Abstract
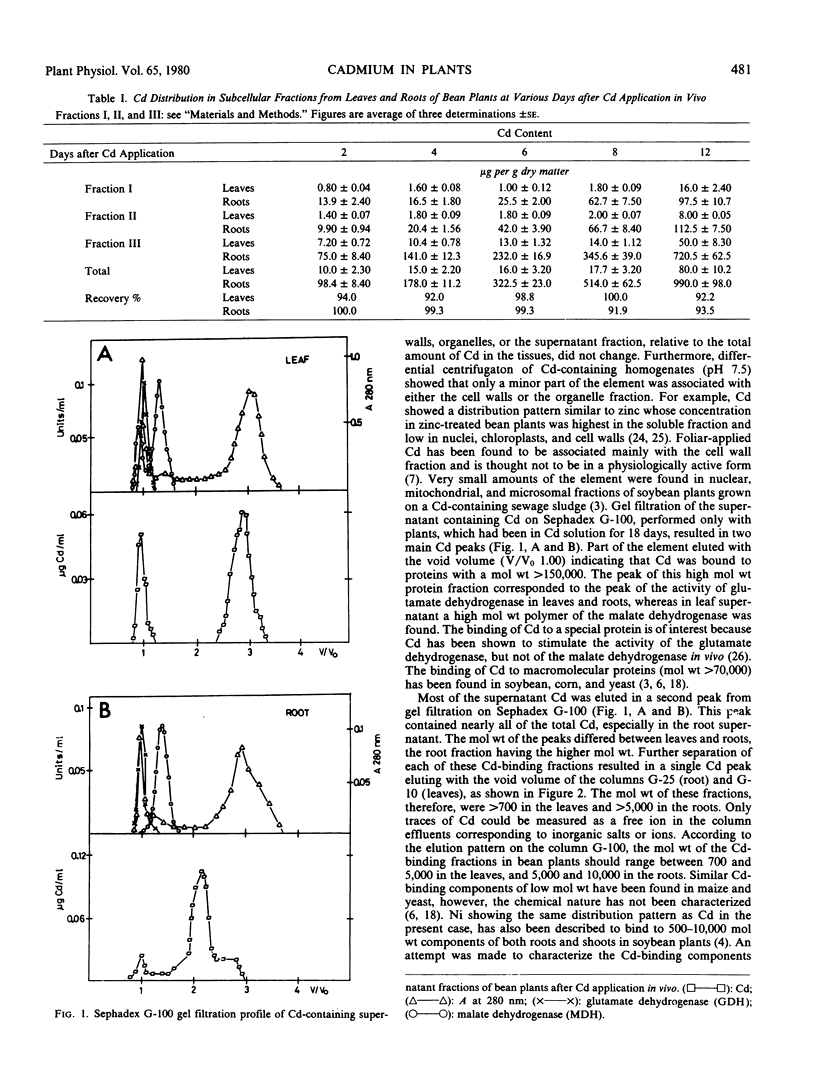
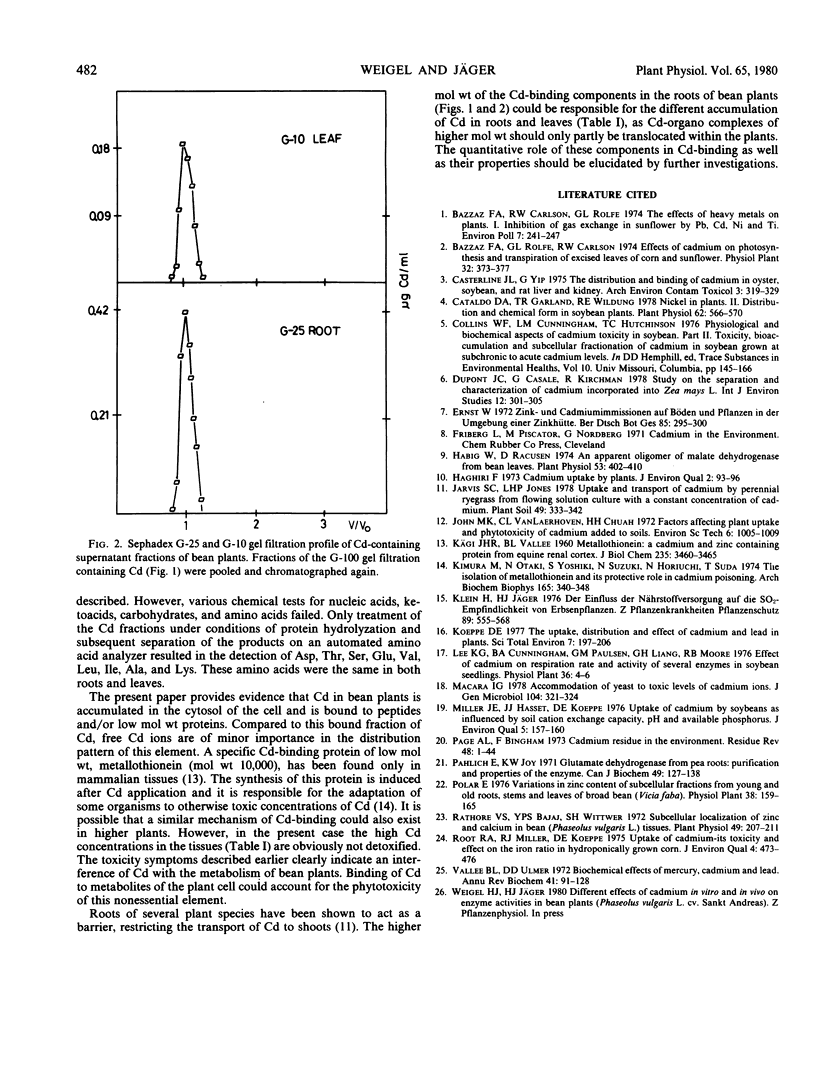
The subcellular distribution and chemical form of Cd in bean plants grown in nutrient solutions containing Cd were investigated. Cd was accumulated mainly in roots and to a minor extent in leaves. Subcellular fractionation of Cd-containing tissues (pH 7.5) showed that more than 70% of the element was localized in the cytoplasmic fraction in roots as well as in leaves. Little Cd (8 to 14%) was bound either to the cell wall fraction or to the organelles. Gel filtration of the soluble fraction showed Cd to be associated mainly with 5,000 to 10,000 molecular weight components in roots, and 700 to 5,000 molecular weight components in leaves. Small amounts of Cd were found in the high molecular weight proteins (molecular weight 150,000). Only traces of Cd could be detected as a free ion. Chemical characterization of the low molecular weight components resulted in the identification of nine amino acids which were identical in roots and leaves. Cd in bean plants is assumed to be bound to peptides and/or proteins of low molecular weight.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casterline J. L., Jr, Yip G. The distribution and binding of cadmium in oyster, soybean, and rat liver and kidney. Arch Environ Contam Toxicol. 1975;3(3):319–329. doi: 10.1007/BF02220744. [DOI] [PubMed] [Google Scholar]
- Cataldo D. A., Garland T. R., Wildung R. E. Nickel in Plants: II. Distribution and Chemical Form in Soybean Plants. Plant Physiol. 1978 Oct;62(4):566–570. doi: 10.1104/pp.62.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W., Racusen D. An apparent oligomer of malate dehydrogenase from bean leaves. Plant Physiol. 1974 Mar;53(3):402–410. doi: 10.1104/pp.53.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- Kimura M., Otaki N., Yoshiki S., Suzuki M., Horiuchi N. The isolation of metallothionein and its protective role in cadmium poisoning. Arch Biochem Biophys. 1974 Nov;165(1):340–348. doi: 10.1016/0003-9861(74)90172-6. [DOI] [PubMed] [Google Scholar]
- Page A. L., Bingham F. T. Cadmium residues in the environment. Residue Rev. 1973;48(0):1–44. doi: 10.1007/978-1-4615-8498-8_1. [DOI] [PubMed] [Google Scholar]
- Pahlich E., Joy K. W. Glutamate dehydrogenase from pea roots: purification and properties of the enzyme. Can J Biochem. 1971 Jan;49(1):127–138. doi: 10.1139/o71-018. [DOI] [PubMed] [Google Scholar]
- Rathore V. S., Bajaj Y. P., Wittwer S. H. Subcellular Localization of Zinc and Calcium in Bean (Phaseolus vulgaris L.) Tissues. Plant Physiol. 1972 Feb;49(2):207–211. doi: 10.1104/pp.49.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Ulmer D. D. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41(10):91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]



