Abstract
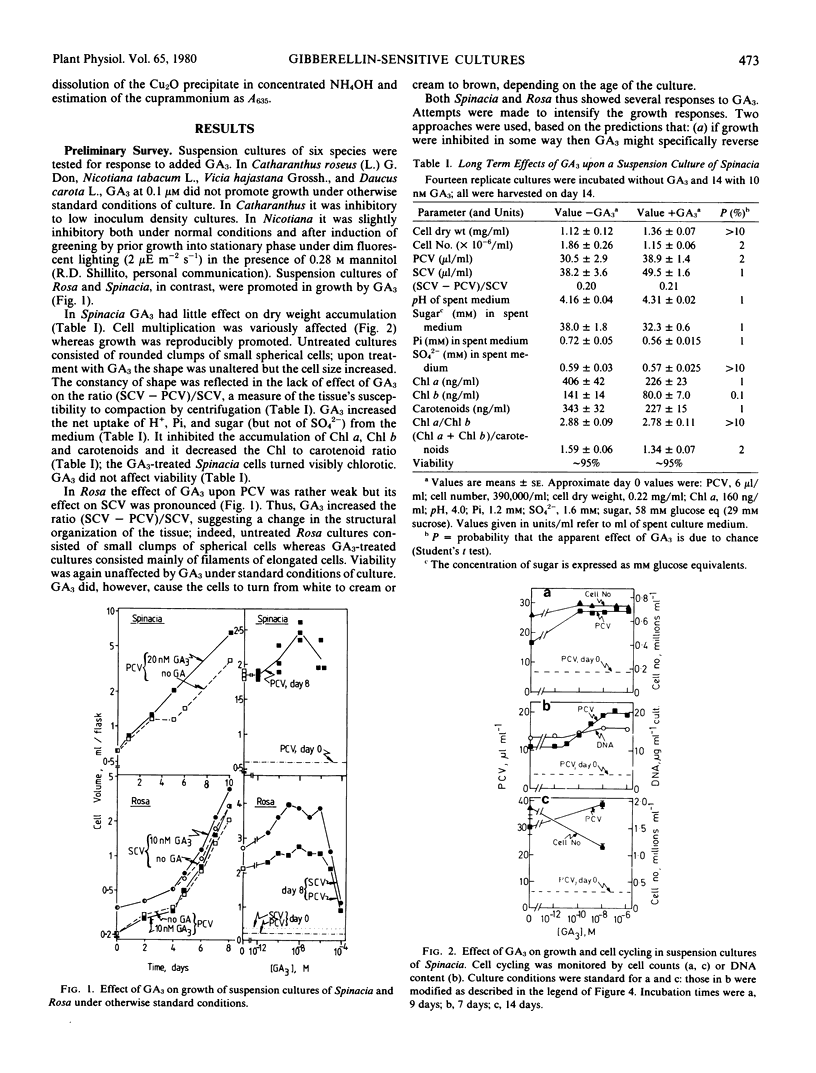
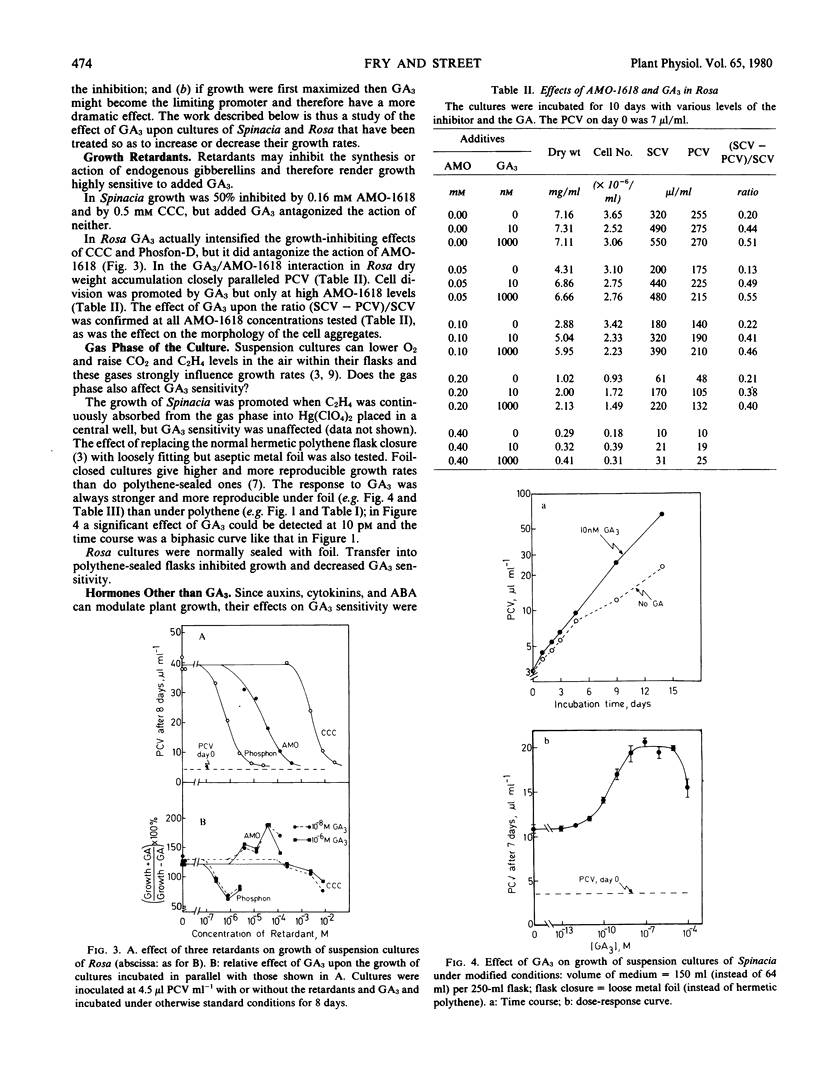
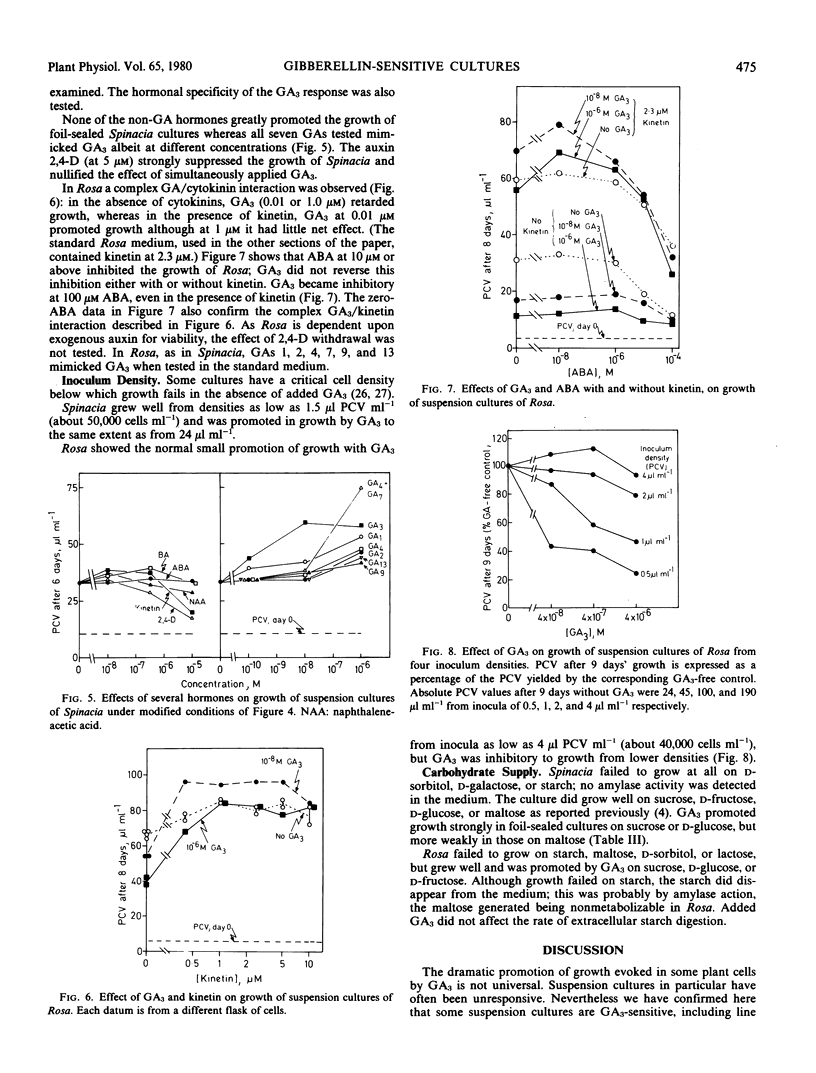
Suspension cultures were incubated in the presence and absence of gibberellic acid (GA3) in an attempt to define a new experimental system for study of the molecular action of gibberellins upon growth. Unlike many suspension cultures, an auxin-independent green clone from spinach (Spinacia oleracea L.) and an auxin-dependent line of “Paul's Scarlet” rose (Rosa sp.) were promoted in expansion growth by GA3 at 10−11 to 10−6 molar. In Rosa the cells also elongated upon GA3 treatment whereas in Spinacia they remained isodiametric.
Attempts were made to intensify the response. The effect of GA3 in Spinacia was stronger when gas exchange between the culture and the laboratory air was facilitated. The response of Rosa was dependent on the presence of a cytokinin, although this culture did not require exogenous cytokinin for serial subculture. GA3 antagonized the growth retardant AMO-1618 in Rosa but not in Spinacia. In general, conditions that enhanced growth also rendered GA3 a more effective promoter; conversely, GA3 tended to become inhibitory under conditions that permitted only slow growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton C. C., Street H. E. The role of the gas phase in the greening and growth of illuminated cell suspension cultures of spinach (Spinacia oleracea, L.). In Vitro. 1976 Jul;12(7):485–494. doi: 10.1007/BF02796491. [DOI] [PubMed] [Google Scholar]
- FELS I. G. Acid-catalyzed oxidation of reduced pyridine nucleotides. Science. 1961 Jul 28;134(3474):280–281. doi: 10.1126/science.134.3474.280-a. [DOI] [PubMed] [Google Scholar]
- Haddon L., Northcote D. H. The influence of gibberellic acid and abscisic acid on cell and tissue differentiation of bean callus. J Cell Sci. 1976 Jan;20(1):47–55. doi: 10.1242/jcs.20.1.47. [DOI] [PubMed] [Google Scholar]
- Helgeson J. P., Upper C. D. Modification of logarithmic growth rates of tobacco callus tissue by gibberellic Acid. Plant Physiol. 1970 Jul;46(1):113–117. doi: 10.1104/pp.46.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzki A., Dela Cruz A., Nickell L. G. Extracellular hydrolysis of starch in sugarcane cell suspensions. Plant Physiol. 1971 Nov;48(5):521–525. doi: 10.1104/pp.48.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. A., Jones R. L. Roles of Extensibility and Turgor in Gibberellin- and Dark-stimulated Growth. Plant Physiol. 1977 Jan;59(1):61–68. doi: 10.1104/pp.59.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]


