10.1111/j.1600-0420.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 50.Galassi F, Giambene B, Varriale R. Systemic vascular dysregulation and retrobulbar hemodynamic in normal-tension glaucoma. Invest Ophthalmol. 2011;52:4467–71. doi: 10.1167/iovs.10-6710. [DOI] [PubMed] [Google Scholar]
- 51.Henry E, Newby DE, Webb DJ, et al. Altered endothelin-1 vasoreactivity in patients with untreated normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2528–32. doi: 10.1167/iovs.05-0240. [DOI] [PubMed] [Google Scholar]
- 52.Kunimatsu S, Mayama C, Tomidokoro A, Araie M. Plasma endothelin-1 level in Japanase normal tension glaucoma patients. Curr Eye Res. 2006;31:727–31. doi: 10.1080/02713680600837382. [DOI] [PubMed] [Google Scholar]
- 53.Shiose Y, Kitazawa Y, Tsukahara S, et al. Epidemiology of glaucoma in Japan – a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35(2):133–55. [PubMed] [Google Scholar]
- 54.Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–52. doi: 10.1016/s0735-1097(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 55.Mocco J, Ransom ER, Komotar RJ, et al. Racial differences in cerebral vasospasm: a systematic review of the literature. Neurosurgery. 2006;58:305–14. doi: 10.1227/01.NEU.0000195009.02412.E8. [DOI] [PubMed] [Google Scholar]
- 56.Nicolela MT, Ferrier SN, Morrison CA, et al. Effect of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci. 2003;44:2565–72. doi: 10.1167/iovs.02-0913. [DOI] [PubMed] [Google Scholar]
- 57.Venkataraman SR, Flanagan JG, Hudson C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma. Microcirculation. 2010;17:568–81. doi: 10.1111/j.1549-8719.2010.00045.x. [DOI] [PubMed] [Google Scholar]
- 58.Good TJ, Kahook MY. The role of endothelin in the pathophysiology of glaucoma. Expert Opin Ther Targets. 2010;14:647–54. doi: 10.1517/14728222.2010.487065. [DOI] [PubMed] [Google Scholar]
- 59.Henry E, Newby DE, Webb DJ, O’Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–14. [PubMed] [Google Scholar]
- 60.Buckley C, Hadoke PW, Henry E, O’Brien C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br J Ophthalmol. 2002;86:227–32. doi: 10.1136/bjo.86.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]