As breast cancer becomes a chronic condition rather than a life-threatening illness, survivors not only have the challenge of dealing with multiple long-term side effects of treatment protocols, but may also be forced to address the preexisting comorbidities of their therapies, which often include multiple other issues. It is imperative that the information available regarding survivorship issues be accessible in an organized and useful format. This article is a modest attempt to provide a comprehensive review of the long-term medical issues.
Abstract
Long-term survival rates after a diagnosis of breast cancer are steadily rising. This is good news, but clinicians must also recognize that this brings new challenges to the medical community. As breast cancer becomes a chronic condition rather than a life-threatening illness owing to advances in early diagnosis and more effective treatments, health care practitioners must recognize and manage the long-term sequelae of the constellation of therapeutic modalities. Survivors of breast cancer represent a unique and extremely complex group of patients; not only do they have the challenge of dealing with multiple long-term side effects of treatment protocols, but many are also forced to address the preexisting comorbidities of their therapies, which often include multiple other issues. Therapies have additional and/or additive side effects that may interfere with treatments directed toward the new primary diagnosis of breast cancer. Our mandate is to establish a smooth transition from patient with breast cancer to survivor of breast cancer while providing ongoing and future guidance.
Certainly, the information and resources to accomplish this transition are readily available; however, they are scattered throughout the literature and therefore are not easily accessible or available to the primary care physician. It is imperative that the information available regarding survivorship issues be accessible in an organized and useful format. This article is a modest attempt to provide a comprehensive review of the long-term medical issues relevant to survivorship after the diagnosis and treatment of breast cancer. A predicted shortage of oncologists by 2020 is well-recognized. Therefore, the bulk of long-term care will become dependent on the primary care physician. This shift of care means that these physicians will need to be well educated in the long-term medical issues related to breast cancer treatment.
INTRODUCTION
It is estimated that there are approximately 2.5 million survivors of breast cancer in the US.1 This figure will expand to 3.4 million in 2015, representing an increase of 31%.2 The millions more worldwide are probably grossly underestimated because of the poor or inefficient reporting systems and the lack of reliable cancer registries in third-world countries.3 In 2006, the Institute of Medicine (IOM) issued a milestone comprehensive report, From Cancer Patient to Cancer Survivor: Lost in Transition.4 Of the 10 recommendations regarding cancer survivorship by the IOM, the issues receiving the utmost attention to date have been the provision of a summary of diagnosis, treatment received (treatment summary), future follow-up care plans, and healthy lifestyle recommendations.5 A recent Special Series Overview6 eloquently described a number of major topics that have been addressed by world-renowned experts since the IOM recommendations were published.6 These include long-term cardiovascular issues secondary to treatment protocols,7 bone health,8 the increased risk of second primary malignancies (SPMs),9 the development of lymphedema,10 and other issues that, although extremely important, may not be life-threatening. Multiple other concerns have been inadequately addressed, including the increased risk of venous thromboembolism in the setting of malignancy,11–13 the failure of adherence and compliance to prescribed adjuvant hormonal therapies,14–16 and lifestyle changes with recommendations for effective modifications.
Although of major importance, issues regarding sexuality and fertility preservation are not addressed in this review. The reader is directed to excellent reviews of breast cancer and sexuality,17,18 as well as extensive guidelines regarding fertility preservation.19 In addition, non-specific symptoms secondary to treatment protocols (eg, fatigue, insomnia, pain, cognition) are omitted because recent and thorough reviews of these issues are readily available.20 Furthermore, these are excluded because many are nonspecific symptoms and are not unique to a diagnosis of breast cancer.
Survivorship care programs provide an important component of the patient treatment pathway but fail in the elaboration and communication to the patient regarding many issues relevant to long-term survival. Most long-term care plans inadequately address the most important medical issues involving the long-term consequences of survivorship. Although the primary recommendations of the 2006 IOM report are sound,4 we would add the importance of patient education regarding some of the long-term sequelae of this disease and its treatment. These include the symptoms of the issues addressed in this article: cardiovascular diseases (CVDs) secondary to treatment modalities, bone health, SPMs, lymphedema, thromboembolic risks, long-term compliance with oral medications, and finally, lifestyle interventions.
Although most people with breast cancer will not die of breast cancer, their comorbidities (eg, obesity, hypertension, hyperlipidemia, and diabetes mellitus [DM]) will most certainly affect disease-free survival (DFS) and, ultimately, overall survival (OS). In this review we address lifestyle changes, which are largely dependent on body mass index (BMI) and include diet and exercise, and review recommendations regarding these issues. Survivors of breast cancer represent a unique group who must be cognizant of the long-term side effects of their treatment protocols and be given information to encourage a proactive approach to their overall health. Finally, a robust reference resource list is included in this article for those who wish to delve into specific issues in greater depth.
CARDIAC ISSUES
Although breast cancer is the most feared disease by most women in the US, it is far from the leading cause of death in women.21,22 CVD is the number 1 killer of women, claiming well over 400,000 lives each year. Sadly, nearly 50% of women are unaware that heart disease is the leading cause of death among women.23 Survival of patients with breast cancer has dramatically increased in the last 2 decades, largely owing to earlier detection by advanced mammographic screening technologies, increased patient awareness, and of course more effective treatment modalities.24 This success, however, may lead to an unintended increase in the incidence of mortality due to CVD. Although each year many women succumb to CVD, the risk of death due to CVD may be greatly increased in survivors of breast cancer by the addition of adjuvant therapies, regardless of cancer stage, at the time of diagnosis.
A further concern, demanding urgent attention, is that the rate of younger women (aged 35 to 44 years) who develop CVD is on the rise.25,26 Overlapping with this age group are the younger and younger women receiving a diagnosis of breast cancer. Therefore, the diagnosis, treatment, and long-term sequelae of treatment may be converging on patients who are currently facing a breast cancer diagnosis and are thus subjected to “modern” protocols. As with the treatment of other malignancies occurring at young ages (eg, Hodgkin disease), the long-term sequelae of breast cancer treatment are just now, decades later, becoming fully realized. In fact, a subset of cardiologists in the US and internationally is focusing on a new sub-specialty—cardio-oncology—a specialty in managing the long-term cardiovascular side effects of the treatment of malignancies.27 Many survivors of breast cancer are at significantly increased risk of death caused by CVD, far exceeding their risk of death resulting from the initial cancer itself or from a recurrent cancer.21,22,28,29
Chemotherapy
The development of multiple antineoplastic agents—many novel but also many older (decades old)—has dramatically increased breast cancer OS. Unfortunately, many of the chemotherapeutic agents used have the potential to cause cardiovascular complications, some acute but most chronic.30,31 The spectrum of CVD in the setting of breast cancer therapy includes congestive heart failure (CHF), myocardial ischemia, hypertension, arrhythmias, QT prolongation, bradycardia, pericarditis, acute coronary syndrome, and thromboembolic events (TEs).31–34
Anthracyclines: Doxorubicin and Epirubicin: Anthracyclines bind to the DNA of malignant cells, interfering with the replication process and resulting in cellular death. Anthracycline therapy has been shown to increase the development of CHF and cardiomyopathy by 2%,34–39 doubling to 4% if used in conjunction or sequence with trastuzumab.34,39–43
Of particular importance to the primary care physician, extensive data are available in the literature about the potential long-term sequelae of the cardiotoxicity of anthracycline-based therapy for survivors of breast cancer.31–34 Furthermore, it should be noted that anthracycline therapy may not result in cardiotoxicity, particularly CHF, which is clinically evident for 10 to 20 years after treatment.33,44,45
Alkylating Agents: Cyclophosphamide: Alkylating agents act by reacting with the proteins of the DNA of cancer cells by adding an alkyl group to them. This disrupts effective DNA replication, resulting in the apoptosis of cancerous cells. Cyclophosphamide therapy can lead to cardiac damage, resulting in heart failure in nearly 30% of patients receiving the drug.46–48
As with most chemotherapeutic agents, the risk of cardiotoxicity appears to be dose related.33,47 In addition to dose, prior anthracycline therapy, a history of mediastinal radiation, and elderly age are further risk factors for cardiotoxicity.29,45–47,49,50
Cytoskeletal Disruptors (Taxanes): Paclitaxel and Docetaxel: Cytoskeletal disruptors inhibit the process of cell division through the interruption of microtubular functions, which are essential for cell division. Taxanes have been incorporated in the treatment of breast cancer only since the 1990s and, as such, their long-term cardiotoxic side effects may not yet have been adequately identified and reported. Furthermore, the multiple-combination chemotherapies used, which have included the addition of a taxane-based agent, make it difficult to decipher the true incidence of cardiotoxicity attributable to these drugs, particularly when administered alone.29,45,51–54
Although the most common side effects of taxane therapies are related to arrhythmias, particularly bradycardia, myocardial ischemia has also been reported. The incidence with paclitaxel use is 0.5% to 5% and with docetaxel appears to approach 2%.33 CHF resulting from docetaxel has ranged from 2.3% to 8%.51,55 It should be noted that most data for the development of cardiotoxicity has accumulated from the use of paclitaxel.56
As noted previously, the cardiotoxicity of systemic therapies may become one of the most devastating consequences of the treatment itself, particularly when an additional comorbidity such as a history of coronary artery disease, hypertension, or smoking is added to the patient’s active problem list (Table 1).45,56–58
Table 1.
Potential cardiotoxicity of therapeutic agentsa
| Agent | Potential cardiotoxicity |
|---|---|
| Anthracyclines: doxorubicin and epirubicin | CHF, cardiomyopathy |
| Alkylating agents: cyclophosphamide | CHF |
| Taxanesb: paclitaxel and docetaxel | CHF, ischemia, arrhythmias |
| Targeted therapies: trastuzumab and lapatinib | CHF |
| Hormonal blockade: tamoxifen | TE |
Long-term effects of conjunctive therapies may be additive.
Long-term cardiovascular toxicities are yet to be determined.
CHF = congestive heart failure; TE = thromboembolic events.
Radiation Therapy
The current standard of care for the treatment of early-stage breast cancer involves giving a patient an informed choice regarding surgical options.59–61 The effectiveness of breast-conserving therapy (BCT), beginning in the 1970s with quadrantectomy vs mastectomy, has been fully verified with numerous studies, some reporting more than 2 decades of follow-up data.62–67 These findings have resulted in BCT as the primary surgical option for most patients during the past 2 decades. An integral part of BCT is the mandatory addition of adjuvant radiation therapy (RT).63,64,68 This surge in BCT has resulted in a large number of patients who receive adjuvant RT for early-stage breast cancer; rates of recurrence and death are markedly reduced.69–75
Cardiac injury resulting from RT to the thorax has long been recognized. Because of the increasing number of patients who have become long-term survivors of breast cancer thanks to BCT and RT, attention has now been directed to the late side effects of RT. These include direct damage to the myocardium and the coronary arteries, resulting in an increase in CHF and myocardial infarction compared with patients who do not receive RT.29,33,76
Since the mid-1980s, the mean centigray cardiac exposure has decreased because of improved technologies, such as computed tomography for simulation for RT; nonetheless, even small amounts of radiation reaching the heart may be damaging.77–82 It is estimated that each centigray exposure the heart receives increases the risk of death due to heart disease by 3%.83,84 The incidence and severity of cardiac morbidity and mortality risk are far greater for left-sided disease by virtue of human anatomy.81,85–88
As with chemotherapy, the risk of death due to RT starts to rise 10 years after treatment and may not be fully manifest until the second decade after therapy.83,88 RT has a long-term effect; therefore, it is important to be cognizant of the lengthy delay in cardiac symptoms, particularly as the patient ages and becomes more vulnerable to the development of CVD.89 Because RT has evolved over the years, incorporating new technologies, administration schedules, and delivery of centigray doses, the side effects for contemporary patients may be somewhat lessened, although long-term follow-up is not yet available.76,90–94
Hormonal Blockade
Tamoxifen: Tamoxifen is a selective estrogen receptor modulator (SERM) and inhibits the growth of breast cancer cells by its antiestrogenic activity through its competitive inhibition of estrogen binding to estrogen receptors.95,96 Since its introduction in the 1970s, tamoxifen has been shown to reduce the risk of breast cancer recurrence and mortality by more than 30%.97,98 Tamoxifen has been heralded as one of the most important advances in the treatment of breast cancer because approximately 70% of these patients have estrogen receptor-positive (ER+) cancer.99–103
The side effects of tamoxifen therapy are typically those that accompany the onset of menopause. These include hot flashes, mood swings, depression, loss of libido, and vaginal dryness.101 In addition, tamoxifen increases the risk of thromboembolic complications, including deep venous thrombosis, pulmonary embolism (PE), and cerebral vascular events.104–107 Tamoxifen has also been associated with an increase in the development of endometrial cancer97,105; therefore, women receiving this form of hormonal blockade who experience spotting require urgent gynecologic referral for uterine biopsy to rule out cancer. Because tamoxifen is a SERM, a beneficial effect is an apparent decrease in the incidence of myocardial infarction and CVD-related death as well as offering protection from osteoporosis and fracture risk in postmenopausal patients.106–108
A final but important consideration for patients receiving tamoxifen therapy centers on the recognized interactions with this drug in two common comorbid conditions: coagulation and depression. An aging population results in an increasing incidence of cardiac arrhythmias and other conditions resulting in the need for long-term anticoagulative therapy. Tamoxifen potentiates the action of warfarin by competing with its metabolizing enzyme, cytochrome P4503A4, which may result in major hemorrhagic consequences.103,109
Antidepressants are one of the most commonly prescribed medications in the US.110–112 Commonly prescribed antidepressants classified as selective serotonin reuptake inhibitors (SSRIs) inhibit the enzyme CYP2D6 and thus may slow the metabolism of tamoxifen, resulting in a decrease of its potency and thereby increasing the risk of recurrence.113–116
Aromatase Inhibitors: Letrozole, Anastrozole, and Exemestane: Aromatase inhibitors (AIs) work by blocking the enzyme aromatase, which converts adrenal androgens into estrogens. Whereas tamoxifen is employed in premenopausal women who have ER+ tumors, AIs are the estrogen blocker of choice in postmenopausal women whose cancer is ER+. AIs have been established as an effective adjuvant treatment in the post-menopausal group.101,117–119 In women, AIs have similar side effects to tamoxifen regarding menopausal symptoms (eg, hot flashes, mood swings, vaginal dryness, and loss of libido).97,101–120 The risk of CVD, including myocardial infarction, CHF, hypertension, and hyperlipidemia, remains controversial because published studies have failed to adequately resolve these issues.106–108,121–126 Until more definitive data are available, it would be prudent to err on the side of caution and consider those patients who are also receiving long-term AI therapy to be at an increased risk for the development of CVD.
Although arthralgias are an important side effect of AI therapy,101,122,123 a potentially more clinically important side effect is the development of bone loss. Osteopenia (a decrease in bone calcium content) and osteoporosis (a decrease in the actual bony matrix) are well-recognized side effects of AI therapy.127 Further discussion of bone loss are addressed in the section, Bone Health.
Targeted Biologic Therapies: Trastuzumab and Lapatinib
Targeted biologic agents are directed at protein kinases and the receptors that activate them. Approximately 15% to 30% of all breast cancers are human epidermal growth factor receptor 2-positive (HER2+) and, as such, the HER2 receptor tyrosine kinase pathway has become an important therapeutic target.128–134 The main function of the HER2/neu oncogene (now also called ERBB2) is to promote the differentiation, growth, and survival of cells, thereby enhancing the aggressiveness of these breast cancers, resulting in an overall outcome that is inferior to those patients not overexpressing this oncogene.135–139 Multiple studies have demonstrated a reduction in mortality and an increase in OS in HER2+ patients when trastuzumab has been incorporated into their treatment regimens.140–142 When used as a single treatment agent, trastuzumab increases the duration of survival, which is augmented by the administration of additional chemotherapeutic agents.128,138,143 The most severe complication of trastuzumab therapy has been its potential to adversely affect cardiac function; however, the exact mechanism of its cardiotoxicity remains unclear.31,144
The risk of trastuzumab-related cardiac events, as with other cardiotoxic agents, increases when additional CVD risk factors are noted, especially a history of coronary artery disease or impaired left ventricular dysfunction.56,145 On a positive note, it appears that the cardiotoxic effects of trastuzumab are reversible as long as they are identified early through rigorous monitoring during administration.29,145–149
Lapatinib, an orally administered medication, appears to be associated with a lower incidence of cardiotoxicity compared with trastuzumab.150 It appears that the cardiotoxicity associated with lapatinib is not as severe and is also reversible.151 Lapatinib is a new targeted modality, and further clinical investigation is needed before definitive conclusions about its cardiac safety are made, especially in light of the fact that many treatment options employed in breast cancer therapy have been demonstrated to have delayed long-term toxicities. In addition, further follow-up studies need to be conducted to determine whether outcomes are comparable to those of trastuzumab therapy.146,152,153
The cardiovascular complications of breast cancer treatment are an extremely complex subject, involving numerous variables that may be difficult to isolate. The multifocal approach to treatment includes many chemotherapeutic agents, alone and/or in combination, as well as RT modalities, options for hormonal blockade depending on menopausal status, and targeted therapies. Dosages, sequence of administration, and concordant or tangential approaches further complicate a thorough understanding of both the short-term and long-term toxicity of the administered therapies. The cluster of therapies such as anthracycline-based chemotherapy, right- or left-sided RT, trastuzumab administration, and hormonal blockade with AIs may contribute to an increased incidence of CVD.39–48,62,67,153–156 In particular, there have been recent concerns calling for further investigation of targeted therapies used in combination with RT and the potential for long-term cardiovascular side effects.92,147,154,157 Table 1 summarizes the potential long-term cardiovascular side effects of chemotherapy.
Further complicating the multiple cardiotoxicities of breast cancer therapy are the long, well-recognized, preexisting conditions that predispose to CVD (obesity, hypertension, dyslipidemias, and DM). Patients with breast cancer, most of whom now are becoming long-term survivors, may harbor one or more of these comorbidities, all of which increase as the population ages. Because of the complexity of the long-term side effects of treatment modalities for breast cancer, those addressing survivorship care must be aware of the need to incorporate a multidisciplinary approach to issues surrounding assessment and management of CVD, which remains the leading cause of mortality in women.158 Lifestyle changes that address these concerns are discussed in greater detail in the section, Lifestyle Management and Breast Cancer.
BONE HEALTH
Women with breast cancer are at an increased risk for the development of bone loss and osteoporosis because of adjuvant therapies; these changes may be extremely rapid in onset. Osteoporosis is a “silent disease” that is often not recognized until a fracture event. Osteoporosis results in the deterioration of the bony microstructure, particularly in the vertebrae, ribs, and hips, culminating in fragility fractures and an increase in overall mortality. Maintenance of bone integrity is an important issue in breast cancer care because weakening of the bony matrix represents a major factor in OS. Current therapies profoundly influence the metabolic effectiveness of the skeletal structure.
Risk factors for osteoporosis, excluding the diagnosis of breast cancer, are well recognized and include both non-modifiable and modifiable variables, particularly in the elderly population.159
Nonmodifiable risk factors include a family history of osteoporosis (genetically based), having a small, thin frame,160,161 increasing age, a prior fracture, and the early onset of menopause. These all contribute to the increased risk of osteopenia and osteoporosis (Table 2).162–167
Table 2.
Risk factors for osteoporosis
| Type of factor | Risk factor |
|---|---|
| Nonmodifiable | Age older than 50 |
| Family history | |
| Small, thin frame | |
| History of previous fracture | |
| Early-onset menopause | |
| Modifiable | Sedentary lifestyle |
Poor nutrition
| |
| Tobacco use | |
| Alcohol use | |
| Concurrent medications | Aromatase inhibitors |
| Glucocorticoids | |
| Proton pump inhibitors | |
| Psychotropic agents | |
| Antidepressants | |
| Thyroid replacement | |
| Anticoagulants | |
| Anticonvulsants |
In addition, indications for the treatment of other medical conditions necessitate certain pharmacologic interventions not specifically related to treatment of the breast cancer itself (Table 2). These include drugs commonly prescribed for gastrointestinal symptoms or diseases, psychotropic agents, glucocorticoids, hormonal therapies for thyroidal malfunction, anticonvulsants, and anticoagulants for treatment of cardiac disease such as atrial fibrillation.
Gastrointestinal complaints, including those related to gastroesophageal reflux and peptic ulcer disease, result in one of the most commonly prescribed medications: proton pump inhibitors, which approach 150 million prescriptions annually.168 These often-prescribed drugs decrease the intestinal absorption of calcium and therefore result in a decrease in bone mineral density (BMD), an effect that is reversible after discontinuation of therapy, usually within 12 months.169–171
Nearly 10% of Americans are prescribed antidepressants annually.172 Second-generation antidepressants, SSRIs, are commonly dispensed and rank third in all drug classes prescribed in the US.173 Serotonin receptors are present in all major bone cells and, as such, the neuroendocrine system of bony structures may be subjected to interference by the administration of SSRIs.174–177
Many survivors of breast cancer are prescribed medications for depression diagnosed either before or after their initial diagnosis of breast cancer. Patients with comorbidities such as depression are therefore at additional risk of BMD depletion. Add to this the compounding issues affecting depressed individuals, such as decrease in exercise, poor eating habits, lack of sun exposure, tobacco use, and an increase in alcohol intake, and the increased risk of fracture events rises even higher.178,179 Those caring for survivors of breast cancer must be aware of patients who are receiving antidepressants, particularly those receiving SSRIs, who may be at an increased risk of osteoporosis and subsequent fractures.178
Long-term corticosteroid therapy, often employed in the treatment of multiple inflammatory and autoimmune diseases, is also a well-recognized risk factor for osteoporosis.164,179 Steroidal therapy leads to osteoporosis by decreasing bone formation through multiple and complex mechanisms, which are beyond the scope of this review.180–183 As with the proton pump inhibitors, the osteoclastic effect of corticosteroid therapy appears to decrease fracture risk after discontinuation of therapy.184 Multiple other drugs prescribed for patients with breast cancer for concurrent diseases, including anticonvulsant and anticoagulation medications, may affect BMD, further contributing to osteoporotic fractures, particularly in the aging population. The literature is conflicting, but one should be aware that these classes of medications might increase the risk of fracture events (Table 2).185–187
AIs, be they steroidal (exemestane) or nonsteroidal (anastrazole and letrozole), are associated with a substantial and often rapid decrease in BMD and an increased fracture risk.188–190 These medications appear to be significantly more effective than tamoxifen adjuvant therapy in ER+ tumors in postmenopausal patients, with longer overall DFS and without the additional risk of endometrial carcinoma.185 Tamoxifen, classified as a SERM, acts like an antiestrogen in some tissues (breast) and an estrogen agonist in others (bone) and therefore is considered as a bone strengthener in women who are postmenopausal, an effect not seen in the premenopausal population.191–195 As opposed to tamoxifen, AIs block the aromatization of androgens and thereby block their conversion to estrogens, resulting in bone loss. Recovery of BMD after completion of aromatase inhibiting therapy may be only partial, especially if exemestane (steroidal-based therapy) has been used as the initial choice of antiestrogen therapy (AET).164,185,194 Such reversible effects on bone density deterioration are similar to those reported with the discontinuation of other medications previously addressed.184
Recent attention has been directed to patients who have been placed on a regimen of hormonal blockade therapy with tamoxifen, which is then discontinued after the appropriate duration. Postmenopausal women upon discontinuation of tamoxifen may suddenly experience an estrogen deprivation syndrome with respect to bone health; this results in the loss of the protective effects against the development of osteoporosis, with a subsequent increase in the risk of fracture.187–193,195 Thus, those providing long-term care for survivors of breast cancer must be aware that the abrupt discontinuation of tamoxifen requires particular attention to bone fragility and its assessment.195–199 A further issue, yet to be adequately addressed, involves those patients in whom discontinuation of tamoxifen is followed by the administration of AIs, putting bone health and density at greater risk.
To further complicate the issue, recent studies suggest a beneficial effect in the extension of hormonal blockade, specifically tamoxifen, to ten years, exceeding the previously recommended years of therapy.198,199 Although these recommendations should be cautiously interpreted, the long-term suppression of bone density must be addressed. It is hoped that by the time patients who are just beginning hormonal blockade therapies reach their five-year mark, they will have a definitive answer and/or evidence-based medicine to strongly recommend continuing those therapies for an additional five years. Additional confounding variables, especially for long-term therapies, are adherence and compliance, which are discussed in the section, Adherence and Compliance.
Finally, an extensive variety of other medical conditions, diagnoses, and pharmacologic interventions have been implicated in contributing to the development or progression of decreasing BMD from osteopenia to osteoporosis.164,168,185,188 Many of these issues are addressed only by observational studies. Therefore, although these risk factors are important, their relevance to absolute risk increase awaits further results from ongoing trials.
Myriad medical conditions have been associated with the development of osteoporosis, but two particularly common diseases must be mentioned: thyroidal conditions and DM.
Hyperthyroidism is a common disorder affecting approximately 1 in 100 individuals, and it is often accompanied by the progression of osteoporosis, especially in postmenopausal women.200 Thyroidal disorders are often a comorbidity of patients with breast cancer, and thyroxine replacement therapy must be recognized because hyperthyroidism and the treatment of hypothyroidism may both result in bone resorption, resulting in an increased risk of osteoporosis.185,201,202 Conflicting results have been reported regarding thyrotoxic conditions and the effect of thyroxine replacement therapy, suggesting that many patients are overmedicated for hypothyroidism and thus may be exposed to increasing their risk of osteoporosis.203,204 The relationship between thyroidal disease and osteoporosis remains controversial, and therefore, a diagnosis of thyroidal disease should be noted in addressing issues of survivorship in patients with breast cancer.185,205–208 Thyroidal dysfunction may be a risk factor for osteoporosis.209,210
DM has emerged as a pandemic disease affecting more than 10% of the world population.211–213 Many patients diagnosed with breast cancer enter the cancer “arena” with a preexisting diagnosis of DM. Both type 1 and type 2 DM have been associated with the exacerbation of osteoporosis214; however, the mechanism of bone weakening appears to differ between the 2 diseases. Type 1 DM (insulin-dependent) is caused by insulin deficiency resulting in hyperglycemia in young patients, and it may lead to a decrease in BMD, particularly in the spine and hips, resulting in an increased risk of fracture.215–222 Conversely, the evidence for type 2 DM (non-insulin-dependent) for the increased risk of fracture appears somewhat conflicting for reasons unknown.215,223–225 It has been suggested that the comorbidities of type 2 DM (visual impairment, gait-related neuropathy, advanced age, and obesity) may offer clues to the increase in fracture risk.215,224 Multiple studies have reported contrary results when analyzing data regarding the association of type 2 DM and osteoporosis. Some studies suggest no differences in BMD and type 2 DM; some, a lower risk of osteoporosis with type 2 diabetes; and still others, a higher risk.214,226–228
Many medical diagnoses have been identified as potential risk factors for osteoporosis. These include gastrointestinal diseases (celiac disease, malabsorption syndromes, and irritable bowel disease), autoimmune disorders (rheumatoid arthritis and lupus), and other diseases or syndromes.200,229 Because an abundance of information exists regarding the development of osteoporosis caused by coexistent morbidities, patients with breast cancer must be thoroughly evaluated for potential comorbidities, particularly in the setting of hormonal blockade with AIs when used as long-term adjuvant therapy. Chronic conditions require the long-term use of medications that may further increase the risk of osteoporotic development. Extensive confusion surrounds the issue of osteoporotic comorbidities (both disease and drug related). Until further evidence is available, it would be prudent to consider these issues as potential risk factors for osteoporosis much the same as hypertension, dyslipidemias, and DM are regarded as risk factors for CVD and cerebral vascular diseases.
There are multiple modifiable risk factors for osteoporosis in the setting of breast cancer therapy. Most of these modifiable risk factors are related to lifestyle and include alcohol and/or tobacco use, nutritional concerns (including eating disorders), maintenance of a near normal BMI, and adequate physical activity.
The excessive consumption of alcohol, defined as greater than 2 U/day to 3 U/day (1 U equals a half-pint of beer [300 mL], a glass of wine [100 mL], 1 shot of distilled spirits [25 mL]), increases the risk of an osteoporotic fracture by up to 40% compared with those with moderate to no alcohol intake.230–232 Excessive alcohol intake results in suppression of bone-forming cells and calcium metabolism. Alcoholism is also associated with multiple nutritional deficiencies, including vita-min D3 deficiency, which results in the increased production of parathyroid hormone, thereby increasing bone resorption, thus further weakening BMD.232,233 Falls, resulting from chronic heavy drinking, further increase a patient’s risk of fracture events.
Tobacco use, both historic and current consumption, affects bone density and increases fracture risk, although the mechanism of action is not well understood.230,234,235 Inhibition of osteoblastic activity, excessive estrogen breakdown, and earlier onset of menopause have been suggested as possible causes of increased bone fragility in smokers.230,236
Adequate nutrition plays a critical and complex role in bone health. Appropriate intake of calcium, phosphorus, and multiple other nutrients are essential in the maintenance of therapeutic levels of vitamin D3.237,238 It was once thought that obesity was protective against osteoporosis,239 but recent evidence fails to support this belief.240,241 Assessment and monitoring of BMD has been established as an effective and appropriate predictor of fracture risk. Osteoporosis is currently defined on the basis of BMD as established by the World Health Organization in 1994 using T-scores.199,242
Multiple technologies are available for assessing BMD; however, dual-energy x-ray absorptiometry is the most commonly employed. For each BMD value calculated, a T-score representing the average peak BMD in a young, normal reference population and a Z-score representing the standard deviation of the patient’s calculated BMD from the patient’s expected age-matched cohort are calculated. Osteopenia (decreased levels of calcium in the bones) is defined as a T-score between −1.0 and −2.5. Osteoporosis (decreased level of the bony matrix itself) is defined as a T-score equal to or less than −2.5.243,244
To further delineate the risk of fracture incidence, the World Health Organization has developed the Fracture Risk Assessment tool (FRAX), which is a risk-assessment software program that attempts to further delineate the absolute risk by combining BMD measurements and clinical and historical factors.199,242 Table 2 summarizes risk factors for osteoporosis.
SECOND PRIMARY MALIGNANCIES
SPMs, those cancers that occur after the diagnosis of a primary cancer, now constitute one-sixth of all malignancies reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.245
Because patients with breast cancer constitute nearly one-fourth of all long-term cancer survivors,246,247 the issue of SPMs is particularly germane. Commonly, SPMs occur in survivors because of a genetic predisposition and increased susceptibility, caustic exposures to environmental toxins yet to be fully identified, and the carcinogenic proaccelerators of treatment modalities currently in use.9,248,249 Because survival rates for women with a breast cancer diagnosis continue to increase,250,251 the risk for development of SPMs also rises. Longer survival also increases the opportunity for SPMs to develop because increasing age is a well-recognized risk factor for all cancers.
The most important risk factor for SPMs appears to be age at the time of diagnosis. The younger one’s age at diagnosis, the more likely the potential for the development of an SPM. Although the development of a new breast cancer may not qualify specifically as an SPM, it is the most common second malignancy in patients with a primary breast cancer; it accounts for nearly 40% of all new malignancies.252 It may present in the ipsilateral or contralateral breast, but most often, such malignancies are found in the opposite breast, especially if the primary treatment of the initially diagnosed cancer included a mastectomy. The increased risk has been reported to approach 70% more than that of the general population during a 10-year follow-up period.252 Again, younger age at diagnosis has been identified as a predictor of increased risk.253,254
Recurrence can be local, developing in or near the original site, resulting from a failure of primary treatment (even after mastectomy); regional, presenting as nodal involvement in the axillary, supraclavicular, or cervical anatomic locations; or distant, appearing in the bones, lung, liver, or brain. Most often, recurrence is predicated on the initial stage at the time of diagnosis; the higher the stage, the more likely a recurrence.255
An issue of major concern and debate centers on the differentiation of an ipsilateral tumor recurrence after BCT vs the development of a true new primary malignancy. The question is simple; the answer is complex. Approximately one in five patients with breast cancer who have completed a five-year course of adjuvant therapy will experience a recurrence vs an SPM. Technologies exist to distinguish between the two and result in the opportunity to offer better-advantage therapeutic approaches depending on the differentiation.253,254,256 In addition, time to occurrence has been demonstrated to be significantly shorter in patients with an ipsilateral recurrence compared with those diagnosed with a new SPM.257
Signs and symptoms of breast cancer, be they a new primary malignancy or a local-regional recurrence, include the following: a new lump in the breast or on the chest wall; dimpling of the skin; nipple retraction; a spontaneous clear or bloody discharge from the nipple; redness, scaling, or thickening of the nipple areolar complex; shrinking or swelling of the breast, especially if it is unilateral; a mass in the axilla; and a rash on the breast that does not resolve with antibiotics. Signs and symptoms of a distant recurrence may include the new onset of localized bone pain in the long bones, ribs, and/or spine lasting longer than two weeks; persistent chest pain with or without a cough; persistent headache and abdominal pain without intended weight loss; personality changes; new-onset seizures; and loss of consciousness. (See Sidebar: Signs and Symptoms of Breast Cancer.)
Signs and Symptoms of Breast Cancer.
Primary or recurrent/local-regional:
Lump in the breast/chest wall/axilla
Dimpling of the skin
Nipple retraction
Clear or bloody nipple discharge (spontaneous)
Redness, scaling, thickening of nipple-areolar complex
Rash on breast, unresponsive to antibiotics
Distant recurrence:
New-onset localized bone pain lasting longer than 2 weeks (long bones, ribs, spine)
Persistent chest pain, with or without cough
Persistent abdominal pain
Unintended weight loss
Persistent headache
Personality changes
New-onset seizures
Loss of consciousness
Follow-up of recommendations for patients who have completed therapy with a curative intent have been published by the American Society of Clinical Oncology.258 Regular physical examinations, varying from three to six months during the first three years and annually thereafter, are recommended. Mammograms are performed annually (with the exception of a six-month follow-up mammogram after completion of RT). Magnetic resonance imaging is indicated as an annual adjunctive screening tool in those patients who are BRCA gene positive and/or have a diagnosis of invasive lobular breast cancer.259,260 Follow-up in an asymptomatic patient does not call for regular bloodwork, advanced radiographic imaging, or surveillance with specific biomarkers.
In addition to the risk of the development of contralateral disease, survivors of breast cancer are at an increased risk for the development of additional SPMs. It is estimated that an SPM will develop in 5% of patients within 10 years of diagnosis because chemotherapy has been linked, specifically, to the development of secondary acute myeloid leukemia (AML) and, more rarely, myelodysplastic syndromes (MDS).261–267 The risk of AML or MDS appears to depend on the cumulative doses of anthracyclines and alkylating agents administered.262,266,268
Recent controversies have questioned whether use of granulocyte colony-stimulating factors contributes to an increased risk of AML or MDS. The leukemogenic effect of granulocyte colony-stimulating factors is unknown at this time, but those involved in the long-term care of survivors of breast cancer should note that the addition of granulocyte colony-stimulating factors as part of the chemotherapy regimen may, in fact, increase the patient’s potential for the development of AML or MDS.269–273 Although the absolute risk of the development of leukemia is likely to be low in survivors of breast cancer, it should be discussed with the patient, to educate about the potential signs and symptoms of these diseases.262
Recent studies have reported the increased risk of SPMs, with the authors hypothesizing that such malignancies are dependent on multiple other factors in addition to treatment effects.274,275 The risk of developing an SPM, aside from a contralateral breast cancer, appears to be in the range of 5% to 7%.274–278 The most common sites for SPMs to develop are the pulmonary, gynecologic (endometrial and/or ovarian), colorectal, and integumentary (melanoma) systems.276,277 The fact that malignancies of the lung and the colon and rectum appear high on the list is not surprising because both are in the top 3 malignancies in women by incidence and mortality.279
Gynecologic malignancies are related to breast cancer through genetic predispositions (BRCA1 and BRCA2 genetic mutations) as well as conjoined risk factors, including obesity, nulliparity, delayed parity, and a history of hormone replacement therapy.280,281 Numerous epidemiologic studies have established the role of family history as an important risk factor for breast, ovarian, and other associated malignancies and have referred to this as “inherited cancer susceptibility syndromes.” In the early 1990s, a genetic link was discovered between breast and ovarian cancers through the identification of the mutated forms of the BRCA1 and BRCA2 genes.282,283 These genes, when healthy, produce tumor suppressor proteins that help repair damaged DNA, but when mutated, the ability to repair DNA is rendered ineffective.
The harmful mutations in BRCA1 or BRCA2 can be inherited from a mother or father, further amplifying the importance of a thorough acquisition of the patient’s family history. Although in the general population breast cancer will develop in about 1 in 8 women (12%) sometime in their lives, it will develop in 55% to 65% of women with a BRCA1 mutation and 45% of women with a BRCA2 mutation assuming they reach age 70 years.284–286 The general female population has slightly more than a 1% chance of ovarian cancer developing, in contrast to a 39% chance in those with a mutated BRCA1 gene penetrance and an 11% to 17% chance if affected by the BRCA2 mutation.284–286 Previous reports may have overstated the increased risk of breast and ovarian cancers associated with BRCA1 and BRCA2. Carriers of BRCA1 were reported to have a risk as high as 87%, and BRCA2 carriers, a risk as high as 84%.287,288 The incidence of ovarian cancers has also been previously overestimated in families with a history of breast cancer.287 In addition, BRCA1 and BRCA2 mutations have been associated with an increased risk of fallopian tube and peritoneal cancers.288–292
Multiple other genes and their subsequent predisposition to the development of syndromes associated with the increased risk of breast cancer have been identified.293,294 Additional cancer susceptibility syndromes that have been noted are also an issue of concern deserving attention when providing care to long-term survivors of breast cancer. Nearly a dozen syndromes have been associated with hereditary breast (and ovarian) cancer mutations, and several excellent reviews of these issues are available.295,296 Other inherited susceptibility syndromes and/or genes that may predispose to the development of breast cancer include the Li-Fraumeni syndrome (soft tissue carcinoma, osteosarcoma, neurologic tumors, adrenocortical tumors, and leukemia),297,298 Cowden syndrome (multiple hematomas, tumors of the thyroid gland and uterus),296,299 and the Lynch syndrome (colon, uterus, pancreas, brain, gastrointestinal tract, and the integumentary system).295 Numerous other syndromes have been described in association with the increased incidence of breast cancer, but their penetrance and incidence appear minimal.295,296
The most common gynecologic malignancy seen in survivors of breast cancer is uterine cancer, which is probably because of the common use of tamoxifen as an adjuvant therapy for ER+ tumors.97,105 Most studies have demonstrated that the increased relative risk of endometrial cancer in patients receiving tamoxifen is 2 to 3 times higher than that of an age-adjusted, cohort population.300–303 Furthermore, the association of uterine cancer and tamoxifen use appears to be dose dependent and also increases with duration of use.303–305 Despite the acknowledged increased risk of endometrial cancer in patients who receive tamoxifen therapy, the 5-year DFS rate for breast cancer approaches a 40% higher rate than patients not receiving the drug; therefore the risk-benefit ratio for significant increases in survival appears to far outweigh the risk of uterine cancer, which, in most cases, is cured by hysterectomy.300,305
The development of colorectal malignancies has long been known to be increased in patients after the diagnosis of breast cancer.252 However, the reported incidence rates of colorectal cancer in association with breast cancer vary widely.306,307 When investigation of the association of the BRCA1 and BRCA2 gene mutations is undertaken regarding a potential increased risk of colon cancer, current results are inconsistent and conflicting. Some studies have shown an elevated risk of colon cancers in BRCA1 and BRCA2 carriers, but these findings have not been confirmed by others.308,309 Further investigation must be undertaken to verify or to refute an association of BRCA1 and BRCA2 mutations with colorectal cancers.
There appears to be a reciprocally elevated risk of skin cancer occurring after the development of breast cancer, and vice versa.310 The reverse increases in the development of breast cancer and melanoma range from a onefold to threefold increased incidence of developing the other malignancy.275,310–313 As with the increased incidence of SPMs, the incidence of cutaneous melanoma is also age dependent; the younger a woman is at time of diagnosis with one or the other malignancy, the higher her risk of the other malignancy.310 These malignancies may share genetic predispositions such as BRCA2 mutations275,281,310 and mutations in the CDKN2A gene, which have been identified as definitive risk factors for melanoma and thus may inversely increase the risk of breast cancer.310–314 Despite the conflicting results reported to date, one should note that the development of an SPM is associated with a significant decrease in OS, which is particularly concerning.276
Treatment of early-stage breast cancer saw a major paradigm shift in the 1990s from modified radical mastectomy with or without adjuvant therapy to the increased use of BCT followed by radiation for control of local and/or local-regional recurrence.252 With this change came the increased use of RT as the preferred adjunctive approach in procedures that aim to conserve the breast.59–75 Survival has increased with BCT and RT, and therefore the use of RT has grown exponentially. Long-term side effects of RT assume an increasingly important role in the development of SPMs.
Thoracic malignancies after RT for BCT have become an area of great concern as the role of RT in the treatment of breast cancer continues to rise. Lung cancer accounts for 5% of SPMs after breast cancer treatment; considering the high survival rate of breast cancer, lung malignancies, as a new SPM in these patients, are of concern because the survival rate of lung cancer is quite low.315–317 Lung SPMs appear to be significantly increased among women who are younger than age 50 years at their diagnosis. Strikingly, the increase in lung cancer appears as early as 1 year after treatment and the risk persists for an extended period of time. This phenomenon is perhaps not explained in the setting of RT and BCT because the long-term effects of RT have been well documented and follow a latent period of 5 to 10 years or longer.71,83,88,261,318–322 There may be an association in younger patients who have a higher increase in estrogen receptor negative tumors and an increased propensity for SPMs occurring as new lung malignancies.318,319,323
Initially, RT was used as an adjuvant therapy for patients undergoing BCT. However, even in the setting of mastectomy, RT has been used as adjuvant therapy in patients with 4 or more axillary lymph nodes involved with metastases.324,325 There is clear and consistent, prospective, randomized data showing an absolute OS benefit approaching 10% in addition to fairly dramatic benefits in local and local-regional control in these patients. On the other hand, the ability to make solid recommendations for adjuvant RT in women with lesser node involvement has been more elusive. Some recent evidence points to the benefits of RT even in patients who undergo mastectomy but have minimal lymph node involvement (1 to 3 positive lymph nodes) reported in the final pathologic synopsis.326
Thus, concerns about SPMs and RT in patients with breast cancer undergoing mastectomy with minimal nodal involvement will require future awareness and education for caregivers of survivors of breast cancer. As breast cancer survival continues to improve, and this improvement is largely attributable to adjuvant RT, understanding the long-term side effects of RT is assuming an increasingly important role. In addition to the commonly recognized SPMs, as described earlier, reports are beginning to emerge of less well-recognized SPMs secondary to RT, including the development of esophageal malignancies.315,327 The development of such malignancies has not been discussed in recent reports addressing SPMs, probably owing to their obscurity.276 Nonetheless, survivors of breast cancer and those providing follow-up care must be aware of these potential long-term complications, which have only recently been recognized. (See Sidebar: Risk Factors for Second Primary Malignancies.)
Risk Factors for Second Primary Malignancies.
Age at diagnosis
Family history
Genetic syndromes
Adjuvant chemotherapy
Use of granulocyte colony-stimulating factors
Adjuvant radiation therapy
Adjuvant antiestrogen therapy
BREAST CANCER-RELATED LYMPHEDEMA
Breast cancer-related lymphedema (BCRL) is a serious, chronic, debilitating, and common consequence of breast cancer treatment and has been addressed as incurable, or at least as refractory, to conventional treatment modalities. Multiple lifelong morbidities include deformity, pain, a reduction in limb use, and extreme emotional distress often resulting in isolation.328–330 Many patients fear the development of lymph-edema even more so than the diagnosis of the cancer itself or the loss of a breast.328,329,331–333 Upwards of one in five patients may face the consequences of this irreversible, lifelong condition.330 In BCRL, there is an ongoing, progressive accumulation of protein-rich fluids and subsequent fibrosis in the affected limb because of the disruption of lymphatic anatomy.10,334–337 This condition remains poorly understood despite extensive research directed in attempts to identify its exact etiology.10,335,338
BCRL is a well-recognized sequela of the treatment of breast cancer, including surgery and adjuvant therapies employed.10,339 The risk of the development of BCRL is a lifetime risk. Fibrosis may be slow to develop, which may account for the delay in the development of BCRL.334,337 BCRL may develop at any time after treatment; however, the condition develops in most patients within the first two to three years after treatment.332,340–342
The incidence of BCRL, as reported, is incredibly misleading and quite confusing because it varies from 6% to 62%. This range represents an enormous variation and underscores our poor understanding of the condition.331,338,343–345 Some estimates of the incidence of BCRL even exceed 80%.332 Discrepancy of the reported incidence of BCRL appears to result from multifactorial variations of the definition of the condition, the absence of any standardized uniform measurements, the lack of patient symptom reporting, inadequate follow-up of complaints relating to BCRL symptoms, varying follow-up periods, weak study designs, and finally, poor documentation by health care professionals involved in the treatment of patients with BCRL.10,338,346–348 In addition, BCRL may develop in other regions, including the chest wall and/or the remaining breast tissue, an issue that has received little attention in the medical community.349–352
Numerous predisposing risk factors for BCRL have been identified. These risks can be stratified into two major categories: disease specific (factors beyond the patient’s control) and lifestyle risks (factors that may be influenced or controlled by the patient’s proactive involvement). Although some of these risk factors may overlap, many nonmedical factors remain beyond the patient’s control. Factors beyond the patient’s control include the age at diagnosis, stage of disease, extent of surgical manipulation, need for adjuvant therapies, development of postoperative infections, and formation of seromas.10,353,354 Age has been addressed in several studies, and the evidence for this as being a definitive risk factor for the development of BCRL remains conflicting.10,353,354 An urgent and more recent concern is that breast cancer is being diagnosed in younger women. Because development of BCRL is a lifelong risk, the long-term survival of younger patients may result in an increased risk of BCRL over time, as with the risk of CVD.25,26
The surgical treatment of breast cancer appears to be the primary predisposing factor for the development of BCRL. Therefore, the risk of BCRL may differ depending on the initial surgical option chosen by the patient. Mastectomy, as opposed to lumpectomy, may result in a significantly higher risk (a twofold to sixfold increase) of BCRL.341,355,356 The extent of axillary dissection and the ratio of positive to negative lymph nodes have also been identified as factors that may increase the potential for BCRL development.330,338,357–359
Intuitively, it appears to make sense that the number of lymph nodes removed and, furthermore, those that are found to be involved with metastatic disease would increase the chance of BCRL developing owing to the disruption of the anatomic flow of lymph. Several studies do not support this concept and, in fact, offer little evidence for the mechanism of this pathologic event.339,354,360 A potential explanation offered is the fact that lymph node involvement early in the disease process allows the development of collateral channels for lymphatic drainage.360 On the other hand, multiple additional studies lend support to the hypothesis that the extent of nodal dissection or involvement with the disease are, indeed, factors that increase the propensity for the BCRL.330,338,340,357–359,361 Replacing the radical axillary dissections of decades ago, sentinel lymph node sampling is now the currently accepted, minimally invasive, approach to breast cancer treatment in early-stage disease.328,362 Compared with traditional axillary dissection, multiple studies have well documented that sentinel lymph node biopsy for assessing and staging breast cancer results in a significant reduction in the development of BCRL.328,355,363–366 Nonetheless, despite the rapid adoption of sentinel lymph node biopsy, BCRL remains a concern; according to recent data, there is still more than a 7% or 8% chance of BCRL developing within the first 6 months after the biopsy procedure.328,353,367
Chemotherapy has been well documented as an extremely effective adjuvant therapy to decrease recurrence and increase the OS of patients with breast cancer.368,369 Interestingly, the percentage of patients receiving such adjuvant therapies as related to the development of BCRL is poorly documented because of incomplete information gathering and secondarily, in large part, because of the outpatient administration of chemotherapy.370 The addition of chemotherapy to the breast cancer treatment regimen and its relationship to the incidence of BCRL remains largely unresolved. As the multidisciplinary approach to breast cancer increases, it is becoming increasingly difficult to separate various therapies and their long-term consequences. This is particularly true in the setting of BCRL. Polyagent therapies have been implicated with an increase in the incidence of BCRL.371–373 Particular attention has focused on the anthracycline-based therapies.374 It remains unclear why the addition of chemotherapy to the treatment of breast cancer may increase the incidence of BCRL. The issue of more advanced disease requiring adjuvant chemotherapy may skew the population that is more likely to experience BCRL. No studies, to our knowledge, have addressed or isolated a primary association. Therefore, it seems wise to be aware of the fact that chemotherapy, particularly anthracycline-based regimens, should be considered as a potential contributing risk factor for BCRL.
As previously mentioned, RT has become a mainstay in the adjuvant treatment of breast cancer.59–75 Although the ultimate role of adjuvant RT in the development of BCRL is currently under review, substantial evidence has been reported supporting the idea that axillary RT increases the risk of BCRL.335,338,375,376 Presumably, RT-induced fibrosis results in scarring of the lymphatic system, resulting in further lymphatic flow disruption and the subsequent development of BCRL. Other studies have reported no increase in the incidence of BCRL after adjuvant axillary RT.372–374 Contemporary therapy involves sophisticated computed tomographic planning for appropriate simulation and allows for a more exact “targeted” zone for RT. As such, the potential for BCRL secondary to RT will, one hopes, be minimized in the near future.
Additional risk factors for BCRL include the postoperative complications of infection and seroma formation.336,352,355 Trauma, such as a shearing chest wall injury or dermal intrusion secondary to activities such as gardening and hiking, may also predispose a patient to the development of late-onset BCRL. Furthermore, surgery on the dominant side may also increase the incidence of BCRL.377
Lifestyle issues that are modifiable by a patient’s behavior may also play an important role in BCRL risk. The most important modifiable risk factor is related to obesity as determined by BMI.330,338,354,355 A sedentary lifestyle contributes to obesity, and therefore increasing physical activity may help decrease a patient’s BMI. In fact, multiple studies have demonstrated the benefits of exercise to not only decrease BMI but also to decrease the risk of BCRL.10,354,378–380 In addition, exercise has been shown to have significant beneficial effects in cancer rehabilitation and, when coupled with an effective diet, including high vegetable and fruit consumption, has been shown to increase OS after breast cancer.381–383 Finally, DM and hypertension, both associated with an increased BMI, have been identified as potential risk factors for BCRL, and these conditions may be altered by an effective diet and exercise program.10,338,354,355,381,383 Obesity, DM, and hypertensive states also increase the risk of CVD as previously discussed. (See Sidebar: Risk Factors for Breast Cancer-Related Lymphedema.)
Risk Factors for Breast Cancer-Related Lymphedema.
Age at time of diagnosis
Stage of disease
Extent of axillary surgical manipulation
Postoperative infection/seroma formation
Adjuvant chemotherapy
Adjuvant radiation therapy
Trauma to chest wall after therapy
Obesity
Comorbid conditions: diabetes mellitus, hypertension
THROMBOEMBOLIC EVENTS
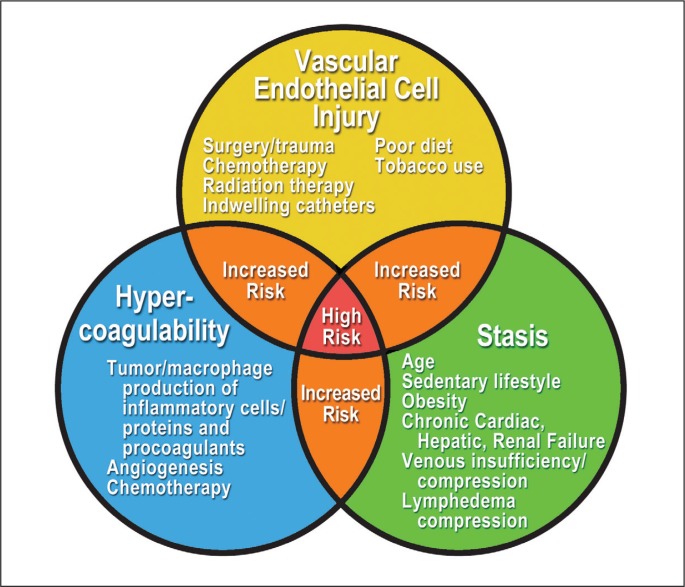
TEs, such as deep venous thrombosis and PE, are uncommon but serious potential consequences related to all malignancies, breast cancer being no exception.384 Cancers are prothrombotic states, and the association of malignancies and the development of hypercoagulability have been well recognized for more than 150 years. Initially described by Rudolf Virchow,385 this association has come to be known as the triad of Virchow or Virchow’s triad: damage to endothelial cells, hypercoagulability (elaboration of procoagulants), and stasis (alteration in blood flow)12,386,387 (Figure 1).
Figure 1.
Virchow’s triad.
CHF = congestive heart failure.
The risk of TE in cancer-affected patients is estimated to range from 15% to 20% and is the second-leading cause of death in those with cancer, although it is often seen in conjunction with multiple additional comorbidities.384,388 The incidence of TE appears to be on the rise because of improved diagnostic imaging technologies, advanced and more effective therapeutic interventions, and, most importantly, increased long-term survival (DFS rates, measured in years after diagnosis and treatment).389 Multiple risk factors for TE in patients with cancer have been identified as significantly affecting morbidity and mortality. It has been estimated that patients in whom a malignancy was diagnosed have a 4- to 7-fold higher risk of TE compared with individuals without a cancer diagnosis.390,391
In addition to the diagnosis of cancer, which itself is a thrombogenic and prothrombotic state,392–394 other risk factors for TE have been identified. As with all diseases, age older than 40 years remains a primary risk factor for TE.395 Stage of disease at the time of diagnosis has also been associated with an increased risk of TE. The more aggressive the disease, the higher the chance of experiencing an episode of TE.390,391,396,397 Stage of disease dictates, in large part, further and more aggressive therapeutic interventions, including the more frequent use of invasive technologies and advanced chemotherapeutic regimens, which further increase the risk of TE.390,398–400 Chemotherapy increases TE risk through multiple pathways and mechanisms involving multifactorial issues, which are beyond the scope of this article. Several excellent reviews on the complex interactions of the association between chemotherapy and the increased risk of TE are available in the references provided.13,292,392 Again, it is important to mention that advanced and/or metastatic disease places patients at an increased risk of TE.400
Most patients with breast cancer (upwards of 70%) have ER+ status, meaning their tumors are being fueled by endogenous estrogen.99–103,401,402 Having an ER+ tumor is important for 2 reasons. First, ER+ tumors tend to be less aggressive, and second, such tumors can be treated with an array of AETs. Tamoxifen has long been considered the primary antiestrogenic drug of choice for ER+ breast cancers. A major side effect, recognized early after its implementation, has been the increased incidence of TE.104–107 The risk of TEs in patients receiving tamoxifen as adjuvant therapy is 1% to 2%105,403 and appears to be highest in the initial 2 years of treatment, although the risk remains throughout all years of therapy.390,404
The recognition of the extremely effective role of AET in the treatment of most breast cancers has resulted in the so-called “third generation” of AETs, the AIs (letrozole, anastrazole, and exemestane).188–190,405 As previously stated, the effects of AIs on the circulatory system remain controversial.106–108,121–126 What seems to have been resolved is the incidence of AIs and TEs. Multiple studies have now documented the decreased incidence of TEs with the use of AIs in direct comparison to tamoxifen.127,406,407 Thus, it now appears that AIs do not increase the risk of TE in patients with breast cancer. What remains controversial are the long-term effects of AIs on lipid levels, which may affect cardiovascular profiles, and subsequent risk factors, which may contribute to CVD. 106–108,121–126 Clearly, for those women with a history of TE, AIs are the drug of choice for adjuvant AET in the postmenopausal patient with an ER+ breast cancer.
Additional risk factors for TE exist in patients with breast cancer. Many contemporary patients receive long-term intravenous therapies, which extend beyond the short-term chemotherapy regimen. These include a one-year cycle of trastuzumab and/or pertuzumab as well as bisphosphonates for bone metastasis and protection against fractures. For the comfort of the patients, indwelling catheters are often placed for administration of such medications as well as to provide easy vascular access to monitor whole blood cell counts. Indwelling catheters may lead to thrombotic complications, the incidence of which is poorly documented in the literature.384,400,408,409 Additional risk factors for TE are not unique to patients with breast cancer but are often identified along with their comorbidities. These include any history of cardiac disease (myocardial infarction, CHF) and a history of TE.395,410 Obesity and its impact on breast cancer is described in the section, Lifestyle Management and Breast Cancer. Relating to the development of TE, obesity is a well-recognized risk factor.395,411,412 Overweight and obesity are often associated with a sedentary lifestyle and general immobility (lack of exercise). It is a modifiable lifestyle risk factor in most patients. Exceptions include increased immobilization caused by hemiplegia after nonfatal cerebral vascular events and fractures of the lower extremities and hips.164,384,392,413
The mechanism by which malignant tumors cause a hypercoagulative state is incompletely understood and is likely multifactorial in nature. Numerous abnormalities in blood composition have been identified, including increased levels of clotting factors, excessive tumor production of inflammatory proteins (cytokines, tumor necrosis factor-α, interleukin-1β), C-reactive protein, and tissue factor from vascular endothelial cells, which all interfere with the normal hemostatic mechanism.387–389,393,414,415
Angiogenesis (the formation of new blood vessels) has been identified recently as a process that may also interfere with the coagulation cascade because both tissue factor and vascular endothelial growth factor are produced by tumorous cells that acquire their own vascular supply.13,388,416,417 In fact, detection of tissue factor in breast cancer vascular endothelium has been shown to be proportionally related to the initiation of new blood vessel formation.418 Further linking angiogenesis and TE are the well-described role of platelet aggregation and the production of platelet dermal growth factor. As such, these also contribute to the risk of TE development.56,392,418 Angiogenesis may also result in the formation of blood vessel structures that are abnormal in their basic anatomic scaffolds and appearance and that display aberrant blood flow patterns.384 Such flow discrepancies may have a role in the development of TE.
A TE significantly decreases long-term survival rates in patients with cancer.384,400 Patients with a malignancy who experience a TE have a fourfold to eightfold higher risk of dying of TE than those who do not have a concurrent malignancy.419–422 Without doubt, patients with malignancies and an episode of TE have a poor prognosis. Furthermore, the risk of a recurrence of TE after an initial episode of TE is higher in patients who have a diagnosis of malignancy. The development of TE is well documented to lead to significantly decreased long-term survival in patients with breast cancer,405,423 and malignancies resulting in death often involve a thrombotic component.
Surprisingly, as high as the incidence of TE is, nearly three-fourths of Americans surveyed were unaware of the condition and its long-term sequelae.413,424 Because TE is a lifetime risk factor after breast cancer,391 it is incumbent on the survivorship care team to educate patients with breast cancer on the signs and symptoms of TE. Usually DVT is heralded by the sudden onset of pain, swelling, tenderness, and occasionally redness and/or warmth in an extremity. A PE, sometimes the sequela of untreated DVT, is heralded by the sudden onset of shortness of breath, chest pain exaggerated by deep breathing, a rapid or irregular pulse, lightheadedness, and occasionally hemoptysis. Education of patients regarding these symptoms can be lifesaving.425,426 (See Sidebar: Risk Factors for Thromboembolic Events.)
Risk Factors for Thromboembolic Events.
Diagnosis of malignancy
Age older than 40 years
History of thromboembolic events
Stage of disease at time of diagnosis
Adjuvant chemotherapy
Adjuvant antiestrogen therapy (long-term administration)
Obesity
Sedentary lifestyle
ADHERENCE AND COMPLIANCE
Many patients who are given prescriptions for medications fail to take them as directed or for the length of time recommended. Adherence and compliance are a concern in the management of malignancies because oral chemotherapeutic agents are increasingly being developed and used in long-term management.427 Of the nearly 400 antineoplastic agents in various stages of development, nearly one-fourth are planned as oral agents.428 Clearly, the increasing percentage of cancer patients who are prescribed or will be prescribed oral therapies will affect current oncologic treatment patterns. Breast cancer survivorship is on a steady rise,427,428 and this cancer is no longer thought of as an acute illness but rather a chronic condition. Therefore, long-term therapies are being increasingly used. Foremost of these interventions is the oral administration of drugs in the outpatient setting, allowing patients to medicate themselves with appropriate dosages and scheduling.
This major advance in cancer treatment comes with new concerns: adherence and compliance. Although adherence and compliance are ultimately related, they are distinct parameters of therapy. Adherence defines the taking of medication as prescribed, whereas compliance more specifically addresses taking the medication for the full term recommended. Compliance is also often referred to as “persistence.”429,430 Some have called for the dismissal of the term compliance because it connotes an onus and dependence on the patients for their ultimate outcomes.431
Regardless of definitions and disparities, the ultimate measure of outcome is OS.432 Although developments in oncology have resulted in major advances in survivorship, many are dependent on long-term administration protocols. As such, the issue of adherence and/or compliance to therapies recommended has become the latest oncologic challenge. This also provides increased impetus for survivorship programs to assume a major role in the care of these patients, that is, follow-up with adherence and compliance.
Adherence and compliance are important for women who are prescribed AET. AET has been definitively demonstrated for more than 30 years to decrease both recurrence and mortality in ER+ patients.15,97,98 Five years of AET, with either tamoxifen or AIs, results in a greater than 30% reduction in breast cancer recurrence and increased OS.97,98,433 Despite the strong documentation of the effectiveness of AET, it is both surprising and disappointing to note the incredibly high rates of noncompliance to a 5-year regimen, which range from 30% to 70%.434 Less than 80% compliance at 2.5 years has been associated with increased mortality.14 Nearly 25% of patients discontinue AET within the first year, and 50% become noncompliant by Year 4,433,435 despite multiple trials that have shown higher recurrence rates and decreased survival.14,436,437
Another point of major concern is that women younger than age 45 years have a greater risk of recurrence owing to more aggressive, higher grade tumors, and yet this group is most likely to discontinue therapy.15 Multiple studies have noted this,435,438,439 yet the issue of age has not been adequately addressed. Patients who are premenopausal when their breast cancer is diagnosed have a higher recurrence rate and increased mortality than those diagnosed in the postmenopausal state.
The poor adherence and compliance to 5 years of AET presents a major challenge. Although this is a large enough issue, we now face new reports strongly supporting a 10-year regimen. Results from the Adjuvant Tamoxifen Longer Against Shorter (ATLAS) trial and the Adjuvant Tamoxifen Treatment Offers More (aTTom) trial have clearly demonstrated improved outcomes by doubling the 5-year recommendation for AET.198,440 The ATLAS trial concluded that recurrence and mortality were lowered in patients given an additional 5 years of tamoxifen. Ten-year recurrence rates decreased by 29% in 6846 patients. Similar findings were reported in aTTom, which followed 6934 women with early-stage breast cancer. Although AIs have been clearly demonstrated to decrease recurrence rates in postmenopausal women with breast cancer,189 their indications for extended length of therapy are less clear and are currently undergoing further investigation. These reports demonstrate the increasing need for adherence and compliance for AET maintenance. Extensions of AET must also take into consideration the long-term side effects of these therapies. Risks of PE were noted in the ATLAS trial as well as the development of endometrial cancer; however, the risk of mortality was lower than the mortality due to breast cancer itself.441,442 Long-term side effects of the AIs have yet to be determined. Particular attention must focus on bone health and osteoporosis as well as the CVD risk associated with AIs.126,443 Clearly, poor adherence and compliance result in less effective disease outcomes and increased mortality.444
AET is one of the most important recent advances in cancer treatment. As simple a treatment as it is, via oral administration, a large number of patients do not take advantage of this intervention. Numerous barriers to adherence and compliance have been identified. These barriers are multifactorial, complex, and often interrelated. Medication side effects are the primary reason for discontinuation of AET. Of patients receiving AET, 94% report mild to severe symptoms directly attributable to AET. These include hot flashes, bone and joint pain, muscle aches, mood swings, loss of libido, dyspareunia, and other menopause-related symptoms.445 Each year, more than 80,000 postmenopausal women begin the 5-year regimen with AIs in an effort to decrease recurrence.446 A major reason for the discontinuation of AIs is the development of incapacitating bone pain and arthralgias in a group that, because of age, has the comorbidity of arthritis.447 Strategies to encourage continuation of AIs include switching to different AIs and the promotion of exercise.448,449
Additional reasons cited for noncompliance include a poor understanding by the patient of the importance of taking the medication to offset recurrence and mortality.450 This has been attributed to inadequate communication by health care practitioners regarding risk vs benefit of the regimens and, in general, poor clinician-patient relationships.432 Patients who fail to understand the importance of their oral medications are likely not to take them as directed. Furthermore, as years of treatment progress and there are no overt symptoms of a malignancy or disease, patients may develop a sense of complacency, resulting in further noncompliance.431 Finally, the cost of medications may play a factor in noncompliance.15,431,432 Those with poor health care coverage or high copayments may not be able to afford the costs of oral agents for an extended time. Although most intravenous medications are a covered benefit, the same is not so for oral therapies. Oral parity legislation has been enacted by multiple states to guarantee payment for outpatient chemotherapy, putting it on par with infusion therapies. The federal government is considering such legislation but has failed to implement such a law. The American College of Surgeons Commission on Cancer’s Advocacy Committee (of which the senior author [BB] is a member) has been active in attempting to push oral parity legislation in Washington, DC. The unequal copay for oral anticancer agents needs correction, as does the poor adherence and compliance in patients with breast cancer receiving AET.
Practices to improve the dismal adherence and compliance rates for AET need to be developed. Interventions should involve a multifaceted approach, beginning with the attending physician and then extending to pharmacists and navigators. Pharmacists, in particular, have the opportunity to expand their scope of practice by actively participating in patient education regarding the importance of taking medications and appropriate scheduling. Navigators can play an important role as well. Regular telephone or texting conversations to check on a patient’s adherence can not only evaluate the patient for compliance but also serve as a motivator for the patient. Although it would appear intuitive that education for patient and family members about the importance of consistent oral therapy would improve compliance, this has not been fully validated. 432 Reminder letters and telephone calls have demonstrated only a minor increase in adherence rates.451 Other studies have noted no improvement in patients given additional education materials or increased support services.452,453 Going forward, technologic advances such as the widespread use of electronic medical records, sophisticated prescription bottles with built-in reminder timers, and effective pharmacy tracking systems may lead to further improvements. Simplified dosing regimens, as well as seamless access to refills, may also help improve compliance. Clearly, many women are not taking their AET as prescribed, and this remains an issue of major concern. Prescribed medications are useless if the patient does not take them. (See Sidebar: Barriers to Adherence and Compliance.)
Barriers to Adherence and Compliance.
Medication side effects or intolerance
Lack of oral parity in health care coverage
Poor understanding of importance of therapy
Inadequate patient education
Complacency
Cost of medications
A CALL TO ACTION
We, as caregivers, are letting our patients die by not taking a strong, proactive role in promoting healthy eating and an active lifestyle, and encouraging emotional resilience. These principles are the cornerstone of the rapidly emerging subspecialty known as lifestyle medicine. Current medical practice is reactive: surgery or a prescription for every illness. This needs to change. A paradigm shift to lifestyle medicine must be implemented immediately.
Dramatic effects using lifestyle interventions have been demonstrated in patients with chronic conditions, which now include breast cancer. Several large studies have conclusively shown that diet and exercise modifications can significantly improve total health.1–6 One prospective study of 23,000 participants evaluated adherence to 4 recommendations: no tobacco use, 30 minutes of exercise 5 times per week, maintaining a body mass index less than 30 kg/m2, and eating a healthy diet (high consumption of fruits, vegetables, legumes, and whole grains, and low consumption of meat).5 People who adhered to these 4 recommendations had an overall 78% lower risk for development of a chronic condition during an approximately 8-year timeframe.5 Furthermore, in those adhering to the recommendations, there was a 93% reduced risk of diabetes mellitus, an 81% reduced risk of myocardial infarction, and a 36% reduction in the risk of cancer.5
Ample evidence exists to support the avocation of a diet based on the recommendations noted in Table 1.1–3,7–12 In addition, a whole-food, plant-based diet tends to promote a healthy body mass index, which is associated with, yet again, a lower risk of all common cancers.13 Dietary principles cannot be fully addressed without consideration of caloric density. Caloric density refers to foods that may or may not provide high amounts of vitamins and nutrients, but contain higher levels of calories. High-nutrient foods have fewer calories per pound in contrast to low-nutrient foods (Figure 1). A healthy diet should remain in the green zone as much as possible and constitute the bulk of food intake.
Table 1.
Daily dietary recommendations
| Decrease or eliminate | Increase or consume heavily |
|---|---|
Bad carbohydrates
|
Good carbohydrates
|
Bad fats
|
Good fats
|
| Salt | |
Meat
| |
| Dairy | |
| Alcohol |
Processed foods are stripped of nutrients and include unhealthy additives.
It is important to remove these from the diet completely.
Figure 1.
Approximate food calories per pound.
Green indicates nutrient-rich foods that should be a major part of a healthy diet; red indicates foods that are low in nutrition and high in calories and should be eliminated completely or consumed in smaller amounts; yellow indicates foods that may be nutrient rich or calorie dense.
Sadly, because profit motives play a large role in the business of health care, the delivery of care and the care of patients is often politicized. Most chronic conditions are influenced by lifestyle and account for more than 75% of health care costs.14 Since 2009, more than 17% of the US gross national product has been spent on health care, amounting to more than $2 trillion.15 Few, if any, of these dollars have been spent on identifying the true underlying etiologies of these chronic conditions. Lifestyle changes have taken a backseat to disease treatment. If we continue on the pathway of treating risk factors and developed disease, we will bankrupt the health care system in the near future.14 Costs for care will continue to escalate; lives will continue to be lost.
It is time for the medical community to intervene and to intervene aggressively. We are not providing the proper treatment when confronting conditions that can be prevented and may even be reversed with lifestyle change and education. Current and future physicians must be trained in lifestyle medicine. The neglect of both the root cause of disease and corrective interventions continues to further the development of chronic conditions and ultimately demise (Figure 2). Lifestyle management courses should be required annual training for all health care employees, optimally as we do annual training for corporate compliance. It is time to prevent disease in all aspects of our lives and the lives of the people we love. It is time to change our health destiny by changing our hearts and minds from an unhealthy lifestyle to a total health lifestyle. It is time to move from disease to health where we live, learn, work, pray, and play. It is time to eat healthy, be active, and resolve conflict.
Figure 2.
Relationship between lifestyle management and death.
The measurement and treatment of high cholesterol, high blood pressure, smoking, and depression are a key strategy to prevent disease and death. Heart attacks, heart failure, strokes, cancer, and cognitive impairment are influenced by unhealthy behaviors and lifestyle. Unhealthy lifestyle choices subsequently lead to endothelial cell injury, endothelial cell dysfunction, and atherosclerosis. Atherosclerosis leads to organ damage and disease that can be prevented and treated through healthy lifestyle interventions. Therefore, basic lifestyle habits—healthy eating, active living, cessation of smoking, and developing emotional resilience—may be a future upstream strategy to help us prevent preventable disease, lower health care costs, and save lives.
The evidence is irrefutable and the message is clear. We are charged with providing patients with the information they need to live a long, healthy life, which can readily be accomplished through lifestyle education. We, as caregivers, owe them that.
References
- 1.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012 Apr 15;118(8 Suppl):2277–87. doi: 10.1002/cncr.27466. DOI: http://dx.doi.org/10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007 Jun 10;25(17):2345–51. doi: 10.1200/JCO.2006.08.6819. DOI: http://dx.doi.org/10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales JF, Barnard ND, Jenkins DJ, et al. Applying the precautionary principle to nutrition and cancer. J Am Coll Nutr. 2014;33(3):239–46. doi: 10.1080/07315724.2013.866527. DOI: http://dx.doi.org/10.1080/07315724.2013.866527. [DOI] [PubMed] [Google Scholar]
- 4.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012 Jan-Feb;62(1):30–67. doi: 10.3322/caac.20140. DOI: http://dx.doi.org/10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Bergmann MM, Kröger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009 Aug 10;169(15):1355–62. doi: 10.1001/archinternmed.2009.237. DOI: http://dx.doi.org/10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Bergmann MM, Boeing H, Li C, Capewell S. Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Prev Med. 2012 Jul;55(1):23–7. doi: 10.1016/j.ypmed.2012.04.016. DOI: http://dx.doi.org/10.1016/j.ypmed.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012 Apr 9;172(7):555–63. doi: 10.1001/archinternmed.2011.2287. DOI: http://dx.doi.org/10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014 Jul 29;349:g4490. doi: 10.1136/bmj.g4490. DOI: http://dx.doi.org/10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev. 2006 Apr;64(4):175–88. doi: 10.1111/j.1753-4887.2006.tb00200.x. DOI: http://dx.doi.org/10.1111/j.1753-4887.2006.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 10.Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1441–8. [PubMed] [Google Scholar]
- 11.Westley RL, May FE. A twenty-first century cancer epidemic caused by obesity: the involvement of insulin, diabetes, and insulin-like growth factors. Int J Endocrinol. 2013;2013:632461. doi: 10.1155/2013/632461. DOI: http://dx.doi.org/10.1155/2013/632461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellavia A, Larsson SC, Bottai M, Wolk A, Orsini N. Fruit and vegetable consumption and all-cause mortality: a dose-response analysis. Am J Clin Nutr. 2013 Aug;98(2):454–9. doi: 10.3945/ajcn.112.056119. DOI: http://dx.doi.org/10.3945/ajcn.112.056119. [DOI] [PubMed] [Google Scholar]
- 13.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009 May;32(5):791–6. doi: 10.2337/dc08-1886. DOI: http://dx.doi.org/10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman MA, Ornish D, Roizen M. Lifestyle medicine: treating the causes of diseases. Altern Ther Health Med. 2009 Nov-Dec;15(6):12–4. [PubMed] [Google Scholar]
- 15.Ornish D. Intensive lifestyle changes and health reform. Lancet Oncol. 2009 Jul;10(7):638–9. doi: 10.1016/S1470-2045(09)70175-5. DOI: http://dx.doi.org/10.1016/S1470-2045(09)70175-5. [DOI] [PubMed] [Google Scholar]
LIFESTYLE MANAGEMENT AND BREAST CANCER
In 2014, the American Institute for Cancer Research reported in their latest review of global research that diet, physical activity, and weight management play a major role in survival among patients with breast cancer.454 Research indicates that a lower BMI and eating a whole-food, plant-based diet (WFPBD), high in fiber and low in fats, improves survival in breast cancer. Maintaining health after a diagnosis of breast cancer requires a lifestyle transformation that helps fight cancer and prevents the development of other diseases that may lower survival. Thus, all patients should consider developing a lifestyle that includes a focus on the vital signs of health called the Wellness Index (WI).455 (The WI is shown in the Sidebar: The Breast Cancer Wellness Index.)
The Breast Cancer Wellness Index.
Biometrics goals:
Body mass index 21 to 25 kg/m2 (be as lean as possible without becoming underweight)
Blood pressure < 140/90 mm Hg
Total blood cholesterol < 200 mg/dL
Fasting blood glucose < 99 mg/dL; hemoglobin A1c < 7.0%
Tobacco use: no smoking
Alcohol abuse: no more than 1 drink/day for women
The goal of the WI is to determine the current state of health and then use the index to identify areas of opportunity to achieve total health during and after treatment. Achieving total health includes a focus on healthy eating, active living, and emotional resilience (HEALER). (See Sidebar: Healthy Eating, Active Living, and Emotional Resilience Goals.) Surviving breast cancer moves one into the HEALER zone, where patients maximize their abilities to prevent cancer recurrence while simultaneously optimizing their ability to treat and prevent chronic diseases such as obesity, DM, hypertension, hyperlipidemia, tobacco and/or alcohol abuse, and coronary artery disease. The WI appears in two parts. The first part is an objective measure of biometrics. This includes a report on BMI, blood pressure, blood glucose and hemoglobin A1c, and any current history of tobacco use or alcohol abuse. Biometrics is an objective measure of current health status. The second part, HEALER, subjectively assesses what lifestyle changes the patient is making to improve survival.
Healthy Eating, Active Living, and Emotional Resilience Goals.
Healthy eating
Eat a whole-food, plant-based diet that includes at least 5 servings of fruits and vegetables per day, legumes, and whole grains
Limit consumption of salty foods and foods processed with salt
Avoid calorie-dense foods, which include sugary drinks
Limit consumption of red meats (eg, beef, pork, and lamb) and avoid all processed meats
Avoid supplements purported to protect against cancer
Active living
Be physically active for at least 30 minutes 5 days per week
Limit sedentary habits
Emotional resilience
Evaluation for depression and treatment of depression, if needed
Biometrics
Surviving breast cancer involves a total-health care strategy. Women with breast cancer who are otherwise healthy have a better chance of survival than do unhealthy women. Biometrics and HEALER are considered total-health balance factors (Figure 2) because they describe the relationship of our current wellness state and our future lifestyle approaches. Healthy eating, active living, and BMI describe an energy balance that is the relationship between energy consumed (healthy eating), energy expended (physical activity), and energy stored (fat). BMI is defined as weight in kilograms divided by height in meters squared. In general, underweight is a BMI below 20 kg/m2, normal weight is a BMI of 21 to 25 kg/m2, overweight is a BMI of 25 to 29.9 kg/m2, and obesity refers to a BMI above 30 kg/m2.456
Figure 2.
The essential components of total health.
A positive energy balance results when energy intake exceeds energy expended (BMI increased); negative energy balance results when energy intake is less than energy expended (BMI decreased). Obesity, inactivity, and unhealthy eating are linked to decreased overall and cancer-specific survival in patients with breast cancer. Studies have demonstrated that interventions to maximize healthy eating and active living can improve quality of life and survival in patients with breast cancer.
Obesity
Obesity is associated with an increased risk of postmenopausal breast cancer in population-based studies.457 Obesity at the time of diagnosis may limit the reduction in breast cancer mortality attainable through the detection and treatment of early-stage disease.458 In addition, obesity at diagnosis is associated with inferior outcomes in ER+ operable breast cancers.459 Obesity is a risk factor for breast cancer recurrence and mortality and an important outcome measure for overall health. Maintaining a healthy weight through programs such as HEALER is one of the most important interventions a patient with breast cancer can make to reduce the risk of breast cancer recurrence, mortality, and development of other chronic diseases.
A systematic literature review and meta-analysis of 82 follow-up studies on the relationship between BMI and breast cancer survival was reported in 2014.456 The report included 213,075 survivors of breast cancer and 41,477 deaths (23,182 deaths were attributed to breast cancer). For each 5 kg/m2 increment of BMI before breast cancer diagnosis, less than 12 months after diagnosis, and 12 or more months after diagnosis, increased risks were observed, respectively, of 17%, 11%, and 8% for overall mortality and 18%, 14%, and 29% for breast cancer-specific mortality. The authors concluded that obesity is associated with poorer OS and breast cancer survival regardless of when BMI is ascertained.456
Despite abundant data linking obesity to a poor prognosis in early-stage breast cancer, there have been relatively few studies evaluating the efficacy and potential benefits of weight loss interventions in survivors of breast cancer. In 2002, researchers performed a systematic review of 5687 literature citations to explore associations among survival and/or recurrence and obesity at diagnosis or weight gain after diagnosis of breast cancer. Results of this observational study showed that women with breast cancer who are overweight or gain weight after diagnosis are found to be at higher risk of breast cancer recurrence and death. The authors concluded that weight loss interventions should be considered in the total-health management of patients with breast cancer.460
Data from the Health, Eating, Activity, & Lifestyle Study suggest that increasing physical activity and decreasing body fat may be a reasonable intervention to decrease insulin and leptin levels, thereby potentially influencing breast cancer prognosis.461 Preventing weight gain by regular aerobic exercise in these women may be important in preventing recurrent disease.462 The strongest evidence that physical activity leading to weight loss and weight maintenance is associated with better outcomes of breast cancer comes from the Nurses’ Health Study.463 Weight management with diet and lifestyle changes should be an integral part of the follow-up of women with breast cancer.
Besides BMI, other biometrics are important for health. Women treated for cancer are also at risk of chronic diseases later in life. Controlling blood pressure, cholesterol, and fasting blood glucose/hemoglobin A1c and avoidance of tobacco and excessive alcohol consumption will help decrease the risk of death caused by chronic disease. For these reasons, women with a breast cancer diagnosis should also monitor and control the other biometrics listed to maintain good health. The HEALER interventions will help maintain a healthy BMI and reduce risks factors associated with the other chronic conditions mentioned.
Epidemiologic evidence shows that the risk of premature death due to coronary artery disease is increased in women who have uncontrolled hypertension, hyperlipidemia, and an elevated hemoglobin A1c level, and in those who smoke tobacco. The strong association observed between mortality and major cardiovascular risk factors makes the undertaking of multifactorial prevention strategies important. Lifestyle strongly influences the development of high blood pressure, high cholesterol, and DM in women. Therefore, women with uncontrolled risk factors for CVD should be seen by their primary care physician and treatment should be initiated to reduce the risk of CVD.464 Because some studies suggest that all types of alcohol may increase the risk of cancer, women with breast cancer should also limit alcohol intake.465
Women who smoke should stop. The relationship between breast cancer risk and active cigarette smoking remains controversial because of unresolved issues of confounding (alcohol intake) and dose response. To investigate these issues further, researchers analyzed data from 73,388 women in the American Cancer Society’s Cancer Prevention Study II Nutrition Cohort.466 Analyses were based on 3721 patients with invasive breast cancer identified during a median follow-up of 13.8 years. The results showed that breast cancer rates were higher in current and former smokers than in never smokers. In addition, the data showed that the risk of invasive breast cancer was highest in women who began smoking at an earlier age.466
Because a large portion of the life of a patient with breast cancer may be spent in survivorship, lifestyle interventions could have time to make a difference and should be included in the overall treatment plan of all patients who receive a breast cancer diagnosis. Assessing biometrics will help us understand opportunities for improvement that can be made as described in the next intervention, which includes healthy eating, active living, and developing emotional resilience as it relates to survivorship.
Healthy Eating, Active Living, and Emotional Resilience
Each year breast cancer is diagnosed in more than 240,000 women in the US. A high proportion of these patients are both obese and sedentary.467 Therefore, lifestyle interventions may be needed to improve health outcomes and prognosis. Recent studies demonstrate that weight loss interventions in breast cancer result in significant weight loss at 6, 12, and 18 months after diagnosis.468 A single-variable analysis in 2007 looked at the association between healthy eating, active living, and obesity with breast cancer survival in a prospective study that included 1490 women who underwent treatment of breast cancer.381 The results showed an association between reduced mortality and higher vegetable-fruit consumption, increased physical activity, and a BMI that was neither underweight nor obese.
An analysis of 85 studies that included more than 164,000 women worldwide demonstrated that the survival of patients with breast cancer may be associated with healthy eating, active living, and a healthy weight.469 These findings support the recommendation that all survivors of breast cancer eat a WFPBD, maintain a healthy weight, and get regular exercise.470 Research suggests that women who have a healthy weight and are physically active have a better chance of surviving breast cancer.
Healthy Eating: On the basis of the aforementioned evidence, women with a breast cancer diagnosis should enroll in a course on lifestyle management. This course should include advice from a WFPBD-trained lifestyle specialist. Consultation should include a discussion on a variety of issues outlined in the Sidebar: The Breast Cancer Wellness Index, including a focus on total health471 and a WFPBD with a substantial reduction, and possibly complete elimination, of all animal-based foods. The dietary focus should emphasize the importance of fruits, vegetables, whole grains, and legumes as the basis for a healthy diet.472,473 Also included in a WFPBD is the elimination of energy-dense foods such as sugary drinks and processed foods high in added sugar, salt, and fat. These types of foods contain more calories per ounce and increase the risk of weight gain. Low-energy-dense foods, like those found in a WFPBD, allow patients to actually eat more food but consume fewer calories. A WFPBD results in decreased intake of foods that increase the risk of coronary artery disease474 and increased intake of foods that may prevent angiogenesis, or the growth of new blood vessels, to cancer cells.475
A large, multiple-database review (MEDLINE, Embase, and The Cochrane Library) to examine and to quantify the potential dose-response relation between fruit and vegetable consumption and the risk of all-cause, cardiovascular, and cancer mortality was reported in 2014.476 The researchers looked at prospective cohort studies that reported mortality risk estimates by levels of fruit and vegetable consumption. Sixteen prospective cohort studies were eligible in this meta-analysis, with follow-up periods ranging from 4.6 to 26 years in which there were 56,423 deaths (including 11,512 deaths caused by CVD and 16,817 due to cancer) among 833,234 participants. Higher consumption of fruits and vegetables was significantly associated with a lower risk of all-cause mortality. The researchers found that there was a threshold at 5 servings of fruits and vegetables per day, after which the risk of all-cause mortality was not further reduced. The results support current recommendations to increase consumption of fruits and vegetables to promote health and overall longevity. Other studies have shown that a diet rich in fruits and vegetables and low in fat lowers blood pressure and reduces the risk of stroke and type 2 DM.477–480 One meta-analysis of prospective cohort studies demonstrated that increased consumption of fruits and vegetables from fewer than 3 servings per day to more than 5 servings per day is related to a 17% reduction in CVD risk.479
In laboratory studies, many individual minerals, vitamins, and phytochemicals demonstrate anticancer effects, yet evidence suggests it is the synergy of compounds working together in the overall diet that offers the strongest cancer protection. No single food or food component can protect against cancer by itself, but strong evidence shows that a diet filled with a variety of plant foods (vegetables, fruits, whole grains, and beans) helps lower the risk of many cancers. A recent meta-analysis of prospective studies reported that a high intake of fruits and vegetables was associated with a reduction in the risk of breast cancer.481 Further data show that there may be an inverse association between dietary fiber intake and breast cancer risk.482 Finally, reducing dietary fat intake, with a modest influence on body weight, may improve relapse-free survival rates of patients with breast cancer receiving conventional cancer treatment.483
Fruits and vegetables contain large amounts of polyphenols. These nutrients have been shown in epidemiologic studies and meta-analyses to offer some protection against the development of cancers, CVD, and DM.484,485 Polyphenols may fight cancer by inhibiting carcinogenesis.486 For example, resveratrol, found in grapes, has been shown to inhibit the growth of a variety of cancer cells. Studies have shown that resveratrol has the potential to modulate all three stages of carcinogenesis (initiation, promotion, and progression), in both chemically and ultraviolet B-induced skin carcinogenesis in mice, as well as in various murine models of human cancers.487
A number of studies have demonstrated that consumption of polyphenols limits the incidence of coronary artery diseases.488 Atherosclerotic lesions may be present and silent for decades before becoming active and causing cardiovascular events.488 Polyphenols may be protective against CVDs by improving endothelial cell function, inhibiting oxidation of low-density lipoproteins, inhibiting platelet aggregation, and preventing macrophage activation and subsequent thrombosis.489,490
Although the association between breast cancer risk and dietary factors has long been identified, the complex relationship between obesity and breast cancer is poorly understood. Obesity in women presenting with breast cancer may be a marker of unhealthy eating and inactivity. However, recent data suggest that even more important than obesity status, women who eat at least five servings of fruits and vegetables per day have a survival advantage over women who do not.476
Active Living: A prospective observational study to determine whether physical activity among women with breast cancer decreases the risk of death caused by breast cancer compared with more sedentary women has demonstrated the relationship between breast cancer survival and physical activity.491 The study was based on responses from 2987 female registered nurses in the Nurses’ Health Study who were diagnosed with Stage I, II, or III breast cancer. Results showed that women who were inactive had a higher risk of death than women who were physically active. The greatest benefit occurred in women who performed the equivalent of walking 3 to 5 hours per week. The authors concluded that physical activity after a breast cancer diagnosis may reduce the risk of death; thus, women with breast cancer who follow physical activity recommendations may improve their survival.463
Additional studies have shown that women who increased physical activity after a breast cancer diagnosis reduce their overall risk of death by 45%, whereas women who decreased physical activity after diagnosis had a 4-fold greater risk of death.492,493 Other studies suggest that exercise after breast cancer diagnosis may improve overall quality of life494 and DFS.495 Healthy eating and active living interventions for women with breast cancer will require behavior change.496 Therefore, strategies for behavior change should be part of lifestyle management programs designed to improve survival in this population. Finally, physical activity has been shown to improve quality of life and balance of life after a breast cancer diagnosis.497
Emotional Resilience: Depression is a major public health problem and often is undiagnosed and untreated in women with breast cancer.498–500 Untreated, depression can cause amplification of physical symptoms, poor treatment adherence, and increased functional impairment.501,502 Physicians are now more aware of the importance of screening and treating depression while managing a particular chronic disease such as breast cancer.503,504 Important advances include routine depression screening at the time of breast cancer diagnosis,505 as well as early interventions and counseling specifically designed to treat depression in patients with cancer.506,507 Cognitive therapy appears to be particularly helpful in treating depression in patients with breast cancer.508 In addition, cognitive therapy may be used to help women with breast cancer achieve the biometric outcomes and weight loss goals associated with improved survival.509 Tamoxifen is commonly used in the treatment of women with breast cancer. As previously mentioned, certain antidepressants, including paroxetine, fluoxetine, and bupropion, may interfere with the metabolism of tamoxifen and should be avoided. Venlafaxine, desvenlafaxine, and mirtazapine do not appear to affect the metabolism of tamoxifen and may be considered the safer choice for the treatment of depression in patients with breast cancer who are receiving tamoxifen.116
Summary of Lifestyle Recommendations
Diet, physical activity, and weight play a major role in survival among patients with breast cancer.454 Looking at improving long-term survival in breast cancer encompasses a total-health strategy that includes a focus on healthy eating, active living, healthy weight, and emotional resilience.471
Five-year breast cancer survival rates have increased, and a total-health care plan will reduce a woman’s risk of cancer recurrence, new cancer formation, and CVD.457
HEALER is a total-health approach to wellness that includes treating the mind, body, and spirit of a patient with breast cancer. Our long-term goal is to help patients with breast cancer understand the importance of energy balance.510 By helping patients with breast cancer achieve a healthy weight and healthy biometrics, we can maximize their chances for long-term survival.
CONCLUSION
Breast cancer survivorship has become a major issue, particularly in the last decade, as early detection and more effective therapies have led to an ever-increasing number of those transitioning from patient to survivor. These successes present a new challenge to the medical community, which must now deal with the long-term complications of past and current treatment modalities.
Although extremely effective in curative intent, many of these therapies result in long-term side effects. Current therapies, which often include polychemotherapeutic agents, RT, and AET, can challenge the cardiovascular system. Cardiovascular disease remains the number one cause of mortality in women in the US, although breast cancer is the most feared.21,22 Bone strength is affected secondary to prolonged estrogen blockade. As younger patients are receiving a breast cancer diagnosis, the incidence of SPMs is becoming more frequently recognized. Thromboembolism risk increases after a cancer diagnosis, and some therapies increase its risk, resulting in death secondary to embolic events.
Advanced therapies call for extended administration of recently developed oral chemotherapy agents. The medical community has been challenged to enforce a five-year regimen for estrogen blockade, and recent findings suggest that doubling the therapy to ten years may decrease recurrence and increase survival. Adherence and compliance for just five years of oral therapy have been poor, and extending such recommendations to ten years appears to be the next challenge for oncologists.
Lifestyle changes, largely focused on reducing BMI, have been demonstrated to play a significant role in extending OS after breast cancer treatment. HEALER provides a tool for clinicians to evaluate the status of survivors of breast cancer. HEALER also summarizes the proactive role that patients may take to enhance their survival.
There is a well-recognized predicted shortage of oncologists by 2020.511 Therefore, the bulk of long-term care will become dependent on the primary care physician. This shift of care means that these physicians will need to be well educated in the long-term medical issues related to breast cancer treatment. Our intent is to share the present information with all those who will be charged with survivorship care in the coming years.
Acknowledgments
The authors wish to acknowledge the assistance of Helene Wolf in the preparation of this manuscript, the assistance of Stephen Beebe in creating the Virchow’s triad figure, and the editorial assistance and support provided by Max L McMillen, ELS.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Abbreviations
- AET
antiestrogen therapy
- AI
aromatase inhibitor
- AML
acute myeloid leukemia
- ATLAS
Adjuvant Tamoxifen Longer Against Shorter
- aTTom
Adjuvant Tamoxifen Treatment Offers More
- BCRL
breast cancer-related lymphedema
- BCT
breast-conserving therapy
- BMD
bone mineral density
- BMI
body mass index
- CHF
congestive heart failure
- CVD
cardiovascular disease
- DFS
disease-free survival
- DM
diabetes mellitus
- ER+
estrogen receptor-positive
- FRAX
Fracture Risk Assessment tool
- HEALER
healthy eating, active living, and emotional resilience
- HER2+
human epidermal growth factor receptor 2-positive
- IOM
Institute of Medicine
- MDS
myelodysplastic syndrome
- OS
overall survival
- PE
pulmonary embolism
- RT
radiation therapy
- SEER
Surveillance, Epidemiology, and End Results
- SERM
selective estrogen receptor modulator
- SPM
second primary malignancy
- SSRI
selective serotonin reuptake inhibitor
- TE
thromboembolic event
- WFPBD
whole-food, plant-based diet
- WI
Wellness Index
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
A Much Brighter Future
In 50 cases operated upon by what we call the complete method we have been able to trace only three local recurrences [6%] … In 34 (73%) … there has never been a local or regionary recurrence, 24 are living and 10 are dead. In 43 of 46 cases (93%) there has been no true local recurrence … . These statistics are so remarkably good that we are encouraged to hope for a much brighter … future for operations for cancer of the breast.
— Halsted WS. I. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889, to January 1894. Ann Surg 1894 Nov;20(5):497–555
References
- 1.Breast Cancer [Internet] Atlanta, GA: American Cancer Society; p. c2014. [cited 2014 Dec 23]. Available from: www.cancer.org/cancer/breastcancer. [Google Scholar]
- 2.De Angelis R, Tavilla A, Verdecchia A, et al. Breast cancer survivors in the United States: geographic variability and time trends, 2005–2015. Cancer. 2009 May 1;115(9):1954–66. doi: 10.1002/cncr.24217. DOI: http://dx.doi.org/10.1002/cncr.24217. [DOI] [PubMed] [Google Scholar]
- 3.Rosedale M, Fu MR. Confronting the unexpected: temporal, situational, and attributive dimensions of distressing symptom experience for breast cancer survivors. Oncol Nurs Forum. 2010 Jan;37(1):E28–33. doi: 10.1188/10.ONF.E28-E33. DOI: http://dx.doi.org/10.1188/10.ONF.E28-E33. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Greenfield S, Stovall E, editors. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academics Press; 2006. [Google Scholar]
- 5.Shockney LD. Perspectives on surveillance and survivorship: when to make the transition. J Natl Compr Canc Netw. 2013 Oct 1;11(10):1298–302. doi: 10.6004/jnccn.2013.0150. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Earle CC, Goodwin PJ. Journal of Clinical Oncology update on progress in cancer survivorship care and research. J Clin Oncol. 2012 Oct 20;30(30):3655–6. doi: 10.1200/JCO.2012.45.3886. DOI: http://dx.doi.org/10.1200/JCO.2012.45.3886. [DOI] [PubMed] [Google Scholar]
- 7.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012 Oct 20;30(30):3657–64. doi: 10.1200/JCO.2012.45.2938. DOI: http://dx.doi.org/10.1200/JCO.2012.45.2938. Erratum in: J Clin Oncol 2012 Dec 20;30(36):4590. DOI: http://dx.doi.org/10.1200/JCO.2012.47.9519. [DOI] [PubMed] [Google Scholar]
- 8.Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol. 2012 Oct 20;30(30):3665–74. doi: 10.1200/JCO.2012.42.2097. DOI: http://dx.doi.org/10.1200/JCO.2012.42.2097. [DOI] [PubMed] [Google Scholar]
- 9.Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012 Oct 20;30(30):3734–45. doi: 10.1200/JCO.2012.41.8681. DOI: http://dx.doi.org/10.1200/JCO.2012.41.8681. [DOI] [PubMed] [Google Scholar]
- 10.Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. 2012 Oct 20;30(30):3726–33. doi: 10.1200/JCO.2012.41.8574. DOI: http://dx.doi.org/10.1200/JCO.2012.41.8574. [DOI] [PubMed] [Google Scholar]
- 11.Flinn WR, Sandager GP, Silva MB, Jr, Benjamin ME, Cerullo LJ, Taylor M. Prospective surveillance for perioperative venous thrombosis. Experience in 2643 patients. Arch Surg. 1996 May;131(5):472–80. doi: 10.1001/archsurg.1996.01430170018002. DOI: http://dx.doi.org/10.1001/archsurg.1996.01430170018002. [DOI] [PubMed] [Google Scholar]
- 12.Rickles FR, Levine MN. Venous thromboembolism in malignancy and malignancy in venous thromboembolism. Haemostasis. 1998;28(Suppl 3):43–9. doi: 10.1159/000022404. DOI: http://dx.doi.org/10.1159/000022404. [DOI] [PubMed] [Google Scholar]
- 13.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465–73. doi: 10.1038/sj.neo.7900263. DOI: http://dx.doi.org/10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008 Dec 2;99(11):1763–8. doi: 10.1038/sj.bjc.6604758. DOI: http://dx.doi.org/10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,679 early-stage breast cancer patients. J Clin Oncol. 2010 Sep 20;28(27):4120–8. doi: 10.1200/JCO.2009.25.9655. DOI: http://dx.doi.org/10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aalto MT. Adherence of hormonal therapy in breast cancer: an advocate’s perspective. Breast Diseases: A Year Book Quarterly. 2013;24(2):130–2. DOI: http://dx.doi.org/10.1016/j.breastdis.2013.04.005. [Google Scholar]
- 17.Krychman ML, Katz A. Breast cancer and sexuality: multi-modal treatment options. J Sex Med. 2012 Jan;9(1):5–13. doi: 10.1111/j.1743-6109.2011.02566.x. DOI: http://dx.doi.org/10.1111/j.1743-6109.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 18.Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol. 2012 Oct 20;30(30):3715–9. doi: 10.1200/JCO.2012.41.7915. DOI: http://dx.doi.org/10.1200/JCO.2012.41.7915. [DOI] [PubMed] [Google Scholar]
- 19.NCCN guidelines for patients: caring for adolescents and young adults [Internet] Fort Washington, PA: National Comprehensive Cancer Network; 2013. [cited 2014 Dec 24]. Available from: www.nccn.org/patients/guidelines/aya/index.html#1/z. [Google Scholar]
- 20.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012 Oct 20;30(30):3687–96. doi: 10.1200/JCO.2012.41.7238. DOI: http://dx.doi.org/10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 21.Canto JG, Kiefe CI. Age-specific analysis of breast cancer versus heart disease mortality in women. Am J Cardiol. 2014 Jan 15;113(2):410–1. doi: 10.1016/j.amjcard.2013.08.055. DOI: http://dx.doi.org/10.1016/j.amjcard.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Robertson RM. Women and cardiovascular disease: the risks of misperception and the need for action. Circulation. 2001 May 15;103(19):2318–20. doi: 10.1161/01.cir.103.19.2318. DOI: http://dx.doi.org/10.1161/01.CIR.103.19.2318. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013 Jan 1;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. DOI: http://dx.doi.org/10.1161/CIR.0b013e31828124ad. Erratum in: Circulation 2013 Jun 11;127(23):e841. DOI: http://dx.doi.org/10.1161/CIR.0b013e31829ae08c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eifel P, Axelson JA, Costa J, et al. National Institution of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001 Jul 4;93(13):979–89. doi: 10.1093/jnci/93.13.979. DOI: http://dx.doi.org/10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 25.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the US from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007 Nov 27;50(22):2128–32. doi: 10.1016/j.jacc.2007.05.056. DOI: http://dx.doi.org/10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 26.Bybee KA, Stevens TL. Matters of the heart: cardiovascular disease in US women. Mo Med. 2013 Jan-Feb;110(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010 Jan 6;102(1):14–25. doi: 10.1093/jnci/djp440. DOI: http://dx.doi.org/10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Health profiles in 5836 long-term cancer survivors. Int J Cancer. 2003 Apr 20;104(4):488–95. doi: 10.1002/ijc.10981. DOI: http://dx.doi.org/10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- 29.Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004 Jun 29;109(25):3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. DOI: http://dx.doi.org/10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 30.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May 14–20;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. DOI: http://dx.doi.org/10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 31.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008 Jan 1;14(1):14–24. doi: 10.1158/1078-0432.CCR-07-1033. DOI: http://dx.doi.org/10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 32.Bovelli D, Plataniotis G, Roila F, ESMO Guidelines Working Group Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010 May;21(Suppl 5):v277–82. doi: 10.1093/annonc/mdq200. DOI: http://dx.doi.org/10.1093/annonc/mdq200. [DOI] [PubMed] [Google Scholar]
- 33.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009 Jun 16;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. DOI: http://dx.doi.org/10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 34.Bowles EJ, Wellman R, Feigelson HS, et al. Pharmacovigilance Study Team Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012 Sep 5;104(17):1293–305. doi: 10.1093/jnci/djs317. DOI: http://dx.doi.org/10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010 Jun 29;10:337. doi: 10.1186/1471-2407-10-337. DOI: http://dx.doi.org/10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackland SP, Anton A, Breitbach GP, et al. HEPI 013 study group Dose-intensive epirubicin-based chemotherapy is superior to an intensive intravenous cyclophosphamide, methotrexate, and fluorouracil regimen in metastatic breast cancer: a randomized multinational study. J Clin Oncol. 2001 Feb 15;19(4):943–53. doi: 10.1200/JCO.2001.19.4.943. [DOI] [PubMed] [Google Scholar]
- 37.Feher O, Vodvarka P, Jassem J, et al. First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol. 2005 Jun;16(6):899–908. doi: 10.1093/annonc/mdi181. DOI: http://dx.doi.org/10.1093/annonc/mdi181. [DOI] [PubMed] [Google Scholar]
- 38.Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N, National Cancer Institute of Canada Clinical Trials Group Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005 Aug 1;23(22):5166–70. doi: 10.1200/JCO.2005.09.423. DOI: http://dx.doi.org/10.1200/JCO.2005.09.423. [DOI] [PubMed] [Google Scholar]
- 39.Martin M, Villar A, Sole-Calvo A, et al. GEICAM Group (Spanish Breast Cancer Research Group), Spain Doxorubicin in combination with fluorouracil and cyclophosphamide (iv FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (iv CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group. Ann Oncol. 2003 Jun;14(6):833–42. doi: 10.1093/annonc/mdg260. DOI: http://dx.doi.org/10.1093/annonc/mdg260. [DOI] [PubMed] [Google Scholar]
- 40.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010 Jul 20;28(21):3416–21. doi: 10.1200/JCO.2009.23.6950. DOI: http://dx.doi.org/10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 41.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008 Mar 10;26(8):1231–8. doi: 10.1200/JCO.2007.13.5467. DOI: http://dx.doi.org/10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010 Jul 20;28(21):3422–8. doi: 10.1200/JCO.2009.26.0463. DOI: http://dx.doi.org/10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 43.Untch M, Muscholl M, Tjulandin S, et al. First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol. 2010 Mar 20;28(9):1473–80. doi: 10.1200/JCO.2009.21.9709. DOI: http://dx.doi.org/10.1200/JCO.2009.21.9709. [DOI] [PubMed] [Google Scholar]
- 44.Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol. 1998 Aug 25;4(Suppl 10):72–85. [PubMed] [Google Scholar]
- 45.Youssef G, Links M. The prevention and management of cardiovascular complications of chemotherapy in patients with cancer. Am J Cardiovasc Drugs. 2005;5(4):233–43. doi: 10.2165/00129784-200505040-00003. DOI: http://dx.doi.org/10.2165/00129784-200505040-00003. [DOI] [PubMed] [Google Scholar]
- 46.Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000 Apr;22(4):263–302. doi: 10.2165/00002018-200022040-00002. DOI: http://dx.doi.org/10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: analysis of dosing as a risk factor. Blood. 1986 Nov;68(5):1114–8. [PubMed] [Google Scholar]
- 48.Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981 May;141(6):758–63. DOI: http://dx.doi.org/10.1001/archinte.1981.00340060066015. [PubMed] [Google Scholar]
- 49.Steinherz LJ, Steinherz PG, Mangiacasale D, et al. Cardiac changes with cyclophosphamide. Med Pediatr Oncol. 1981;9(5):417–22. doi: 10.1002/mpo.2950090502. DOI: http://dx.doi.org/10.1002/mpo.2950090502. [DOI] [PubMed] [Google Scholar]
- 50.Yeh ET. Cardiotoxicity induced by chemotherapy and antibody therapy. Annu Rev Med. 2006;57:485–98. doi: 10.1146/annurev.med.57.121304.131240. DOI: http://dx.doi.org/10.1146/annurev.med.57.121304.131240. [DOI] [PubMed] [Google Scholar]
- 51.Martin M, Pienkowski T, Mackey J, et al. Breast Cancer International Research Group 001 Investigators Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005 Jun 2;352(22):2302–13. doi: 10.1056/NEJMoa043681. DOI: http://dx.doi.org/10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 52.Del Mastro L, Perrone F, Repetto L, et al. Gruppo Italiano di Oncologia Geriatrica (GIOGer) Weekly paclitaxel as first-line chemotherapy in elderly advanced breast cancer patients: a phase II study of the Gruppo Italiano di Oncologia Geriatrica (GIOGer) Ann Oncol. 2005 Feb;16(2):253–8. doi: 10.1093/annonc/mdi056. DOI: http://dx.doi.org/10.1093/annonc/mdi056. [DOI] [PubMed] [Google Scholar]
- 53.Giordano SH, Booser DJ, Murray JL, et al. A detailed evaluation of cardiac toxicity: a phase II study of doxorubicin and one- or three-hour-infusion paclitaxel in patients with metastatic breast cancer. Clin Cancer Res. 2002 Nov;8(11):3360–8. [PubMed] [Google Scholar]
- 54.Jassem J, Pieńkowski T, Płuzańska A, et al. Central & Eastern Europe and Israel Pacitaxel Breast Cancer Study Group Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol. 2001 Mar 15;19(6):1707–15. doi: 10.1200/JCO.2001.19.6.1707. [DOI] [PubMed] [Google Scholar]
- 55.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005 Jul 1;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. DOI: http://dx.doi.org/10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 56.Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2011 Jun;37(4):300–11. doi: 10.1016/j.ctrv.2010.11.001. DOI: http://dx.doi.org/10.1016/j.ctrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Slørdal L, Spigset O. Heart failure induced by non-cardiac drugs. Drug Saf. 2006;29(7):567–86. doi: 10.2165/00002018-200629070-00003. DOI: http://dx.doi.org/10.2165/00002018-200629070-00003. [DOI] [PubMed] [Google Scholar]
- 58.Carver JR, Shapiro CL, Ng A, et al. ASCO Cancer Survivorship Expert Panel American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007 Sep 1;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. DOI: http://dx.doi.org/10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 59.Katz SJ, Hawley ST. From policy to patients and back: surgical treatment decision making for patients with breast cancer. Health Aff (Millwood) 2007 May-Jun;26(3):761–9. doi: 10.1377/hlthaff.26.3.761. DOI: http://dx.doi.org/10.1377/hlthaff.26.3.761. [DOI] [PubMed] [Google Scholar]
- 60.Lantz PV, Zemencuk JK, Katz SJ. Is mastectomy overused? A call for an expanded research agenda. Health Serv Res. 2002 Apr;37(2):417–31. doi: 10.1111/1475-6773.030. DOI: http://dx.doi.org/10.1111/1475-6773.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “the tide is changing”. Ann Surg Oncol. 2009 Oct;16(10):2669–72. doi: 10.1245/s10434-009-0634-y. DOI: http://dx.doi.org/10.1245/s10434-009-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood WC. The future of surgery in the treatment of breast cancer. Breast. 2003 Dec;12(6):472–4. doi: 10.1016/s0960-9776(03)00154-1. DOI: http://dx.doi.org/10.1016/S0960-9776(03)00154-1. [DOI] [PubMed] [Google Scholar]
- 63.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002 Oct 17;347(16):1233–41. doi: 10.1056/NEJMoa022152. DOI: http://dx.doi.org/10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 64.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002 Oct 17;347(16):1227–32. doi: 10.1056/NEJMoa020989. DOI: http://dx.doi.org/10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 65.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995 Apr 6;332(14):907–11. doi: 10.1056/NEJM199504063321402. DOI: http://dx.doi.org/10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 66.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003 Aug 15;98(4):697–702. doi: 10.1002/cncr.11580. DOI: http://dx.doi.org/10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 67.Arriagada R, Lê MG, Guinebretière JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol. 2003 Nov;14(11):1617–22. doi: 10.1093/annonc/mdg452. DOI: http://dx.doi.org/10.1093/annonc/mdg452. [DOI] [PubMed] [Google Scholar]
- 68.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000 Jul;92(14):1143–50. doi: 10.1093/jnci/92.14.1143. DOI: http://dx.doi.org/10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 69.Albain KS, Green SR, Lichter AS, et al. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: an analysis of two intergroup trials. J Clin Oncol. 1996 Nov;14(11):3009–17. doi: 10.1200/JCO.1996.14.11.3009. [DOI] [PubMed] [Google Scholar]
- 70.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992 Apr 23;326(17):1102–7. doi: 10.1056/NEJM199204233261702. DOI: http://dx.doi.org/10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 71.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 Dec 17;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. DOI: http://dx.doi.org/10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 72.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011 Nov 12;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. DOI: http://dx.doi.org/10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000 May 20;355(9217):1757–70. DOI: http://dx.doi.org/10.1016/S0140-6736(00)02263-7. [PubMed] [Google Scholar]
- 74.Rutqvist LE, Rose C, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncol. 2003;42(5–6):532–45. doi: 10.1080/02841860310014444. DOI: http://dx.doi.org/10.1080/02841860310014444. [DOI] [PubMed] [Google Scholar]
- 75.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998 Feb;16(2):441–52. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 76.Recht A. Which breast cancer patients should really worry about radiation-induced heart disease—and how much? J Clin Oncol. 2006 Sep 1;24(25):4058–61. doi: 10.1200/JCO.2006.07.7909. DOI: http://dx.doi.org/10.1200/JCO.2006.07.7909. [DOI] [PubMed] [Google Scholar]
- 77.Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys. 2007 Dec 1;69(95):1484–95. doi: 10.1016/j.ijrobp.2007.05.034. DOI: http://dx.doi.org/10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 78.Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol. 2011 Aug;100(2):241–6. doi: 10.1016/j.radonc.2011.01.004. DOI: http://dx.doi.org/10.1016/j.radonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Aznar MC, Korreman SS, Pedersen AN, Persson GF, Josipovic M, Specht L. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol. 2011 Aug;84(1104):743–6. doi: 10.1259/bjr/12497075. DOI: http://dx.doi.org/10.1259/bjr/12497075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008 Oct 1;72(2):501–7. doi: 10.1016/j.ijrobp.2007.12.058. DOI: http://dx.doi.org/10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 81.Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012 Feb 1;30(4):380–6. doi: 10.1200/JCO.2011.34.5900. DOI: http://dx.doi.org/10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 82.Gagliardi G, Constine L, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S77–85. doi: 10.1016/j.ijrobp.2009.04.093. DOI: http://dx.doi.org/10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 83.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011 Feb;50(2):187–93. doi: 10.3109/0284186X.2010.533190. DOI: http://dx.doi.org/10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- 84.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3):656–65. doi: 10.1016/j.ijrobp.2009.09.064. DOI: http://dx.doi.org/10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ. 2003 Feb 1;326(7383):256–7. doi: 10.1136/bmj.326.7383.256. DOI: http://dx.doi.org/10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Møller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007 Jan 15;7:9. doi: 10.1186/1471-2407-7-9. DOI: http://dx.doi.org/10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005 Aug;6(8):557–65. doi: 10.1016/S1470-2045(05)70251-5. DOI: http://dx.doi.org/10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 88.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006 Sep 1;24(25):4100–6. doi: 10.1200/JCO.2005.05.1037. DOI: http://dx.doi.org/10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 89.Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013 Apr 9;127(14):1476–85. doi: 10.1161/CIRCULATIONAHA.113.001435. DOI: http://dx.doi.org/10.1161/CIRCULATIONAHA.113.001435. [DOI] [PubMed] [Google Scholar]
- 90.Borger JH, Hooning MJ, Boersma LJ, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys. 2007 Nov 15;69(4):1131–8. doi: 10.1016/j.ijrobp.2007.04.042. DOI: http://dx.doi.org/10.1016/j.ijrobp.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 91.Doyle JJ, Neugut AI, Jacobson JS, et al. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. DOI: http://dx.doi.org/10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 92.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013 Mar 14;368(11):987–98. doi: 10.1056/NEJMoa1209825. DOI: http://dx.doi.org/10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 93.Witteles RM. Radiation therapy for breast cancer: buyer beware. J Am Coll Cardiol. 2011 Jan 25;57(4):453–4. doi: 10.1016/j.jacc.2010.08.637. DOI: http://dx.doi.org/10.1016/j.jacc.2010.08.637. [DOI] [PubMed] [Google Scholar]
- 94.Luini A, Gatti G, Zurrida S, et al. The evolution of the conservative approach to breast cancer. Breast. 2007 Apr;16(2):120–9. doi: 10.1016/j.breast.2006.11.001. DOI: http://dx.doi.org/10.1016/j.breast.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Jordan VC. The development of tamoxifen for breast cancer therapy. In: Jordan VC, editor. Long-term tamoxifen treatment for breast cancer. Madison, WI: University of Wisconsin Press; 1994. pp. 3–26. [Google Scholar]
- 96.Osborne CK, Elledge RM, Fuqua SAW. Estrogen receptors in breast cancer therapy. Science & Medicine. 1996 Feb;3(1):32–41. [Google Scholar]
- 97.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 May 16;351(9114):1451–67. DOI: http://dx.doi.org/10.1016/S0140-6736(97)11423-4. [PubMed] [Google Scholar]
- 98.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 May;365(9472):14–20. 1687–717. doi: 10.1016/S0140-6736(05)66544-0. DOI: http://dx.doi.org/10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 99.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. DOI: http://dx.doi.org/10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lumachi F, Brunello A, Maruzzo M, Basso U, Basso SM. Treatment of estrogen receptor-positive breast cancer. Cure Med Chem. 2013;20(5):596–604. doi: 10.2174/092986713804999303. DOI: http://dx.doi.org/10.2174/092986713804999303. [DOI] [PubMed] [Google Scholar]
- 101.Kennecke HF, Ellard S, O’Reilly S, Gelmon KA. New guidelines for treatment of early hormone-positive breast cancer with tamoxifen and aromatase inhibitors. B C Med J. 2006 Apr;48(3):121–6. [Google Scholar]
- 102.Montemurro F, Aglietta M. Hormone receptor-positive early breast cancer: controversies in the use of adjuvant chemotherapy. Endocr Relat Cancer. 2009 Dec;16(4):1091–102. doi: 10.1677/ERC-09-0033. DOI: http://dx.doi.org/10.1677/ERC-09-0033. [DOI] [PubMed] [Google Scholar]
- 103.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609–18. doi: 10.1056/NEJM199811263392207. DOI: http://dx.doi.org/10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 104.Vogel VG, Costantino JP, Wickerham DL, et al. National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006 Jun 21;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. DOI: http://dx.doi.org/10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 105.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998 Sep 16;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. DOI: http://dx.doi.org/10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 106.Vogelvang TE, van der Mooren MJ, Mijatovic V, Kenemans P. Emerging selective estrogen receptor modulators: special focus on effects on coronary heart disease in postmenopausal women. Drugs. 2006;66(2):191–221. doi: 10.2165/00003495-200666020-00005. DOI: http://dx.doi.org/10.2165/00003495-200666020-00005. [DOI] [PubMed] [Google Scholar]
- 107.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003 Nov;18(11):937–47. doi: 10.1046/j.1525-1497.2003.20724.x. DOI: http://dx.doi.org/10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nandur R, Kumar K, Villablanca AC. Cardiovascular actions of selective estrogen receptor modulators and phytoestrogens. Prev Cardiol. 2004 Spring;7(2):73–9. doi: 10.1111/j.1520-037x.2006.2527.x. DOI: http://dx.doi.org/10.1111/j.1520-037X.2006.2527.x. [DOI] [PubMed] [Google Scholar]
- 109.Lodwick R, McConkey B, Brown AM. Life threatening interaction between tamoxifen and warfarin. Br Med J (Clin Res Ed) 1987 Oct 31;295(6606):1141. doi: 10.1136/bmj.295.6606.1141-b. DOI: http://dx.doi.org/10.1136/bmj.295.6606.1141-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsiao CJ, Cherry DK, Beatty PC, Rechsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010 Nov 3;(27):1–32. [PubMed] [Google Scholar]
- 111.Mojtabai R, Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff (Millwood) 2011 Aug;30(8):1434–42. doi: 10.1377/hlthaff.2010.1024. DOI: http://dx.doi.org/10.1377/hlthaff.2010.1024. [DOI] [PubMed] [Google Scholar]
- 112.Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009 Oct 15;339:b3999. doi: 10.1136/bmj.b3999. DOI: http://dx.doi.org/10.1136/bmj.b3999. Erratum in: BMJ 2009;339:b4361. DOI: http://dx.doi.org/10.1136/bmj.b4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmes FA, Liticker JD. Pharmacogenomics of tamoxifen in a nutshell—and who broke the nutcracker? J Oncol Pract. 2005 Nov;1(4):155–9. doi: 10.1200/jop.2005.1.4.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henry NL, Stearns V, Flockhart DA, Hayes DF, Riba M. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. AM J Psychiatry. 2008 Oct;165(10):1251–5. doi: 10.1176/appi.ajp.2008.08040482. DOI: http://dx.doi.org/10.1176/appi.ajp.2008.08040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lash TL, Cronin-Fenton D, Ahern TP, et al. Breast cancer recurrence risk related to concurrent use of SSRI antidepressants and tamoxifen. Acta Oncol. 2010 Apr;49(3):305–12. doi: 10.3109/02841860903575273. DOI: http://dx.doi.org/10.3109/02841860903575273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry. 2009 Dec;70(12):1688–97. doi: 10.4088/JCP.08r04856blu. DOI: http://dx.doi.org/10.4088/JCP.08r04856blu. [DOI] [PubMed] [Google Scholar]
- 117.Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010 Sep-Oct;53(2):94–104. doi: 10.1016/j.pcad.2010.05.006. DOI: http://dx.doi.org/10.1016/j.pcad.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 118.Lamb HM, Adkins JC. Letrazole. A review of its use in postmenopausal women with advanced breast cancer. Drugs. 1998 Dec;56(6):1125–40. doi: 10.2165/00003495-199856060-00020. DOI: http://dx.doi.org/10.2165/00003495-199856060-00020. [DOI] [PubMed] [Google Scholar]
- 119.Howell A, Downey S, Anderson E. New endocrine therapies for breast cancer. Eur J Cancer. 1996 Apr;32A(4):576–88. doi: 10.1016/0959-8049(96)00032-9. DOI: http://dx.doi.org/10.1016/0959-8049(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 120.Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol. 2004 Nov 1;22(21):4261–71. doi: 10.1200/JCO.2004.08.029. DOI: http://dx.doi.org/10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 121.Howell A, Cuzick J, Baum M, et al. ATAC Trialists’ Group Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005 Jan 1–7;365(9453):60–2. doi: 10.1016/S0140-6736(04)17666-6. DOI: http://dx.doi.org/10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 122.Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008 Jan;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. DOI: http://dx.doi.org/10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 123.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007 Feb 10;25(5):486–92. doi: 10.1200/JCO.2006.08.8617. DOI: http://dx.doi.org/10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 124.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005 Sep 7;97(17):1262–71. doi: 10.1093/jnci/dji250. DOI: http://dx.doi.org/10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 125.Nabholtz JM, Gligorov J. Cardiovascular safety profiles of aromatase inhibitors: a comparative review. Drug Saf. 2006;29(9):785–801. doi: 10.2165/00002018-200629090-00003. DOI: http://dx.doi.org/10.2165/00002018-200629090-00003. Erratum in: Drug Saf 2007;30(3):201. [DOI] [PubMed] [Google Scholar]
- 126.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011 Sep 7;103(17):1299–309. doi: 10.1093/jnci/djr242. DOI: http://dx.doi.org/10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 127.Coombes RC, Hall E, Gibson LJ, et al. Intergroup Exemestane Study A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004 Mar 11;350(11):1081–92. doi: 10.1056/NEJMoa040331. DOI: http://dx.doi.org/10.1056/NEJMoa040331. Erratum in: N Engl J Med 2004 Dec 2;351(23):2461. DOI: http://dx.doi.org/10.1056/NEJM200412023512330. Erratum in: N Engl J Med 2006 Oct 19;355(16)1746. DOI: http://dx.doi.org/10.1056/NEJMx060059. [DOI] [PubMed] [Google Scholar]
- 128.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Feb 1;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. DOI: http://dx.doi.org/10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- 129.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011 Feb;62:233–47. doi: 10.1146/annurev-med-070909-182917. DOI: http://dx.doi.org/10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–82. doi: 10.1126/science.3798106. DOI: http://dx.doi.org/10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 131.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–12. doi: 10.1126/science.2470152. DOI: http://dx.doi.org/10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 132.Campiglio M, Somenzi G, Olgiati C, et al. Role of proliferation in HER2 status predicted response to doxorubicin. Int J Cancer. 2003 Jul 1;105(4):568–73. doi: 10.1002/ijc.11113. DOI: http://dx.doi.org/10.1002/ijc.11113. [DOI] [PubMed] [Google Scholar]
- 133.Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: present and promise. Crit Revs Oncol Hematol. 2005 Apr;54(1):11–29. doi: 10.1016/j.critrevonc.2004.10.011. DOI: http://dx.doi.org/10.1016/j.critrevonc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 134.Barroso-Sousa R, Santana IA, Testa L, de Melo Gagliato D, Mano MS. Biological therapies in breast cancer: common toxicities and management strategies. Breast. 2013 Dec;22(6):1009–18. doi: 10.1016/j.breast.2013.09.009. DOI: http://dx.doi.org/10.1016/j.breast.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 135.Ahn ER, Vogel CL. Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Res Treat. 2012 Jan;131(2):371–83. doi: 10.1007/s10549-011-1781-y. DOI: http://dx.doi.org/10.1007/s10549-011-1781-y. [DOI] [PubMed] [Google Scholar]
- 136.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009 Apr;14(4):320–68. doi: 10.1634/theoncologist.2008-0230. DOI: http://dx.doi.org/10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 137.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010 Jan 1;28(1):92–8. doi: 10.1200/JCO.2008.19.9844. DOI: http://dx.doi.org/10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burstein H. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005 Oct 20;353(16):1652–4. doi: 10.1056/NEJMp058197. DOI: http://dx.doi.org/10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 139.Pritchard KI, Shepherd LE, O’Malley FP, et al. National Cancer Institute of Canada Clinical Trials Group HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006 May 18;354(20):2103–11. doi: 10.1056/NEJMoa054504. DOI: http://dx.doi.org/10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 140.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1673–84. doi: 10.1056/NEJMoa052122. DOI: http://dx.doi.org/10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 141.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1659–72. doi: 10.1056/NEJMoa052306. DOI: http://dx.doi.org/10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 142.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. FinHer Study Investigators Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006 Feb 23;354(8):809–20. doi: 10.1056/NEJMoa053028. DOI: http://dx.doi.org/10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 143.Bartsch R, Wenzel L, Hussian D, et al. Analysis of trastuzumab and chemotherapy in advanced breast cancer after the failure of at least one earlier combination: an observational study. BMC Cancer. 2006 Mar 15;6:63. doi: 10.1186/1471-2407-6-63. DOI: http://dx.doi.org/10.1186/1471-2407-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007 May;7(5):332–44. doi: 10.1038/nrc2106. DOI: http://dx.doi.org/10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 145.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007 Aug 10;25(23):3525–33. doi: 10.1200/JCO.2007.11.0106. DOI: http://dx.doi.org/10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 146.Hayes DF, Picard MH. Heart of darkness: the downside of trastuzumab. J Clin Oncol. 2006 Sep 1;24(25):4056–8. doi: 10.1200/JCO.2006.07.5143. DOI: http://dx.doi.org/10.1200/JCO.2006.07.5143. [DOI] [PubMed] [Google Scholar]
- 147.Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004 Jan 15;22(2):322–9. doi: 10.1200/JCO.2004.01.120. DOI: http://dx.doi.org/10.1200/JCO.2004.01.120. [DOI] [PubMed] [Google Scholar]
- 148.Godkar D, Bachu K, Dave B, Megna R, Niranjan S, Khanna A. Comparison and co-relation of invasive and noninvasive methods of ejection fraction measurement. J Natl Med Assoc. 2007 Nov;99(11):1227–8. 1231–4. [PMC free article] [PubMed] [Google Scholar]
- 149.Sengupta PP, Northfelt DW, Gentile F, Zamorano JL, Khanderia BK. Trastuzumab-induced cardiotoxicity: heart failure at the crossroads. Mayo Clin Proc. 2008 Feb;83(2):197–203. doi: 10.4065/83.2.197. DOI: http://dx.doi.org/10.4065/83.2.197. [DOI] [PubMed] [Google Scholar]
- 150.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib; pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008 Jun;83(6):679–86. doi: 10.4065/83.6.679. DOI: http://dx.doi.org/10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 151.Bilancia D, Rasati G, Dinota A, Germano D, Romano R, Manzione L. Lapatinib in breast cancer. Ann Oncol. 2007 Jun;18(Suppl 6):vi26–30. doi: 10.1093/annonc/mdm220. DOI: http://dx.doi.org/10.1093/annonc/mdm220. [DOI] [PubMed] [Google Scholar]
- 152.Magné N, Chargari C, MacDermed D, et al. Tomorrow’s targeted therapies in breast cancer patients: what is the risk for increased radiation-induced cardiac toxicity? Crit Rev Oncol Hematol. 2010 Dec;76(3):186–95. doi: 10.1016/j.critrevonc.2010.01.012. DOI: http://dx.doi.org/10.1016/j.critrevonc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 153.Moy B, Goss PE. Lapatinib-associated toxicity and practical management recommendations. Oncologist. 2007 Jul;12(7):756–65. doi: 10.1634/theoncologist.12-7-756. DOI: http://dx.doi.org/10.1634/theoncologist.12-7-756. [DOI] [PubMed] [Google Scholar]
- 154.Magné N, Védrine L, Chargari C. Impact on cardiac toxicity with trastuzumab and radiotherapy: the question is still ongoing. J Clin Oncol. 2009 Dec 1;27(34):e239. doi: 10.1200/JCO.2009.24.6918. DOI: http://dx.doi.org/10.1200/JCO.2009.24.6918. [DOI] [PubMed] [Google Scholar]
- 155.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005 Dec 1;23(34):8597–605. doi: 10.1200/JCO.2005.02.5841. DOI: http://dx.doi.org/10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 156.Mouridsen H, Keshaviah A, Coates AS, et al. Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1–98 trial. J Clin Oncol. 2007 Dec 20;25(36):5715–22. doi: 10.1200/JCO.2007.12.1665. DOI: http://dx.doi.org/10.1200/JCO.2007.12.1665. [DOI] [PubMed] [Google Scholar]
- 157.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783–92. doi: 10.1056/NEJM200103153441101. DOI: http://dx.doi.org/10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 158.Lenihan DJ, Esteva FJ. Multidisciplinary strategy for managing cardiovascular risks when treating patients with early breast cancer. Oncologist. 2008 Dec;13(12):1224–34. doi: 10.1634/theoncologist.2008-0112. DOI: http://dx.doi.org/10.1634/theoncologist.2008-0112. [DOI] [PubMed] [Google Scholar]
- 159.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2005 Feb;194(2 Suppl):S3–11. doi: 10.1016/j.ajog.2005.08.047. DOI: http://dx.doi.org/10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 160.Ralston SH. Genetic determinants of osteoporosis. Curr Opin Rheumatol. 2005 Jul;17(4):475–9. doi: 10.1097/01.bor.0000166385.62851.92. DOI: http://dx.doi.org/10.1097/01.bor.0000166385.62851.92. [DOI] [PubMed] [Google Scholar]
- 161.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005 Dec;115(2):3318–25. doi: 10.1172/JCI27071. DOI: http://dx.doi.org/10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.US Preventive Services Task Force Screening for osteoporosis: US preventive services task force recommendation statement. Ann Intern Med. 2011 Mar 1;154(5):356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. DOI: http://dx.doi.org/10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 163.Chen Z, Maricic M, Bassford TL, et al. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005 Mar 14;165(5):552–8. doi: 10.1001/archinte.165.5.552. DOI: http://dx.doi.org/10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 164.Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011 Aug 29;11:384. doi: 10.1186/1471-2407-11-384. DOI: http://dx.doi.org/10.1186/1471-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reid IR, Plank LD, Evans MC. Fat mass as an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992 Sep;75(3):779–82. doi: 10.1210/jcem.75.3.1517366. DOI: http://dx.doi.org/10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 166.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005 Jul;20(7):1185–94. doi: 10.1359/JBMR.050304. DOI: http://dx.doi.org/10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 167.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005 Jun;16(6):581–9. doi: 10.1007/s00198-004-1780-5. DOI: http://dx.doi.org/10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 168.Targownik LE, Leslie WD, Davison KS, et al. CaMos Research Group The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012 Sep;107(9):1361–9. doi: 10.1038/ajg.2012.200. DOI: http://dx.doi.org/10.1038/ajg.2012.200. Erratum in: Am J Gastroenterol 2013 Jan;108(1)157. DOI: http://dx.doi.org/10.1038/ajg.2012.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006 Dec 27;296(24):2947–53. doi: 10.1001/jama.296.24.2947. DOI: http://dx.doi.org/10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 170.Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int. 2009 Jan;84(1):13–9. doi: 10.1007/s00223-008-9188-4. DOI: http://dx.doi.org/10.1007/s00223-008-9188-4. [DOI] [PubMed] [Google Scholar]
- 171.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006 Aug;79(2):76–83. doi: 10.1007/s00223-006-0021-7. DOI: http://dx.doi.org/10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 172.Diem SJ, Blackwell TL, Stone KL, et al. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007 Jun 25;167(12):1240–5. doi: 10.1001/archinte.167.12.1240. DOI: http://dx.doi.org/10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- 173.Williams JW, Jr, Mulrow CD, Chiquette E, Noël PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000 May 2;132(9):743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. DOI: http://dx.doi.org/10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- 174.Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012 Sep;51(3):606–13. doi: 10.1016/j.bone.2012.05.018. DOI: http://dx.doi.org/10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 175.Battaglino R, Fu J, Späte U, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004 Sep;19(9):1420–31. doi: 10.1359/JBMR.040606. DOI: http://dx.doi.org/10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 176.Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001 Nov;29(5):477–86. doi: 10.1016/s8756-3282(01)00593-2. DOI: http://dx.doi.org/10.1016/S8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 177.Bliziotes M, Eshleman A, Burt-Pichat B, et al. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006 Dec;39(6):1313–21. doi: 10.1016/j.bone.2006.06.009. DOI: http://dx.doi.org/10.1016/j.bone.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saag K. Mend the mind, but mind the bones!: balancing benefits and potential skeletal risks of serotonin reuptake inhibitors. Arch Intern Med. 2007 Jun 25;167(12):1231–2. doi: 10.1001/archinte.167.12.1231. DOI: http://dx.doi.org/10.1001/archinte.167.12.1231. [DOI] [PubMed] [Google Scholar]
- 179.Schneeweiss S, Wang PS. Association between SSRI use and hip fractures and the effect of residual confounding bias in claims database studies. J Clin Psychopharmacol. 2004 Dec;24(6):632–8. doi: 10.1097/01.jcp.0000145344.76288.39. DOI: http://dx.doi.org/10.1097/01.jcp.0000145344.76288.39. [DOI] [PubMed] [Google Scholar]
- 180.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004 Apr;15(4):323–8. doi: 10.1007/s00198-003-1548-3. DOI: http://dx.doi.org/10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 181.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007 Oct;18(10):1319–28. doi: 10.1007/s00198-007-0394-0. DOI: http://dx.doi.org/10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 182.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000 Jun;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. DOI: http://dx.doi.org/10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 183.Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006 Aug;39(2):253–9. doi: 10.1016/j.bone.2006.02.005. DOI: http://dx.doi.org/10.1016/j.bone.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 184.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int. 2008 Apr;82(4):249–57. doi: 10.1007/s00223-008-9124-7. DOI: http://dx.doi.org/10.1007/s00223-008-9124-7. [DOI] [PubMed] [Google Scholar]
- 185.Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010 Oct;123(10):877–84. doi: 10.1016/j.amjmed.2010.02.028. DOI: http://dx.doi.org/10.1016/j.amjmed.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 186.Lee RH, Lyles KW, Colón-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010 Feb;8(1):34–46. doi: 10.1016/j.amjopharm.2010.02.003. DOI: http://dx.doi.org/10.1016/j.amjopharm.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pilon D, Castilloux AM, Dorais M, LeLorier J. Oral anticoagulants and the risk of osteoporotic fractures among elderly. Pharmacoepidemiol Drug Saf. 2004 May;13(5):289–94. doi: 10.1002/pds.888. DOI: http://dx.doi.org/10.1002/pds.888. [DOI] [PubMed] [Google Scholar]
- 188.Coleman RE, Banks LM, Girgis SI, et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat. 2010 Nov;124(1):153–61. doi: 10.1007/s10549-010-1121-7. DOI: http://dx.doi.org/10.1007/s10549-010-1121-7. [DOI] [PubMed] [Google Scholar]
- 189.Cuzick J, Sestak L, Baum M, et al. ATAC/LATTE Investigators Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10 year analysis of the ATAC trial. Lancet Oncol. 2010 Dec;11(12):1135–41. doi: 10.1016/S1470-2045(10)70257-6. DOI: http://dx.doi.org/10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 190.Rabaglio M, Sun Z, Price KN, et al. BIG 1–98 Collaborative and International Breast Cancer Study Groups Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol. 2009 Sep;20(9):1489–98. doi: 10.1093/annonc/mdp033. DOI: http://dx.doi.org/10.1093/annonc/mdp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy X-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996 Jan;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 192.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992 Mar 26;326(13):852–6. doi: 10.1056/NEJM199203263261302. DOI: http://dx.doi.org/10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 193.Turken S, Siris E, Seldin D, Flaster E, Hyman G, Lindsay R. Effects of tamoxifen on spinal bone density in women with breast cancer. J Natl Cancer Inst. 1989 Jul 19;81(14):1086–8. doi: 10.1093/jnci/81.14.1086. DOI: http://dx.doi.org/10.1093/jnci/81.14.1086. [DOI] [PubMed] [Google Scholar]
- 194.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006 Feb 1;24(4):675–80. doi: 10.1200/JCO.2005.02.3515. DOI: http://dx.doi.org/10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 195.Cohen A, Fleischer JB, Johnson MK, et al. Prevention of bone loss after withdrawal of tamoxifen. Endocr Pract. 2008 Mar;14(2):162–7. doi: 10.4158/EP.14.2.162. DOI: http://dx.doi.org/10.4158/EP.14.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Coleman RE, Banks LM, Girgis SI, et al. Intergroup Exemestane Study group Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007 Feb;8(2):119–27. doi: 10.1016/S1470-2045(07)70003-7. DOI: http://dx.doi.org/10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 197.Resch A, Biber E, Seifert M, Resch H. Evidence that tamoxifen preserves bone density in late postmenopausal women with breast cancer. Acta Oncol. 1998;37(7–8):661–4. doi: 10.1080/028418698430007. DOI: http://dx.doi.org/10.1080/028418698430007. [DOI] [PubMed] [Google Scholar]
- 198.Davies C, Pan H, Godwin J, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 Mar 9;381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1. DOI: http://dx.doi.org/10.1016/S0140-6736(12)61963-1. Erratum in: Lancet 2013 Mar 9;381(9869):804. DOI: http://dx.doi.org/10.1016/S0140-6736(13)60252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gralow JR, Biermann JS, Farooki A, et al. NCC Task Force Report: Bone Health In Cancer Care. J Natl Compr Canc Netw. 2013 Aug;11(Suppl 3):S1–50. doi: 10.6004/jnccn.2013.0215. [DOI] [PubMed] [Google Scholar]
- 200.Kanis JA, McCloskey EV. Risk factors in osteoporosis. Maturitas. 1998 Nov 16;30(3):229–33. doi: 10.1016/s0378-5122(98)00090-5. DOI: http://dx.doi.org/10.1016/S0378-5122(98)00090-5. [DOI] [PubMed] [Google Scholar]
- 201.Sawin CT, Geller A, Hershman JM, Castelli W, Bacharach P. The aging thyroid: the use of thyroid hormone in older persons. JAMA. 1989 May 12;261(18):2653–5. doi: 10.1001/jama.261.18.2653. DOI: http://dx.doi.org/10.1001/jama.1989.03420180077034. [DOI] [PubMed] [Google Scholar]
- 202.Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract. 1993 Mar;43(368):107–9. [PMC free article] [PubMed] [Google Scholar]
- 203.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008 Feb;29(1):76–131. doi: 10.1210/er.2006-0043. DOI: http://dx.doi.org/10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 204.Franklyn JA, Betteridge J, Daykin J, et al. Long-term thyroxine treatment and bone mineral density. Lancet. 1992 Jul 4;340(8810):9–13. doi: 10.1016/0140-6736(92)92423-d. DOI: http://dx.doi.org/10.1016/0140-6736(92)92423-D. [DOI] [PubMed] [Google Scholar]
- 205.Gorka J, Taylor-Gjevre RM, Arnason T. Metabolic and clinical consequences of hyperthyroidism on bone density. Int J Endocrinol. 2013;2013:638727. doi: 10.1155/2013/638727. DOI: http://dx.doi.org/10.1155/2013/638727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tárraga López PJ, López CF, de Mora FN, et al. Osteoporosis in patients with subclinical hypothyroidism treated with thyroid hormone. Clin Cases Miner Bone Metab. 2011 Sep;8(3):44–8. [PMC free article] [PubMed] [Google Scholar]
- 207.Dhanwal DK. Thyroid disorders and bone mineral metabolism. Indian J Endocrinol Metab. 2011 Jul;15(Suppl 2):S107–12. doi: 10.4103/2230-8210.83339. http://dx.doi.org/10.4103/2230-8210.83339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reddy PA, Harinarayan CV, Sachan A, Suresh V, Rajagopal G. Bone disease in thyrotoxicosis. Indian J Med Res. 2012 Mar;135:277–86. [PMC free article] [PubMed] [Google Scholar]
- 209.Abe E, Sun L, Mechanick J, et al. Bone loss in thyroid disease: role of low TSH and high thyroid hormone. Ann N Y Acad Sci. 2007 Nov;1116:383–91. doi: 10.1196/annals.1402.062. DOI: http://dx.doi.org/10.1196/annals.1402.062. [DOI] [PubMed] [Google Scholar]
- 210.Lee WY, Oh KW, Rhee EJ, et al. Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. Arch Med Res. 2006 May;37(4):511–6. doi: 10.1016/j.arcmed.2005.09.009. DOI: http://dx.doi.org/10.1016/j.arcmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 211.Rodbard HW, Blonde L, Braithwaite SS, et al. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007 May-Jun;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. DOI: http://dx.doi.org/10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 212.Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 2005 Sep;28(9):2130–5. doi: 10.2337/diacare.28.9.2130. DOI: http://dx.doi.org/10.2337/diacare.28.9.2130. [DOI] [PubMed] [Google Scholar]
- 213.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. DOI: http://dx.doi.org/10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 214.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007 Apr;18(4):427–44. doi: 10.1007/s00198-006-0253-4. DOI: http://dx.doi.org/10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 215.Wongdee K, Charoenphandhu N. Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J Diabetes. 2011 Mar 15;2(3):41–8. doi: 10.4239/wjd.v2.i3.41. DOI: http://dx.doi.org/10.4239/wjd.v2.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009 Jan;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. DOI: http://dx.doi.org/10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hamilton EJ, Rakic V, Davis WA, et al. Prevalence and predictors of osteopenia and osteoporosis in adults with Type 1 diabetes. Diabet Med. 2009 Jan;26(1):45–52. doi: 10.1111/j.1464-5491.2008.02608.x. DOI: http://dx.doi.org/10.1111/j.1464-5491.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 218.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care. 2008 Sep;31(9):1729–35. doi: 10.2337/dc07-2426. DOI: http://dx.doi.org/10.2337/dc07-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Saha MT, Sievänen H, Salo MK, Tulokas S, Saha HH. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos Int. 2009 Aug;20(8):1401–6. doi: 10.1007/s00198-008-0810-0. DOI: http://dx.doi.org/10.1007/s00198-008-0810-0. [DOI] [PubMed] [Google Scholar]
- 220.Lumachi F, Camozzi V, Tombolan V, Luisetto G. Bone mineral density, osteocalcin, and bone-specific alkaline phosphatase in patients with insulin-dependent diabetes mellitus. Ann N Y Acad Sci. 2009 Sep;1173(Suppl 1):E64–7. doi: 10.1111/j.1749-6632.2009.04955.x. DOI: http://dx.doi.org/10.1111/j.1749-6632.2009.04955.x. [DOI] [PubMed] [Google Scholar]
- 221.Soto N, Pruzzo R, Eyzaguirre F, et al. Bone mass and sex steroids in postmenarcheal adolescents and adult women with Type 1 diabetes mellitus. J Diabetes Complications. 2011 Jan-Feb;25(1):19–24. doi: 10.1016/j.jdiacomp.2009.10.002. DOI: http://dx.doi.org/10.1016/j.jdiacomp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 222.Heilman K, Zilmer M, Zilmer K, Tillmann V. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab. 2009;27(5):598–604. doi: 10.1007/s00774-009-0076-4. DOI: http://dx.doi.org/10.1007/s00774-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 223.Yamaguchi T, Kanazawa I, Yamamoto M, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009 Aug;45(2):174–9. doi: 10.1016/j.bone.2009.05.003. DOI: http://dx.doi.org/10.1016/j.bone.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 224.Petit MA, Paudel ML, Taylor BC, et al. Osteoporotic Fractures in Men (MrOs) Study Group Bone mass and strength in older men with type 2 diabetes: the Osteoporotic Fractures in Men Study. J Bone Miner Res. 2010 Feb;25(2):285–91. doi: 10.1359/jbmr.090725. DOI: http://dx.doi.org/10.1359/jbmr.090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yaturu S, Humphrey S, Landry C, Jain SK. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit. 2009 Jan;15(1):CR5–9. [PubMed] [Google Scholar]
- 226.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep. 2010 Jun;12(3):186–91. doi: 10.1007/s11926-010-0097-y. DOI: http://dx.doi.org/10.1007/s11926-010-0097-y. [DOI] [PubMed] [Google Scholar]
- 227.Räkel A, Sheehy O, Rahme E, LeLorier J. Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008 Jun;34(3):193–205. doi: 10.1016/j.diabet.2007.10.008. DOI: http://dx.doi.org/10.1016/j.diabet.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 228.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006 Sep;91(9):3404–10. doi: 10.1210/jc.2006-0614. DOI: http://dx.doi.org/10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 229.Abdulameer SA, Sulaiman SA, Hassali MA, Subramaniam K, Sahib MN. Osteoporosis and type 2 diabetes mellitus: what do we know, and what we can do? Patient Prefer Adherence. 2012;6:435–48. doi: 10.2147/PPA.S32745. DOI: http://dx.doi.org/10.2147/PPA.S32745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Poole KE, Compston JE. Osteoporosis and its management. BMJ. 2006 Dec 16;333(7581):1251–6. doi: 10.1136/bmj.39050.597350.47. DOI: http://dx.doi.org/10.1136/bmj.39050.597350.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008 May;121(5):406–18. doi: 10.1016/j.amjmed.2007.12.012. DOI: http://dx.doi.org/10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005 Jul;16(7):737–42. doi: 10.1007/s00198-004-1734-y. DOI: http://dx.doi.org/10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 233.Nieves JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005 May;81(5):1232S–1239S. doi: 10.1093/ajcn/81.5.1232. [DOI] [PubMed] [Google Scholar]
- 234.Kanis JA, Johnell O, Oden A. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005 Feb;16(2):155–62. doi: 10.1007/s00198-004-1640-3. DOI: http://dx.doi.org/10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 235.Wong PK, Christie JJ, Wark JD. The effects of smoking on bone health. Clin Sci (Lond) 2007 Sep;113(5):233–41. doi: 10.1042/CS20060173. DOI: http://dx.doi.org/10.1042/CS20060173. [DOI] [PubMed] [Google Scholar]
- 236.Ilich JZ, Kerstetter JE. Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr. 2000 Nov-Dec;19(6):715–37. doi: 10.1080/07315724.2000.10718070. [DOI] [PubMed] [Google Scholar]
- 237.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005 Jul;16(7):713–6. doi: 10.1007/s00198-005-1867-7. DOI: http://dx.doi.org/10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 238.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006 Jun;136(6):1453–6. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Brown SA, Sharpless JL. Osteoporosis: an under-appreciated complication of diabetes. Clin Diabetes. 2004 Jan;22(1):10–20. DOI: http://dx.doi.org/10.2337/diaclin.22.1.10. [Google Scholar]
- 240.Greco EA, Fornari R, Rossi F, et al. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract. 2010 May;64(6):817–20. doi: 10.1111/j.1742-1241.2009.02301.x. DOI: http://dx.doi.org/10.1111/j.1742-1241.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 241.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994 Aug;9(8):1137–41. doi: 10.1002/jbmr.5650090802. DOI: http://dx.doi.org/10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 242.National Osteoporosis Foundation . Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. [revised 2010 Jan]. [Google Scholar]
- 243.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003 Nov 1;21(21):4042–57. doi: 10.1200/JCO.2003.08.017. DOI: http://dx.doi.org/10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 244.WHO Scientific Group on the Prevention and Management of Osteoporosis . WHO technical report series; 921. Geneva, Switzerland: World Health Organization; 2003. Prevention and management of osteoporosis: report of a WHO scientific group. [Google Scholar]
- 245.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2007 [Internet] Bethesda, MD: National Cancer Institute; 2010. [cited 2015 Jan 16]. Available from: http://seer.cancer.gov/csr/1975_2007/. [Google Scholar]
- 246.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012 Jul-Aug;62(4):220–41. doi: 10.3322/caac.21149. DOI: http://dx.doi.org/10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 247.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117–28. doi: 10.1093/jnci/djq495. DOI: http://dx.doi.org/10.1093/jnci/djq495. Erratum in: J Natl Cancer Inst 2011 Apr 20;103(8):699. DOI: http://dx.doi.org/10.1093/jnci/djr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship—genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006 Jan;98(1):15–25. doi: 10.1093/jnci/djj001. DOI: http://dx.doi.org/10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 249.Travis LB. Therapy-associated solid tumors. Acta Oncol. 2002;41(4):323–33. doi: 10.1080/028418602760169361. DOI: http://dx.doi.org/10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 250.Berry DA, Cronin KA, Plevritis SK, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005 Oct 27;353(17):1784–92. doi: 10.1056/NEJMoa050518. DOI: http://dx.doi.org/10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 251.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005 Oct 5;97(19):1407–27. doi: 10.1093/jnci/dji289. DOI: http://dx.doi.org/10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 252.Curtis RE, Freedman DM, Ron E, editors. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. NIH pub no. 05-5320. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 253.Komoike Y, Akiyama F, Iino Y, et al. Analysis of ipsilateral breast tumor recurrences after breast-conserving treatment based on the classification of true recurrences and new primary tumors. Breast Cancer. 2005;12(2):104–11. doi: 10.2325/jbcs.12.104. DOI: http://dx.doi.org/10.2325/jbcs.12.104. [DOI] [PubMed] [Google Scholar]
- 254.Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006 Jan 1;106(1):35–41. doi: 10.1002/cncr.21551. DOI: http://dx.doi.org/10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 255.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008 Aug 20;100(16):1179–83. doi: 10.1093/jnci/djn233. DOI: http://dx.doi.org/10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schlechter BL, Yang Q, Larson PS, et al. Quantitative DNA fingerprinting may distinguish new primary breast cancer from disease recurrence. J Clin Oncol. 2004 May 15;22(10):1830–8. doi: 10.1200/JCO.2004.05.123. DOI: http://dx.doi.org/10.1200/JCO.2004.05.123. [DOI] [PubMed] [Google Scholar]
- 257.Panet-Raymond V, Truong PT, McDonald RE, et al. True recurrence versus new primary: an analysis of ipsilateral breast tumor recurrences after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2011 Oct 1;81(2):409–17. doi: 10.1016/j.ijrobp.2010.05.063. DOI: http://dx.doi.org/10.1016/j.ijrobp.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 258.Khatcheressian JL, Hurley P, Bantug E, et al. American Society of Clinical Oncology Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013 Mar 1;31(7):961–5. doi: 10.1200/JCO.2012.45.9859. DOI: http://dx.doi.org/10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 259.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007 Mar-Apr;57(2):78–89. doi: 10.3322/canjclin.57.2.75. DOI: http://dx.doi.org/10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 260.Heil J, Buehler A, Golatta M, et al. Do patients with invasive lobular breast cancer benefit in terms of adequate change in surgical therapy from a supplementary preoperative breast MRI? Ann Oncol. 2012 Jan;23(1):98–104. doi: 10.1093/annonc/mdr064. DOI: http://dx.doi.org/10.1093/annonc/mdr064. [DOI] [PubMed] [Google Scholar]
- 261.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008 Mar 10;26(8):1239–46. doi: 10.1200/JCO.2007.11.9081. DOI: http://dx.doi.org/10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 262.Azim HA, Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011 Sep;22(9):1939–47. doi: 10.1093/annonc/mdq683. DOI: http://dx.doi.org/10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 263.Beadle G, Baade P, Fritschi L. Acute myeloid leukemia after breast cancer: a population-based comparison with hematological malignancies and other cancers. Ann Oncol. 2009 Jan;20(1):103–9. doi: 10.1093/annonc/mdn530. DOI: http://dx.doi.org/10.1093/annonc/mdn530. [DOI] [PubMed] [Google Scholar]
- 264.Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005 Jun 20;23(18):4179–91. doi: 10.1200/JCO.2005.05.029. DOI: http://dx.doi.org/10.1200/JCO.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 265.Tallman MS, Gray R, Bennett JM, et al. Leukemogenic potential of adjuvant chemotherapy for early-stage breast cancer: the Eastern Cooperative Oncology Group experience. J Clin Oncol. 1995 Jul;13(7):1557–63. doi: 10.1200/JCO.1995.13.7.1557. [DOI] [PubMed] [Google Scholar]
- 266.Smith RE, Bryant J, DeCillis A, Anderson S, National Surgical Adjuvant Breast and Bowel Project Experience Acute myeloid leukemia and myelodysplastic syndrome after doxorubicincyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003 Apr 1;21(7):1195–204. doi: 10.1200/JCO.2003.03.114. DOI: http://dx.doi.org/10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 267.Burnell M, Levine MN, Chapman JA, et al. Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by Paclitaxel verses Doxorubicin and cyclophosphamide followed by Paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010 Jan 1;28(1):77–82. doi: 10.1200/JCO.2009.22.1077. DOI: http://dx.doi.org/10.1200/JCO.2009.22.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Diamandidou E, Buzdar AU, Smith TL, Frye D, Witjaksono M, Hortobagyi GN. Treatment-related leukemia in breast cancer patients treated with fluorouracil-doxorubicin-cyclophosphamide combination adjuvant chemotherapy: the University of Texas MD Anderson Cancer Center experience. J Clin Oncol. 1996 Oct;14(10):2722–30. doi: 10.1200/JCO.1996.14.10.2722. [DOI] [PubMed] [Google Scholar]
- 269.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007 Feb 7;99(3):196–205. doi: 10.1093/jnci/djk028. DOI: http://dx.doi.org/10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 270.Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol. 2007 Sep 1;25(25):3871–6. doi: 10.1200/JCO.2007.12.0832. DOI: http://dx.doi.org/10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 271.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003 Apr 15;21(18):1431–9. doi: 10.1200/JCO.2003.09.081. DOI: http://dx.doi.org/10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 272.Confer DL, Miller JP. Long-term safety of filgrastim (rhG-CSF) administration. Br J Haematol. 2007 Apr;137(1):77–8. doi: 10.1111/j.1365-2141.2007.06524.x. DOI: http://dx.doi.org/10.1111/j.1365-2141.2007.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003 Dec 15;21(24):4524–31. doi: 10.1200/JCO.2003.05.002. DOI: http://dx.doi.org/10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 274.Brown LM, Chen BE, Pfeiffer RM, et al. Risk of second non-hematological malignancies among 376,825 breast cancer survivors. Breast Cancer Res Treat. 2007 Dec;106(3):439–51. doi: 10.1007/s10549-007-9509-8. DOI: http://dx.doi.org/10.1007/s10549-007-9509-8. [DOI] [PubMed] [Google Scholar]
- 275.Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer. 2006 May 1;118(9):2285–92. doi: 10.1002/ijc.21651. DOI: http://dx.doi.org/10.1002/ijc.21651. [DOI] [PubMed] [Google Scholar]
- 276.Yi M, Cormier JN, Xing Y, et al. Other primary malignancies in breast cancer patients treated with breast conserving surgery and radiation therapy. Ann Surg Oncol. 2013 May;20(5):1514–21. doi: 10.1245/s10434-012-2774-8. DOI: http://dx.doi.org/10.1245/s10434-012-2774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Galper S, Gelman R, Recht A, et al. Second nonbreast malignancies after conservative surgery and radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2002 Feb 1;52(2):406–14. doi: 10.1016/s0360-3016(01)02661-x. DOI: http://dx.doi.org/10.1016/S0360-3016(01)02661-X. [DOI] [PubMed] [Google Scholar]
- 278.Sánchez L, Lana A, Hidalgo A, et al. Risk factors for second primary tumours in breast cancer survivors. Eur J Cancer Prev. 2008 Oct;17(5):406–13. doi: 10.1097/CEJ.0b013e3282f75ee5. DOI: http://dx.doi.org/10.1097/CEJ.0b013e3282f75ee5. [DOI] [PubMed] [Google Scholar]
- 279.Cancer facts and figures 2013 [Internet] Atlanta, GA: American Cancer Society; 2013. [cited 2015 Jan 16]. Available from: www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/2013-cancer-facts-and-figures.pdf. [Google Scholar]
- 280.Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004 Jun 15;22(12):2328–35. doi: 10.1200/JCO.2004.04.033. DOI: http://dx.doi.org/10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 281.Thompson D, Easton D. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia. 2004 Jul;9(3):221–36. doi: 10.1023/B:JOMG.0000048770.90334.3b. DOI: http://dx.doi.org/10.1023/B:JOMG.0000048770.90334.3b. [DOI] [PubMed] [Google Scholar]
- 282.Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–9. doi: 10.1126/science.2270482. DOI: http://dx.doi.org/10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 283.Narod SA, Feunteun J, Lynch HT, et al. Familial breast-ovarian cancer locus on chromosome 17q12–q23. Lancet. 1991 Jul 13;338(8759):82–3. doi: 10.1016/0140-6736(91)90076-2. DOI: http://dx.doi.org/10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- 284.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003 May;72(5):1117–30. doi: 10.1086/375033. DOI: http://dx.doi.org/10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007 Apr 10;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. DOI: http://dx.doi.org/10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Howlader N, Noone AM, Krapcho M, editors. SEER cancer statistics review, 1975–2011 [Internet] Bethesda, MD: National Cancer Institute; updated 2014 Dec 17[cited 2015 Jan 20]. Available from: http://seer.cancer.gov/csr/1975_2011/. [Google Scholar]
- 287.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998 Mar;62(3):676–89. doi: 10.1086/301749. DOI: http://dx.doi.org/10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–5. doi: 10.1016/s0140-6736(94)91578-4. DOI: http://dx.doi.org/10.1016/S0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 289.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002 Sep 18;94(18):1365–72. doi: 10.1093/jnci/94.18.1365. DOI: http://dx.doi.org/10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 290.Finch A, Beiner M, Lubinski J, et al. Hereditary Ovarian Cancer Clinical Study Group Salpingooophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with BRCA1 or BRCA2 mutation. JAMA. 2006 Jul 12;296(2):185–92. doi: 10.1001/jama.296.2.185. DOI: http://dx.doi.org/10.1097/01.ogx.0000251480.69322.50. [DOI] [PubMed] [Google Scholar]
- 291.Levine DA, Argenta PA, Yee CJ, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003 Nov 15;21(22):4222–7. doi: 10.1200/JCO.2003.04.131. DOI: http://dx.doi.org/10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- 292.Thompson D, Easton DF, Breast Cancer Linkage Consortium Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002 Sep 18;94(18):1358–65. doi: 10.1093/jnci/94.18.1358. DOI: http://dx.doi.org/10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 293.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008 Aug;124(1):31–42. doi: 10.1007/s00439-008-0529-1. DOI: http://dx.doi.org/10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 294.Walsh T, Casadei S, Coats KH, et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA. 2006 Mar 22;295(12):1379–88. doi: 10.1001/jama.295.12.1379. DOI: http://dx.doi.org/10.1001/jama.295.12.1379. [DOI] [PubMed] [Google Scholar]
- 295.Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010 May;12(5):245–59. doi: 10.1097/GIM.0b013e3181d38f2f. DOI: http://dx.doi.org/10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- 296.Nusbaum R, Vogel KJ, Ready K. Susceptibility to breast cancer: hereditary syndromes and low penetrance genes. Breast Dis. 27:2006–2007. 21–50. doi: 10.3233/bd-2007-27103. [DOI] [PubMed] [Google Scholar]
- 297.Olivier M, Goldgar DE, Sodha N, et al. Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003 Oct 15;63(20):6643–50. [PubMed] [Google Scholar]
- 298.Garber JE, Goldstein AM, Kantor AF, Dreyfus MG, Fraumeni JF, Jr, Li FP. Follow-up study of twenty-four families with Li-Fraumeni syndrome. Cancer Res. 1991 Nov 15;51(22):6094–7. [PubMed] [Google Scholar]
- 299.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003 Sep;22(3):183–98. doi: 10.1002/humu.10257. DOI: http://dx.doi.org/10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 300.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994 Apr 6;86(7):527–37. doi: 10.1093/jnci/86.7.527. DOI: http://dx.doi.org/10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 301.Sismondi P, Biglia N, Volpi E, Giai M, de Grandis T. Tamoxifen and endometrial cancer. Ann N Y Acad Sci. 1994 Sep 30;734:310–21. doi: 10.1111/j.1749-6632.1994.tb21761.x. DOI: http://dx.doi.org/10.1111/j.1749-6632.1994.tb21761.x. [DOI] [PubMed] [Google Scholar]
- 302.Bissett D, Davis JA, George WD. Gynecological monitoring during tamoxifen therapy. Lancet. 1994 Nov 5;344(8932):1244. doi: 10.1016/s0140-6736(94)90747-1. DOI: http://dx.doi.org/10.1016/S0140-6736(94)90747-1. [DOI] [PubMed] [Google Scholar]
- 303.Curtis RE, Boice JD, Jr, Shriner DA, Hankey BF, Fraumeni JF., Jr Second cancers after adjuvant tamoxifen therapy for breast cancer. J Natl Cancer Inst. 1996 Jun 19;88(12):832–4. doi: 10.1093/jnci/88.12.832. DOI: http://dx.doi.org/10.1093/jnci/88.12.832. [DOI] [PubMed] [Google Scholar]
- 304.Swerdlow AJ, Jones ME, British Tamoxifen Second Cancer Study Group Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005 Mar 2;97(5):375–84. doi: 10.1093/jnci/dji057. DOI: http://dx.doi.org/10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 305.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice ACOG committee opinion. No. 336. Tamoxifen and uterine cancer. Obstet Gynecol. 2006 Jun;107(6):1475–8. doi: 10.1097/00006250-200606000-00057. [DOI] [PubMed] [Google Scholar]
- 306.Kmet LM, Cook LS, Weiss NS, Schwartz SM, White E. Risk factors for colorectal cancer following breast cancer. Breast Cancer Res Treat. 2003 May;79(2):143–7. doi: 10.1023/a:1023926401227. DOI: http://dx.doi.org/10.1023/A:1023926401227. [DOI] [PubMed] [Google Scholar]
- 307.Soerjomataram I, Louwman WJ, de Vries E, Lemmens VE, Klokman WJ, Coebergh JW. Primary malignancy after primary female breast cancer in the South of the Netherlands, 1972–2001. Breast Cancer Res Treat. 2005 Sep;93(1):91–5. doi: 10.1007/s10549-005-4016-2. DOI: http://dx.doi.org/10.1007/s10549-005-4016-2. [DOI] [PubMed] [Google Scholar]
- 308.Rubino C, de Vathaire F, Diallo I, Shamsaldin A, Lê MG. Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat. 2000 Jun;61(3):183–95. doi: 10.1023/a:1006489918700. DOI: http://dx.doi.org/10.1023/A:1006489918700. [DOI] [PubMed] [Google Scholar]
- 309.Levi F, Te VC, Randimbison L, La Vecchia C. Cancer risk in women with previous breast cancer. Ann Oncol. 2003 Jan;14(1):71–3. doi: 10.1093/annonc/mdg028. DOI: http://dx.doi.org/10.1093/annonc/mdg028. [DOI] [PubMed] [Google Scholar]
- 310.Goggins W, Gao W, Tsao H. Association between female breast cancer and cutaneous melanoma. Int J Cancer. 2004 Sep 20;111(5):792–4. doi: 10.1002/ijc.20322. DOI: http://dx.doi.org/10.1002/ijc.20322. [DOI] [PubMed] [Google Scholar]
- 311.Bhatia S, Estrada-Batres L, Maryon T, Bogue M, Chu D. Second primary tumors in patients with cutaneous malignant melanoma. Cancer. 1999 Nov 15;86(10):2014–20. doi: 10.1002/(sici)1097-0142(19991115)86:10<2014::aid-cncr19>3.0.co;2-4. DOI: http://dx.doi.org/10.1002/(SICI)1097-0142(19991115)86:10%3C2014::AIDCNCR19%3E3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 312.Borg A, Sandberg T, Nilsson K, et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst. 2000 Aug 2;92(15):1260–6. doi: 10.1093/jnci/92.15.1260. DOI: http://dx.doi.org/10.1093/jnci/92.15.1260. [DOI] [PubMed] [Google Scholar]
- 313.Schmid-Wendtner MH, Baumert J, Wendtner CM, Plewig G, Volkenandt M. Risk of second primary malignancies in patients with cutaneous melanoma. Br J Dermatol. 2001 Dec;145(6):981–5. doi: 10.1046/j.1365-2133.2001.04507.x. DOI: http://dx.doi.org/10.1046/j.1365-2133.2001.04507.x. [DOI] [PubMed] [Google Scholar]
- 314.Monnerat C, Chompret A, Kannengiesser C, et al. BRCA1, BRCA2, TP53, and CDKN2A germline mutations in patients with breast cancer and cutaneous melanoma. Fam Cancer. 2007;6(4):453–61. doi: 10.1007/s10689-007-9143-y. DOI: http://dx.doi.org/10.1007/s10689-007-9143-y. [DOI] [PubMed] [Google Scholar]
- 315.Reis LAG, Melbert D, Krapcho M, editors. SEER cancer statistics review, 1975–2005[Internet] Bethesda, MD: National Cancer Institute; 2008. [cited 2015 Jan 20]. Available from: http://seer.cancer.gov/archive/csr/1975_2005/. [Google Scholar]
- 316.Schonfeld SJ, Curtis RE, Anderson WF, Berrington de González A. The risk of a second primary lung cancer after a first invasive breast cancer according to estrogen receptor status. Cancer Causes Control. 2012 Oct;23(10):1721–8. doi: 10.1007/s10552-012-0054-3. DOI: http://dx.doi.org/10.1007/s10552-012-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, editors. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. NIH pub 05-5302. Bethesda, MD: National Cancer Institute; 2006. pp. 181–205. [Google Scholar]
- 318.Roychoudhuri R, Evans H, Robinson D, Møller H. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004 Aug 31;91(5):868–72. doi: 10.1038/sj.bjc.6602084. DOI: http://dx.doi.org/10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010 Jan 5;102(1):220–6. doi: 10.1038/sj.bjc.6605435. DOI: http://dx.doi.org/10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lorigan P, Califano R, Faivre-Finn C, Howell A, Thatcher N. Lung cancer after treatment for breast cancer. Lancet Oncol. 2010 Dec;11(12):1184–92. doi: 10.1016/S1470-2045(10)70056-5. DOI: http://dx.doi.org/10.1016/S1470-2045(10)70056-5. [DOI] [PubMed] [Google Scholar]
- 321.Ng J, Shuryak I, Xu Y, Clifford Chao KS, Brenner DJ, Burri RJ. Predicting the risk of secondary lung malignancies associated with whole-breast radiation therapy. Int J Radiat Oncol Biol Phys. 2012 Jul 15;83(4):1101–6. doi: 10.1016/j.ijrobp.2011.09.052. DOI: http://dx.doi.org/10.1016/j.ijrobp.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Goldhirsch A, Winer EP, Coates AS, et al. Panel members Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013 Sep;24(9):2206–23. doi: 10.1093/annonc/mdt303. DOI: http://dx.doi.org/10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Recht A, Edge SB, Solin LJ, et al. American Society of Clinical Oncology Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001 Mar 1;19(5):1539–69. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 324.Taylor ME, Haffty BG, Ravinovitch R, et al. ACR appropriateness criteria on postmastectomy radiotherapy expert panel on radiation oncology-breast. Int J Radiat Oncol Biol Phys. 2009 Mar 15;73(4):997–1002. doi: 10.1016/j.ijrobp.2008.10.080. DOI: http://dx.doi.org/10.1016/j.ijrobp.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 325.Sautter-Bihl ML, Souchon R, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer II. Postmastectomy radiotherapy, irradiation of regional lymphatics, and treatment of locally advanced disease. Strahlenther Onkol. 2008 Jul;184(7):347–53. doi: 10.1007/s00066-008-1901-8. DOI: http://dx.doi.org/10.1007/s00066-008-1901-8. [DOI] [PubMed] [Google Scholar]
- 326.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014 Jun 21;383(9935):2127–35. doi: 10.1016/S0140-6736(14)60488-8. DOI: http://dx.doi.org/10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lamart S, Stovall M, Simon SL, et al. Radiation dose to the esophagus from breast cancer radiation therapy, 1943–1996: an international population-based study of 414 patients. Int J Radiat Oncol Biol Phys. 2013 Jul 15;86(4):694–701. doi: 10.1016/j.ijrobp.2013.03.014. DOI: http://dx.doi.org/10.1016/j.ijrobp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lucci A, McCall LM, Beitsch PD, et al. American College of Surgeons Oncology Group Surgical complications associated with sentinel lymph node dissection (SNLD) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007 Aug 20;25(24):3657–63. doi: 10.1200/JCO.2006.07.4062. DOI: http://dx.doi.org/10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 329.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008 Nov 10;26(32):5213–9. doi: 10.1200/JCO.2008.16.3725. DOI: http://dx.doi.org/10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Soran A, D’Angelo G, Begovic M, et al. Breast cancer-related lymphedema—what are the significant predictors and how they affect the severity of lymphedema? . Breast J. 2006 Nov-Dec;12(6):536–43. doi: 10.1111/j.1524-4741.2006.00342.x. DOI: http://dx.doi.org/10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 331.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998 Dec 15;83(12 Suppl American):2776–81. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. DOI: http://dx.doi.org/10.1002/(SICI)1097-0142(19981215)83:12B+%3C2776::AIDCNCR25%3E3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 332.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008 Jul 20;26(21):3536–42. doi: 10.1200/JCO.2007.14.4899. DOI: http://dx.doi.org/10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 333.Stamatakos M, Stefanaki C, Kontzoglou K. Lymphedema and breast cancer: a review of the literature. Breast Cancer. 2011 Jul;18(3):174–80. doi: 10.1007/s12282-010-0246-1. DOI: http://dx.doi.org/10.1007/s12282-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 334.Szuba A, Rockson SG. Lymphedema: anatomy, physiology, and pathogenesis. Vasc Med. 1997 Nov;2(4):321–6. doi: 10.1177/1358863X9700200408. [DOI] [PubMed] [Google Scholar]
- 335.Rockson SG. Precipitating factors in lymphedema: myths and realities. Cancer. 1998 Dec 15;83(12 Suppl American):2814–6. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2814::aid-cncr31>3.3.co;2-5. DOI: http://dx.doi.org/10.1002/(SICI)1097-0142(19981215)83:12B+%3C2814::AIDCNCR31%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 336.Armer JM, Stewart BR, Shook RP. 30-month post-breast cancer treatment lymphoedema. J Lymphoedema. 2009 Apr 1;4(1):14–8. [PMC free article] [PubMed] [Google Scholar]
- 337.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010 Sep;43(3):118–27. [PMC free article] [PubMed] [Google Scholar]
- 338.Herd-Smith A, Russo A, Muraca MG, Del Turco MR, Cardona G. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer. 2001 Oct 1;92(7):1783–7. doi: 10.1002/1097-0142(20011001)92:7<1783::aid-cncr1694>3.0.co;2-g. DOI: http://dx.doi.org/10.1002/1097-0142(20011001)92:7<1783::AIDCNCR1694>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 339.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010 Nov 15;116(22):5138–49. doi: 10.1002/cncr.25458. DOI: http://dx.doi.org/10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 340.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM. 2005 May;98(5):343–8. doi: 10.1093/qjmed/hci053. DOI: http://dx.doi.org/10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 341.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001 Sep 15;92(6):1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. DOI: http://dx.doi.org/10.1002/1097-0142(20010915)92:6%3C1368::AIDCNCR1459%3E3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 342.Edwards TL. Prevalence and aetiology of lymphoedema after breast cancer treatment in southern Tasmania. Aust N Z J Surg. 2000 Jun;70(6):412–8. doi: 10.1046/j.1440-1622.2000.01839.x. [DOI] [PubMed] [Google Scholar]
- 343.Deutsch M, Land S, Begovic M, Sharif S. The incidence of arm edema in women with breast cancer randomized on the National Surgical Adjuvant Breast and Bowel Project study B-04 to radical mastectomy versus total mastectomy and radiotherapy versus total mastectomy alone. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1020–4. doi: 10.1016/j.ijrobp.2007.07.2376. DOI: http://dx.doi.org/10.1016/j.ijrobp.2007.07.2376. [DOI] [PubMed] [Google Scholar]
- 344.Brennan MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. J Pain Symptom Manage. 1992 Feb;7(2):110–6. doi: 10.1016/0885-3924(92)90122-x. DOI: http://dx.doi.org/10.1016/0885-3924(92)90122-X. [DOI] [PubMed] [Google Scholar]
- 345.Ferrandez JC, Serin D, Bouges S. [Frequency of lymphedema of the upper limb after treatment of breast cancer. Risk factors. Apropos of 683 cases]. [Article in French] Bull Cancer. 1996 Dec;83(12):989–95. [PubMed] [Google Scholar]
- 346.Kwan ML, Darbinian J, Schmitz KH, et al. Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg. 2010 Nov;145(11):1055–63. doi: 10.1001/archsurg.2010.231. DOI: http://dx.doi.org/10.1001/archsurg.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported armlymphedema and functional impairment after breast cancer treatment—a nationwide study of prevalence and associated factors. Breast. 2010 Dec;19(6):506–15. doi: 10.1016/j.breast.2010.05.015. DOI: http://dx.doi.org/10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 348.Czerniec SA, Ward LC, Refshauge KM, et al. Assessment of breast cancer-related arm lymphedema—a comparison of physical measurement methods and self-report. Cancer Invest. 2010 Jan;28(1):54–62. doi: 10.3109/07357900902918494. DOI: http://dx.doi.org/10.3109/07357900902918494. [DOI] [PubMed] [Google Scholar]
- 349.Clarke D, Martinez A, Cox RS, Goffinet DR. Breast edema following staging axillary node dissection in patients with breast carcinoma treated by radical radiotherapy. Cancer. 1982 Jun 1;49(11):2295–9. doi: 10.1002/1097-0142(19820601)49:11<2295::aid-cncr2820491116>3.0.co;2-g. DOI: http://dx.doi.org/10.1002/1097-0142(19820601)49:11%3C2295::AIDCNCR2820491116%3E3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 350.Rönkä RH, Pamilo MS, von Smitten KA, Leidenius MH. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43(6):551–7. doi: 10.1080/02841860410014867. DOI: http://dx.doi.org/10.1080/02841860410014867. [DOI] [PubMed] [Google Scholar]
- 351.Degnim AC, Miller J, Hoskin TL, et al. A prospective study of breast lymphedema: frequency, symptoms, and quality of life. Breast Cancer Res Treat. 2012 Aug;134(3):915–22. doi: 10.1007/s10549-012-2004-x. DOI: http://dx.doi.org/10.1007/s10549-012-2004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Boughey JC, Hoskin TL, Cheville AL, et al. Risk factors associated with breast lymphedema. Ann Surg Oncol. 2014 Apr;21(4):1202–8. doi: 10.1245/s10434-013-3408-5. DOI: http://dx.doi.org/10.1245/s10434-013-3408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006 Apr;13(4):491–500. doi: 10.1245/ASO.2006.05.013. DOI: http://dx.doi.org/10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 354.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010 Jan-Feb;16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x. DOI: http://dx.doi.org/10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 355.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007 Oct;59(4):464–72. doi: 10.1097/01.sap.0000257149.42922.7e. DOI: http://dx.doi.org/10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 356.Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004 Jan;187(1):69–72. doi: 10.1016/j.amjsurg.2002.12.003. DOI: http://dx.doi.org/10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- 357.Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2003 Apr 1;55(5):1209–15. doi: 10.1016/s0360-3016(02)04273-6. DOI: http://dx.doi.org/10.1016/S0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- 358.Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003 May;29(4):341–50. doi: 10.1053/ejso.2002.1385. DOI: http://dx.doi.org/10.1053/ejso.2002.1385. [DOI] [PubMed] [Google Scholar]
- 359.Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001 Jan 17;93(2):96–111. doi: 10.1093/jnci/93.2.96. DOI: http://dx.doi.org/10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 360.Purushotham AD, Bennett Britton TM, Klevesath MB, Chou P, Agbaje OF, Duffy SW. Lymph node status and breast cancer-related lymphedema. Ann Surg. 2007 Jul;246(1):42–5. doi: 10.1097/01.sla.0000259390.51203.7b. DOI: http://dx.doi.org/10.1097/01.sla.0000259390.51203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Graham PH. Compression prophylaxis may increase the potential for flight-associated lymphoedema after breast cancer treatment. Breast. 2002 Feb;11(1):66–71. doi: 10.1054/brst.2001.0370. DOI: http://dx.doi.org/10.1054/brst.2001.0370. [DOI] [PubMed] [Google Scholar]
- 362.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011 Feb 9;305(6):569–75. doi: 10.1001/jama.2011.90. DOI: http://dx.doi.org/10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Langer S, Guenther JM, Haigh PI, Difronzo LA. Lymphatic mapping improves staging and reduces morbidity in women undergoing total mastectomy for breast carcinoma. Am Surg. 2004 Oct;70(10):881–5. [PubMed] [Google Scholar]
- 364.Armer J, Fu MR, Wainstock JM, Zagar E, Jacobs LK. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology. 2004 Jun;37(2):73–91. [PubMed] [Google Scholar]
- 365.Leidenius M, Leivonen M, Vironen J, von Smitten K. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005 Oct 1;92(1):23–31. doi: 10.1002/jso.20373. DOI: http://dx.doi.org/10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 366.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005 Jul 1;23(19):4312–21. doi: 10.1200/JCO.2005.03.228. DOI: http://dx.doi.org/10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 367.Schunemann E, Jr, Dória MT, Silvestre JB, Gasperin P, Jr, Cavalcanti TC, Budel VM. Prospective study evaluating oncological safety of axillary reverse mapping. Ann Surg Oncol. 2014 Jul;21(7):2197–202. doi: 10.1245/s10434-014-3626-5. DOI: http://dx.doi.org/10.1245/s10434-014-3626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: findings from Medicare claims data. J Clin Oncol. 2001 Mar 1;19(5):1455–61. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 369.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998 Sep 19;352(9132):930–42. DOI: http://dx.doi.org/10.1016/S0140-6736(98)03301-7. [PubMed] [Google Scholar]
- 370.Du XL, Key CR, Osborne C, Mahnken JD, Goodwin JS. Discrepancy between consensus recommendations and actual community use of adjuvant chemotherapy in women with breast cancer. Ann Intern Med. 2003 Jan 21;138(2):90–7. doi: 10.7326/0003-4819-138-2-200301210-00009. DOI: http://dx.doi.org/10.7326/0003-4819-138-2-200301210-00009. Erratum in: Ann Intern Med 2003 Nov 18;139(10):873. DOI: http://dx.doi.org/10.7326/0003-4819-139-10-200311180-00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):775–82. doi: 10.1158/1055-9965.EPI-06-0168. DOI: http://dx.doi.org/10.1158/1055-9965.EPI-06-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in black women and white women. Breast Cancer Res Treat. 2009 Jan;113(2):383–91. doi: 10.1007/s10549-008-9940-5. DOI: http://dx.doi.org/10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]
- 373.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009 Apr 20;27(12):2007–14. doi: 10.1200/JCO.2008.18.3517. DOI: http://dx.doi.org/10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 374.Norman SA, Localio AR, Kallan MJ, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev. 2010 Nov;19(11):2734–46. doi: 10.1158/1055-9965.EPI-09-1245. DOI: http://dx.doi.org/10.1158/1055-9965.EPI-09-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Perre CI, Hoefnagel CA, Kroon BB, Zoetmulder FA, Rutgers EJ. Altered lymphatic drainage after lymphadenectomy or radiotherapy of the axilla in patients with breast cancer. Br J Surg. 1996 Sep;83(9):1258. doi: 10.1046/j.1365-2168.1996.02349.x. DOI: http://dx.doi.org/10.1046/j.1365-2168.1996.02349.x. [DOI] [PubMed] [Google Scholar]
- 376.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006 Jun 20;24(18):2765–72. doi: 10.1200/JCO.2005.03.6749. DOI: http://dx.doi.org/10.1200/JCO.2005.03.6749. Erratum in: J Clin Oncol 2006 Aug 1;24(22):3716. DOI: http://dx.doi.org/10.1200/JCO.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 377.van der Veen P, De Voogdt N, Lievens P, Duquet W, Lamote J, Sacre R. Lymphedema development following breast cancer surgery with full axillary node resection. Lymphology. 2004 Dec;37(4):206–8. [PubMed] [Google Scholar]
- 378.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001 Apr;10(4):287–301. [PubMed] [Google Scholar]
- 379.Kwan ML, Cohn JC, Armer JM, Stewart BR, Cormier JN. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv. 2011 Dec;5(4):320–36. doi: 10.1007/s11764-011-0203-9. DOI: http://dx.doi.org/10.1007/s11764-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 380.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012 Apr 15;118(8 Suppl):2277–87. doi: 10.1002/cncr.27466. DOI: http://dx.doi.org/10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007 Jun 10;25(17):2345–51. doi: 10.1200/JCO.2006.08.6819. DOI: http://dx.doi.org/10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Gonzales JF, Barnard ND, Jenkins DJ, et al. Applying the precautionary principle to nutrition and cancer. J Am Coll Nutr. 2014;33(3):239–46. doi: 10.1080/07315724.2013.866527. DOI: http://dx.doi.org/10.1080/07315724.2013.866527. [DOI] [PubMed] [Google Scholar]
- 383.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012 Jan-Feb;62(1):30–67. doi: 10.3322/caac.20140. DOI: http://dx.doi.org/10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 384.Letai A, Kuter DJ. Cancer, coagulation, and anticoagulation. Oncologist. 1999;4(6):443–9. [PubMed] [Google Scholar]
- 385.Lyman GH, Khorana AA. Cancer, clots and consensus: new understanding of an old problem. J Clin Oncol. 2009 Oct 10;27(29):4821–6. doi: 10.1200/JCO.2009.22.3032. DOI: http://dx.doi.org/10.1200/JCO.2009.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010 Dec;8(3–4):168–72. doi: 10.3121/cmr.2009.866. DOI: http://dx.doi.org/10.3121/cmr.2009.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996 Apr;10(2):449–530. doi: 10.1016/s0889-8588(05)70349-x. DOI: http://dx.doi.org/10.1016/S0889-8588(05)70349-X. [DOI] [PubMed] [Google Scholar]
- 388.Donati MB. Cancer and thrombosis. Haemostasis. 1994 Mar-Apr;24(2):128–31. doi: 10.1159/000217092. DOI: http://dx.doi.org/10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 389.Font C, Carmona-Bayonas A, Fernández-Martinez A, et al. Outpatient management of pulmonary embolism in cancer: data on a prospective cohort of 138 consecutive patients. J Natl Compr Canc Netw. 2014 Mar 1;12(3):365–73. doi: 10.6004/jnccn.2014.0038. [DOI] [PubMed] [Google Scholar]
- 390.Mandalà M, Tondini C. Adjuvant therapy in breast cancer and venous thromboembolism. Thromb Res. 2012 Oct;130(Suppl 1):S66–70. doi: 10.1016/j.thromres.2012.08.280. DOI: http://dx.doi.org/10.1016/j.thromres.2012.08.280. [DOI] [PubMed] [Google Scholar]
- 391.Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol. 2005 May;16(5):696–701. doi: 10.1093/annonc/mdi165. DOI: http://dx.doi.org/10.1093/annonc/mdi165. [DOI] [PubMed] [Google Scholar]
- 392.Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract. 2011 Mar 3;2011:394740. doi: 10.4061/2011/394740. DOI: http://dx.doi.org/10.4061/2011/394740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lip GY, Chin BS, Blann AD. Cancer and the prothombotic state. Lancet Oncol. 2002 Jan;3(1):27–34. doi: 10.1016/s1470-2045(01)00619-2. DOI: http://dx.doi.org/10.1016/S1470-2045(01)00619-2. [DOI] [PubMed] [Google Scholar]
- 394.Prandoni P. Antithrombotic strategies in patients with cancer. Thromb Haemost. 1997 Jul;78(1):141–4. [PubMed] [Google Scholar]
- 395.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003 Jun 17;107(23 Suppl 1):I-9–I-16. doi: 10.1161/01.CIR.0000078469.07362.E6. DOI: http://dx.doi.org/10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 396.Lieberman JS, Borrero J, Urdaneta E, Wright IS. Thrombophlebitis and cancer. JAMA. 1961 Aug 26;177:542–5. doi: 10.1001/jama.1961.03040340006002. DOI: http://dx.doi.org/10.1001/jama.1961.03040340006002. [DOI] [PubMed] [Google Scholar]
- 397.Rahr HB, Sørensen JV. Venous thromboembolism and cancer. Blood Coagul Fibrinolysis. 1992 Aug;3(4):451–60. DOI: http://dx.doi.org/10.1097/00001721-199203040-00012. [PubMed] [Google Scholar]
- 398.Clahsen PC, van de Velde CJ, Julien JP, Floiras JL, Mignolet FY. Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: a European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group Study. J Clin Oncol. 1994 Jun;12(6):1266–71. doi: 10.1200/JCO.1994.12.6.1266. [DOI] [PubMed] [Google Scholar]
- 399.Goodnough LT, Saito H, Manni A, Jones PK, Pearson OH. Increased incidence of thromboembolism in stage IV breast cancer patients treated with a five-drug chemotherapy regimen. A study of 159 patients. Cancer. 1984 Oct 1;54(7):1264–8. doi: 10.1002/1097-0142(19841001)54:7<1264::aid-cncr2820540706>3.0.co;2-r. DOI: http://dx.doi.org/10.1002/1097-0142(19841001)54:7<1264::AIDCNCR2820540706>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 400.Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003 Jun 17;107(23 Suppl 1):I17–21. doi: 10.1161/01.CIR.0000078466.72504.AC. DOI: http://dx.doi.org/10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 401.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999 May;17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 402.Fabian CJ, Kimler BF. Selective estrogen-receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005 Mar 10;23(8):1644–55. doi: 10.1200/JCO.2005.11.005. DOI: http://dx.doi.org/10.1200/JCO.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 403.Cummings FJ, Gray R, Davis TE, et al. Tamoxifen versus placebo: double-blind adjuvant trial in elderly women with stage II breast cancer. NCI Monogr. 1986;(1):119–23. [PubMed] [Google Scholar]
- 404.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007 Jan 1;25(1):70–6. doi: 10.1200/JCO.2006.07.4393. DOI: http://dx.doi.org/10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 405.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007 Dec;61(12):2051–63. doi: 10.1111/j.1742-1241.2007.01587.x. DOI: http://dx.doi.org/10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Baum M, Budzar AU, Cuzick J, et al. ATAC Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002 Jun 22;359(9324):2131–9. doi: 10.1016/s0140-6736(02)09088-8. DOI: http://dx.doi.org/10.1016/S0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 407.Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005 Aug;93(Suppl 1):S23–7. doi: 10.1038/sj.bjc.6602692. DOI: http://dx.doi.org/10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Bona RD. Thrombotic complications of central venous catheters in cancer patients. Semin Thromb Hemost. 1999;25(2):147–55. doi: 10.1055/s-2007-994916. DOI: http://dx.doi.org/10.1055/s-2007-994916. [DOI] [PubMed] [Google Scholar]
- 409.Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol. 2002 Aug 1;20(15):3276–81. doi: 10.1200/JCO.2002.11.135. DOI: http://dx.doi.org/10.1200/JCO.2002.11.135. [DOI] [PubMed] [Google Scholar]
- 410.Simmons AV, Sheppard MA, Cox AF. Deep venous thrombosis after myocardial infarction. Br Heart J. 1973 Jun;35(6):623–5. doi: 10.1136/hrt.35.6.623. DOI: http://dx.doi.org/10.1136/hrt.35.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007 Nov 15;110(10):2339–46. doi: 10.1002/cncr.23062. DOI: http://dx.doi.org/10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 412.Sousou T, Khorana AA. New insights into cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2009 Mar;29(3):316–20. doi: 10.1161/ATVBAHA.108.182196. DOI: http://dx.doi.org/10.1161/ATVBAHA.108.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Wolberg AS, Mackman N. Venous thromboembolism: risk factors, biomarkers, and treatment. Arterioscler Thromb Vasc Biol. 2009 Mar;29(3):296–7. doi: 10.1161/ATVBAHA.109.184580. DOI: http://dx.doi.org/10.1161/ATVBAHA.109.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Colucci M, Balconi G, Lorenzet R, et al. Cultured human endothelial cells generate tissue factor in resonse to endotoxin. J Clin Invest. 1983 Jun;71(6):1893–6. doi: 10.1172/JCI110945. DOI: http://dx.doi.org/10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–7. doi: 10.1073/pnas.83.12.4533. DOI: http://dx.doi.org/10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996 Feb;2(2):209–15. doi: 10.1038/nm0296-209. DOI: http://dx.doi.org/10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 417.Li WW, Li VW, Hutnik M, Chiou AS. Tumor angiogenesis as a target for dietary cancer prevention. J Oncol. 2012;2012:879623. doi: 10.1155/2012/879623. DOI: http://dx.doi.org/10.1155/2012/879623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Belting M, Ahamed J, Ruf W. Signaling of tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler Thromb Vasc Biol. 2005 Aug;25(8):1545–50. doi: 10.1161/01.ATV.0000171155.05809.bf. DOI: http://dx.doi.org/10.1161/01.ATV.0000171155.05809.bf. [DOI] [PubMed] [Google Scholar]
- 419.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996 Jul 1;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. DOI: http://dx.doi.org/10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 420.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992 May 7;326(19):1240–5. doi: 10.1056/NEJM199205073261902. DOI: http://dx.doi.org/10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 421.Lee AY. Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol. 2005 Feb;128(3):291–302. doi: 10.1111/j.1365-2141.2004.05292.x. DOI: http://dx.doi.org/10.1111/j.1365-2141.2004.05292.x. [DOI] [PubMed] [Google Scholar]
- 422.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000 Dec 21;343(25):1846–50. doi: 10.1056/NEJM200012213432504. DOI: http://dx.doi.org/10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 423.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002 Nov 15;100(10):3484–8. doi: 10.1182/blood-2002-01-0108. DOI: http://dx.doi.org/10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 424.Kahn SR, Ducruet T, Lamping DL, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005 May 23;165(10):1173–8. doi: 10.1001/archinte.165.10.1173. DOI: http://dx.doi.org/10.1001/archinte.165.10.1173. [DOI] [PubMed] [Google Scholar]
- 425.Khorana AA. Venous thromboembolism prevention in cancer outpatients. J Natl Compr Canc Netw. 2013 Nov;11(11):1431–8. doi: 10.6004/jnccn.2013.0164. [DOI] [PubMed] [Google Scholar]
- 426.Sousou T, Khorana AA. Cancer patients and awareness of venous thromboembolism. Cancer Invest. 2010 Jan;28(1):44–5. doi: 10.3109/07357900902744544. DOI: http://dx.doi.org/10.3109/07357900902744544. [DOI] [PubMed] [Google Scholar]
- 427.Bedard PL, Siu LL. Tilting the balance of dose modification for oral anticancer drugs? J Clin Oncol. 2014 May 20;32(15):1537–9. doi: 10.1200/JCO.2014.55.2372. DOI: http://dx.doi.org/10.1200/JCO.2014.55.2372. [DOI] [PubMed] [Google Scholar]
- 428.Weingart SN, Brown E, Bach PB, et al. NCCN task force report: oral chemotherapy. J Natl Compr Canc Netw. 2008 Mar;6(Suppl 3):S1–14. [PubMed] [Google Scholar]
- 429.Touchette DR, Shapiro NL. Medication compliance, adherence, and persistence: current status of behaviorial and educational intervention to improve outcomes. J Manag Care Pharm. 2008;14(6 suppl S-d):S2–S10. [Google Scholar]
- 430.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008 Jan-Feb;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. DOI: http://dx.doi.org/10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 431.Gould E, Mitty E. Medication adherence is a partnership, medication compliance is not. Geriatr Nurs. 2010 Jul-Aug;31(4):290–8. doi: 10.1016/j.gerinurse.2010.05.004. DOI: http://dx.doi.org/10.1016/j.gerinurse.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 432.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005 Aug 4;353(3):487–97. doi: 10.1056/NEJMra050100. DOI: http://dx.doi.org/10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 433.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014 Jul 20;32(21):2255–69. doi: 10.1200/JCO.2013.54.2258. DOI: http://dx.doi.org/10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012 Jul;134(2):459–78. doi: 10.1007/s10549-012-2114-5. DOI: http://dx.doi.org/10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003 Feb 15;21(4):602–6. doi: 10.1200/JCO.2003.07.071. DOI: http://dx.doi.org/10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 436.Geiger AM, Thwin SS, Lash TL, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007 Mar 1;109(5):966–74. doi: 10.1002/cncr.22472. DOI: http://dx.doi.org/10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 437.Yood MU, Owusu C, Buist DS, et al. Mortality impact on less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008 Jan;206(1):66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. DOI: http://dx.doi.org/10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 438.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007 Mar 1;109(5):832–9. doi: 10.1002/cncr.22485. DOI: http://dx.doi.org/10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 439.Ruddy KJ. Partridge. Adherence with adjuvant hormonal therapy for breast cancer. Ann Oncol. 2009 Mar;20(3):401–2. doi: 10.1093/annonc/mdp039. DOI: http://dx.doi.org/10.1093/annonc/mdp039. [DOI] [PubMed] [Google Scholar]
- 440.Gray RG, Rea D, Handley K, et al. aTTOM Collaborative Group aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer [ASCP Annual Meeting Abstracts] J Clin Oncol. 2013;31(15_suppl May 20):5. [Google Scholar]
- 441.Litton J, Buzdar A, Mac Gregor MC, Gonzalez-Angulo A, Hortobagyi G. Tamoxifen therapy for patients with breast cancer. Lancet. 2013 Jun 15;381(9883):2077–8. doi: 10.1016/S0140-6736(13)61236-2. DOI: http://dx.doi.org/10.1016/S0140-6736(13)61236-2. [DOI] [PubMed] [Google Scholar]
- 442.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Lonati V, Barni S. Five or more years of adjuvant endocrine therapy in breast cancer: a meta-analysis of published randomised trials. Breast Cancer Res Treat. 2013 Jul;140(2):233–40. doi: 10.1007/s10549-013-2629-4. DOI: http://dx.doi.org/10.1007/s10549-013-2629-4. [DOI] [PubMed] [Google Scholar]
- 443.Ewer MS, Glück S. A woman’s heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009 May 1;115(9):1813–26. doi: 10.1002/cncr.24219. DOI: http://dx.doi.org/10.1002/cncr.24219. [DOI] [PubMed] [Google Scholar]
- 444.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013 Apr 16;108(7):1515–24. doi: 10.1038/bjc.2013.116. DOI: http://dx.doi.org/10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012 Nov;8(6):e149–57. doi: 10.1200/JOP.2012.000543. DOI: http://dx.doi.org/10.1200/JOP.2012.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Castel LD, Hartmann KE, Mayer IA, et al. Time course of arthralgia among women initiating aromatase inhibitor therapy and postmenopausal comparison group in a prospective cohort. Cancer. 2013 Jul 1;119(13):2375–82. doi: 10.1002/cncr.28016. DOI: http://dx.doi.org/10.1002/cncr.28016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012 Mar 20;30(9):936–42. doi: 10.1200/JCO.2011.38.0261. DOI: http://dx.doi.org/10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Partridge AH, LaFountain A, Meyer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008 Feb 1;26(4):556–62. doi: 10.1200/JCO.2007.11.5451. DOI: http://dx.doi.org/10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 449.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat. 2010 Feb;120(1):127–34. doi: 10.1007/s10549-009-0692-7. DOI: http://dx.doi.org/10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 450.Liu Y, Pérez M, Aft RL, et al. Accuracy of perceived risk of recurrence among patients with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2010 Mar;19(3):675–80. doi: 10.1158/1055-9965.EPI-09-1051. DOI: http://dx.doi.org/10.1158/1055-9965.EPI-09-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ziller V, Kyvernitakis I, Knöll D, Storch A, Hars O, Hadji P. Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment—the COMPAS study. BMC Cancer. 2013 Sep 4;13:407. doi: 10.1186/1471-2407-13-407. DOI: http://dx.doi.org/10.1186/1471-2407-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Hadji P, Blettner M, Harbeck N, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013 Jun;24(6):1505–12. doi: 10.1093/annonc/mds653. DOI: http://dx.doi.org/10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 453.Neven P, Markopoulos C, Tanner M, et al. The impact of educational materials on compliance and persistence rates with adjuvant aromatase inhibitor treatment: first-year results from the Compliance of ARomatase Inhibitors AssessmenT In Daily practice through Educational approach (CARIATIDE) study. Breast. 2014 Aug;23(4):393–9. doi: 10.1016/j.breast.2014.02.009. DOI: http://dx.doi.org/10.1016/j.breast.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 454.What breast cancer survivors can do [Internet] Washington, DC: American Institute for Cancer Research; p. c2014. [cited 2014 Nov 3]. Available from: www.aicr.org/learn-more-about-cancer/infographics/what-breast-cancer-survivors-cando.html. [Google Scholar]
- 455.Tuso PJ. Behavior medicine specialist. Perm J. 2014 Fall;18(4):52–7. doi: 10.7812/TPP/14-035. DOI: http://dx.doi.org/10.7812/TPP/14-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014 Oct;25(10):1901–14. doi: 10.1093/annonc/mdu042. DOI: http://dx.doi.org/10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res (Phila) 2012 Apr;5(4):515–21. doi: 10.1158/1940-6207.CAPR-12-0091. DOI: http://dx.doi.org/10.1158/1940-6207.CAPR-12-0091. [DOI] [PubMed] [Google Scholar]
- 458.Senie RT, Rosen PP, Rhodes P, Lesser ML, Kinne DW. Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med. 1992 Jan 1;116(1):26–32. doi: 10.7326/0003-4819-116-1-26. DOI: http://dx.doi.org/10.7326/0003-4819-116-1-26. [DOI] [PubMed] [Google Scholar]
- 459.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012 Dec 1;118(23):5937–46. doi: 10.1002/cncr.27527. DOI: http://dx.doi.org/10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002 Feb 15;20(4):1128–43. doi: 10.1200/JCO.2002.20.4.1128. DOI: http://dx.doi.org/10.1200/JCO.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 461.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005 Dec;14(12):2881–8. doi: 10.1158/1055-9965.EPI-05-0185. DOI: http://dx.doi.org/10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Schwartz AL. Exercise and weight gain in breast cancer patients receiving chemotherapy. Cancer Pract. 2000 Sep-Oct;8(5):231–7. doi: 10.1046/j.1523-5394.2000.85007.x. DOI: http://dx.doi.org/10.1046/j.1523-5394.2000.85007.x. [DOI] [PubMed] [Google Scholar]
- 463.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005 May 25;293(20):2479–86. doi: 10.1001/jama.293.20.2479. DOI: http://dx.doi.org/10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 464.Sclavo M. [Cardiovascular risk factors and prevention in women: similarities and differences]. [Article in Italian] Ital Heart J Suppl. 2001 Feb;2(2):125–41. [PubMed] [Google Scholar]
- 465.Nelson DE, Jarman DW, Rehm J, et al. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013 Apr;103(4):641–8. doi: 10.2105/AJPH.2012.301199. DOI: http://dx.doi.org/10.2105/AJPH.2012.301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013 Apr 17;105(8):515–25. doi: 10.1093/jnci/djt023. DOI: http://dx.doi.org/10.1093/jnci/djt023. [DOI] [PubMed] [Google Scholar]
- 467.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010 May;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. DOI: http://dx.doi.org/10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 468.Segal R, Pond GR, Vallis M, et al. Randomized trial of a lifestyle intervention for women with early-stage breast cancer (BC) receiving adjuvant hormone therapy: initial results [Abstract] J Clin Oncol. 2011 May;29(15 suppl; abstr 512) [Google Scholar]
- 469.World Cancer Research Fund International [Internet] London, England: World Cancer Research Fund International; p. c2014. [cited 2015 Feb 3]. Available from: www.wcrf.org/. [Google Scholar]
- 470.Diet, nutrition, physical activity and breast cancer survivors [Internet] London, England: World Cancer Research Fund International; 2014. [cited 2014 Nov 4]. Available from: www.aicr.org/assets/docs/pdf/reports/2014-breast-cancer-survivorship-cup.pdf. [Google Scholar]
- 471.Tuso P. Physician update: total health. Perm J. 2014 Spring;18(2):58–63. doi: 10.7812/TPP/13-120. DOI: http://dx.doi.org/10.7812/TPP/13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. DOI: http://dx.doi.org/10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Tuso P, Stoll SR, Li WW. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015 Winter;19(1):62–7. doi: 10.7812/TPP/14-036. DOI: http://dx.doi.org/10.7812/TPP/14-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tuso P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J. 2014 Summer;18(3):88–93. doi: 10.7812/TPP/14-002. DOI: http://dx.doi.org/10.7812/TPP/14-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012 Sep;9(9):498–509. doi: 10.1038/nrclinonc.2012.120. DOI: http://dx.doi.org/10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 476.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014 Jul 29;349:g4490. doi: 10.1136/bmj.g4490. DOI: http://dx.doi.org/10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997 Apr 17;336(16):1117–24. doi: 10.1056/NEJM199704173361601. DOI: http://dx.doi.org/10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 478.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013 Aug 28;347:f5001. doi: 10.1136/bmj.f5001. DOI: http://dx.doi.org/10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007 Sep;21(9):717–28. doi: 10.1038/sj.jhh.1002212. DOI: http://dx.doi.org/10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 480.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006 Jan 28;367(9507):320–6. doi: 10.1016/S0140-6736(06)68069-0. DOI: http://dx.doi.org/10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 481.Aune D, Chan DS, Vieira AR, et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012 Jul;134(2):479–93. doi: 10.1007/s10549-012-2118-1. DOI: http://dx.doi.org/10.1007/s10549-012-2118-1. [DOI] [PubMed] [Google Scholar]
- 482.Aune D, Chan DS, Greenwood DC, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012 Jun;23(6):1394–402. doi: 10.1093/annonc/mdr589. DOI: http://dx.doi.org/10.1093/annonc/mdr589. [DOI] [PubMed] [Google Scholar]
- 483.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006 Dec 20;98(24):1767–76. doi: 10.1093/jnci/djj494. DOI: http://dx.doi.org/10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 484.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004 Nov 3;96(21):1577–84. doi: 10.1093/jnci/djh296. DOI: http://dx.doi.org/10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 485.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009 Nov-Dec;2(5):270–8. doi: 10.4161/oxim.2.5.9498. DOI: http://dx.doi.org/10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. DOI: http://dx.doi.org/10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 487.Athar M, Back JH, Tang X, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007 Nov 1;224(3):274–83. doi: 10.1016/j.taap.2006.12.025. DOI: http://dx.doi.org/10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005 Jan;81(1 Suppl):292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 489.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009 Sep;58(9):537–52. doi: 10.1007/s00011-009-0037-3. DOI: http://dx.doi.org/10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 490.Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000 May;71(5):1062–76. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 491.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. DOI: http://dx.doi.org/10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 492.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008 Feb;17(2):379–86. doi: 10.1158/1055-9965.EPI-07-0771. DOI: http://dx.doi.org/10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 493.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008 Aug 20;26(24):3958–64. doi: 10.1200/JCO.2007.15.9822. DOI: http://dx.doi.org/10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009 May 13;301(18):1883–91. doi: 10.1001/jama.2009.643. DOI: http://dx.doi.org/10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Chen X, Lu W, Zheng W, et al. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila) 2011 Sep;4(9):1409–18. doi: 10.1158/1940-6207.CAPR-10-0355. DOI: http://dx.doi.org/10.1158/1940-6207.CAPR-10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD005001. doi: 10.1002/14651858.CD005001.pub2. DOI: http://dx.doi.org/10.1002/14651858.CD005001.pub2. [DOI] [PubMed] [Google Scholar]
- 497.Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res. 2009;11(5):106. doi: 10.1186/bcr2351. DOI: http://dx.doi.org/10.1186/bcr2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Fann JR, Thomas-Rich AM, Katon WJ, et al. Major depression after breast cancer: a review of epidemiology and treatment. Gen Hosp Psychiatry. 2008 Mar-Apr;30(2):112–26. doi: 10.1016/j.genhosppsych.2007.10.008. DOI: http://dx.doi.org/10.1016/j.genhosppsych.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 499.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011 Feb;12(2):160–74. doi: 10.1016/S1470-2045(11)70002-X. DOI: http://dx.doi.org/10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 500.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. DOI: http://dx.doi.org/10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 501.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008 Jul;110(1):9–17. doi: 10.1007/s10549-007-9706-5. DOI: http://dx.doi.org/10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 502.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007 Sep 8;370(9590):851–8. doi: 10.1016/S0140-6736(07)61415-9. DOI: http://dx.doi.org/10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 503.Tuso P. Treatment progress indicator: application of a new assessment tool to objectively monitor the therapeutic progress of patients with depression, anxiety, or behavioral health impairment. Perm J. 2014 Summer;18(3):55–9. doi: 10.7812/TPP/13-091. DOI: http://dx.doi.org/10.7812/TPP/13-091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Sharpe M, Strong V, Allen K, et al. Major depression in outpatients attending a regional cancer centre: screening and unmet treatment needs. Br J Cancer. 2004 Jan 26;90(2):314–20. doi: 10.1038/sj.bjc.6601578. DOI: http://dx.doi.org/10.1038/sj.bjc.6601578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009 Jan;18(1):14–22. doi: 10.1002/pon.1368. DOI: http://dx.doi.org/10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Walker J, Sharpe M. Depression care for people with cancer: a collaborative care intervention. Gen Hosp Psychiatry. 2009 Sep-Oct;31(5):436–41. doi: 10.1016/j.genhosppsych.2009.05.010. DOI: http://dx.doi.org/10.1016/j.genhosppsych.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 507.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008 Jul 5;372(9632):40–8. doi: 10.1016/S0140-6736(08)60991-5. DOI: http://dx.doi.org/10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 508.Caplette-Gingras A, Savard J. Depression in women with metastatic breast cancer: a review of the literature. Palliat Support Care. 2008 Dec;6(4):377–87. doi: 10.1017/S1478951508000606. DOI: http://dx.doi.org/10.1017/S1478951508000606. [DOI] [PubMed] [Google Scholar]
- 509.Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007 Aug;104(2):145–52. doi: 10.1007/s10549-006-9410-x. DOI: http://dx.doi.org/10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 510.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012 Jul 3;126(1):126–32. doi: 10.1161/CIRCULATIONAHA.111.087213. DOI: http://dx.doi.org/10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Hortobagyi GN, American Society of Clinical Oncology A shortage of oncologists? The American Society of Clinical Oncology workforce study. J Clin Oncol. 2007 Apr 20;25(12):1468–9. doi: 10.1200/JCO.2007.10.9397. DOI: http://dx.doi.org/10.1200/JCO.2007.10.9397. [DOI] [PubMed] [Google Scholar]