Abstract
Gliomas are amongst the most insidious and destructive types of brain cancer and are associated with a poor prognosis, frequent recurrences, and extremely high lethality despite combination treatment of surgery, radiotherapy, and chemotherapy. The existence of the blood–brain barrier (BBB) restricts the delivery of therapeutic molecules into the brain and offers the clinical efficacy of many pharmaceuticals that have been demonstrated to be effective for other kinds of tumors. This challenge emphasizes the need to be able to deliver drugs effectively across the BBB to reach the brain parenchyma. Enhancement of the permeability of the BBB and being able to transport drugs across it has been shown to be a promising strategy to improve drug absorption and treatment efficacy. This review highlights the innovative technologies that have been introduced to enhance the permeability of the BBB and to obtain an optimal distribution and concentration of drugs in the brain to treat gliomas, such as nanotechniques, hyperthermia techniques, receptor-mediated transport, cell-penetrating peptides, and cell-mediated delivery.
Keywords: glioma; blood–brain barrier; drug delivery, nanotechnology; hyperthermia; receptor-mediated transport; cell-penetrating peptides; cell-mediated delivery
Introduction
Gliomas are the most frequent tumors occurring in the central nervous system (CNS) and are responsible for more than 32% of all primary brain and CNS tumors and 80% of all malignancies of the brain and CNS.1,2 Despite decades of advancement in both the understanding of their molecular pathogenesis and the clinical protocols available, malignant gliomas remain almost always fatal.3 None of the current state-of-the-art treatments for malignant glioma could be regarded as effective. The current 5-year and 10-year survival rates for patients with malignant glioma are 4.5% and 2.7%, respectively, and the median survival is only about 14.6 months, even after combination treatments of cytoreductive surgical resection, radiotherapy, and adjuvant oral chemotherapy with temozolomide.4,5
Compared with other types of tumors, gliomas are more challenging to treat because of the shield of the blood–brain barrier (BBB).6 All the treatments available have the problem of not being able to penetrate the BBB to reach the tumor mass. New opportunities for efficient drug delivery across the BBB are urgently needed. Considerable research indicates that enhancement of the permeability of the BBB is needed to improve therapeutic outcomes. In the present review, we outline the latest innovative strategies for enhancing the permeability of the BBB and transporting therapeutic agents across it for the treatment of glioma.
Glioma
Although accounting for less than 2% of adult cancers, gliomas are the most common form of malignant primary brain tumor in adults.7 Despite their rarity, gliomas are notorious for being the leading cause of cancer-related death in men aged 20–39 years and the second leading cause of cancer-related death in children.8 Glioblastoma multiforme (World Health Organization grade IV glioma), the most prevalent primary malignant manifestation of glioma, accounts for approximate three quarters of all gliomas9 and presents an almost unparalleled clinical challenge that culminates in death shortly after diagnosis.10–12
A number of factors have been identified as limiting the successful treatment of glioma, including a hypoxia environment,13 the extreme phenotypic and genotypic heterogeneity of the disease,14 aberrant signaling pathways,15 and the existence of glioma stem cells.16 Another factor that has often been overlooked is the impaired delivery of drugs to their intracellular targets because of the existence of the BBB, which limits the efficacy of many systemically administered chemotherapeutics.6
The BBB in glioma
The BBB is composed primarily of specialized endothelial cells characterized by so-called tight junctions that maintain homeostasis between the blood circulation and the CNS17 such that essentially 100% of large-molecule pharmaceutics and more than 98% of small molecules cannot cross this barrier.18,19 With its extremely selective permeability, the BBB acts as a fortress to protect the vulnerable parenchyma from insults by potentially detrimental foreign material. At the same time, the BBB presents an insurmountable obstacle to potentially effective therapeutic agents in patients with CNS disease.20 This is the reason why many chemotherapeutic strategies cannot be used to treat glioma, despite being effective in other malignancies.21,22
The integrity of the BBB is notably heterogeneous during the development of glioma even within a single tumor tissue,23 giving a profound challenge for drug delivery across this barrier. Generally, the gradual progression of glioma leads to abnormal structural features in endothelial cells,24 resulting in enhanced permeability of the BBB when compared with normal brain tissue.25 However, the BBB in peripheral glioma remains essentially intact.26,27 If surgical resection is used to remove all visible tumor, the BBB near the tumor core is destroyed,27,28 but is still intact in the infiltrative pool, a region that may be several centimeters away from the visible tumor due to migration of tumor cells that have escaped into the surrounding brain parenchyma.29 This is thought to be the reason for the highly refractory nature of glioblastoma multiforme within a 2–3 cm margin of the surgical resection cavity.30
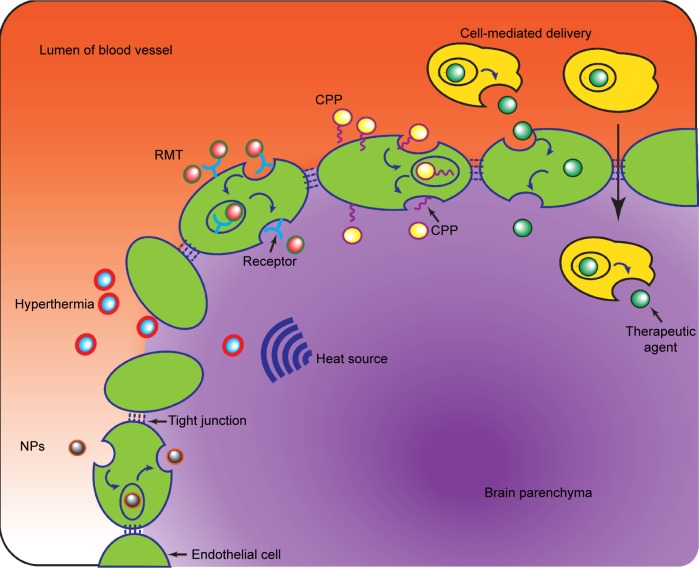
The perplexity of the diffuse, limited-permeable BBB has renewed interest of pursuing effective delivery concepts and strategies to increase the delivery of drugs cross the BBB to target tumor mass. Generally, the strategies potentially available can be classified into three groups, ie, circumventing or bypassing the BBB, widespread opening of the BBB (paracellular approach), and delivery across the BBB (transcellular approach).19 The first approach includes direct intratumoral injection,31 implantation of drug-releasing polymers,32 convention-enhanced delivery,33 and intranasal delivery.34 The second approach involves enhancing the permeability of the BBB with hypertonic mannitol, alkylglycerols, or a bradykinin analog. This approach is associated with better therapeutic outcomes but may cause permanent damage due to lack of specific targeting.35 The third approach, ie, the transcellular strategy, which can enhance the permeability of the BBB in a less invasive manner, represents a promising strategy for improving therapeutic efficacy. In recent years, several innovative technologies have succeeded in obtaining an optimal drug distribution and concentration in the CNS to treat glioma, as outlined in Figure 1 and discussed further below.
Figure 1.
Representative schematic of several strategies to enhance the permeability of the blood–brain barrier for the delivery of therapeutic agents to the brain parenchyma.
Abbreviations: CPP, cell-penetrating peptides; NPs, nanoparticles; RMT, receptor-mediated transport.
Nanotechnology
A wide variety of nanoparticles has been created to enable delivery of therapeutic drugs across the BBB.36,37 Nanoparticles are capable of overcoming the obstacle of the BBB because they can be administered intracerebrally and release their payload in a sustained manner.38 When administered systemically, nanoparticles can protect the loaded drugs from degradation.39 Small therapeutic molecular agents that are normally poorly distributed can be incorporated into nanoparticles via a variety of chemical methods, including encapsulation, adsorption, and covalent linkage, while macromolecules can be attached to the surface of nanoparticles to improve targeting.40,41 For example, researchers have encapsulated doxorubicin in nanoparticles using poly(butyl) cyanoacrylate and then coated Tween-80 nanoparticles to ensure apolipoprotein E binding to the nanoparticles.35 These coating-masked nanoparticles were observed to be endocytosed by the endothelium of the BBB, allowing entry of nanoparticles and drugs into endothelial cells and being effluxed into the brain parenchyma at a higher level. Malatesta et al42 used chitosan nanoparticles to deliver a hypometabolizing synthetic opioid, D-Ala2- D-Leu5-enkephalin, and reported a decrease in both the amount of transcription factors and the proliferation rate, suggesting that these nanoparticles were able to traverse the BBB and release their payload in the brain parenchyma.
Recently, biodegradable polymer-based nanoparticles and gold nanoparticles have been shown to be highly attractive vehicles for carrying drugs across the BBB to treat glioma.36 In particular, gold nanoparticles are thought to be a worthwhile candidate with a better ability to permeate the BBB via an endocytic pathway. For example, Gromnicova et al43 found that 4 nm glucose-coated gold nanoparticles across primary human brain endothelium at a transfer speed at least three times faster than that of non-brain endothelium. Another interesting example was reported by Jensen et al44 who designed an RNA interference-based gold nanoparticle platform, termed spherical nucleic acid, which was able to penetrate the BBB and blood−brain tumor barrier in vivo after systemic injection. These findings suggest that gold nanoparticles have the necessary properties to be efficient and selective carriers of therapeutic agents across the BBB.
Undoubtedly, the nanoparticle-based delivery systems have just begun their promising steps to improve specific and efficient intracerebral delivery of drugs for the treatment of glioma.45 These nanoparticles are better able to penetrate the BBB and enable efficient uptake of therapeutics by the tumor parenchyma.46 Nowadays, nanoparticles are frequently integrated with other techniques (such as hyperthermia or a molecular “Trojan horse”, discussed later in this review) to further increase their cellular transduction.47
Hyperthermia techniques
The limitation of inadequate drug delivery to tumor tissue and the adjacent parenchyma has led to development of the novel concept of creating a transient opening or disruption of the integrity of the BBB by breaking down the tight junctions to promote focused pharmacological delivery, and among these is hyperthermia.48
Hyperthermia is a therapeutic procedure using increased temperature to change the functionality of cellular structures in body tissues.49 Its activity is based on the fact that elevated temperatures (41°C–43°C, or even lower) can synergistically and selectively kill cancer cells, which are more sensitive to a sudden increase in temperature than adjacent normal cells.50 The underlying molecular mechanisms of hyperthermia are not clearly understood. The main mechanism probably lies in the irreversible protein denaturation, DNA damage, and ultimate apoptosis that is triggered by the increase in temperature.51 Also triggered by hyperthermia is a transient and highly localized, site-specific disruption of the BBB.52 Hyperthermia research during the last decade has focused on drug delivery as an effective and feasible therapy for treatment of malignant glioma using various heat sources tools, such as focused ultrasound, radiofrequency, microwaves, laser, and magnetic energy.
Focused ultrasound
Focused ultrasound (FUS) can concentrate acoustic energy into a focal spot to produce selective disruption and increased permeability of the BBB.53,54 Given that FUS is compatible with currently available drugs, it is anticipated to be a benign procedure that can be easily repeated to match chemoschedule.55 In recent years, commercially available contrast agents, ie, microbubbles (MBs),56 have been incorporated into FUS approaches to confine the FUS effects to the blood vessel walls with minimal damage to surrounding brain tissue. In MB-facilitated FUS, circulating MBs interact closely with the low-intensity FUS, resulting in transient disassembly of tight junctions and enhanced permeability of the BBB.57,58 In one animal study,52 MB-facilitated FUS temporary disrupted the BBB transcranially and enhanced the penetration of bis-chloroethylnitrosourea up to 202% in rat xenografts without causing hemorrhage. Successful MB-FUS-induced disruption of BBB was applied to a variety of therapeutic molecules, from doxorubicin,59 temozolomide,60 and methotrexate61 to macroagents such as magnetic nanoparticles (MNPs),62 small interfering (si)RNA,63 and even stem cells.64 These studies provide preclinical evidence that FUS can enhance the permeability of the BBB and increase local concentrations of antitumor drugs to further impede tumor progression.
Electrohyperthermia
Electrohyperthermia is an advanced hyperthermia technique that is considered to be selective because of the higher conductivity and higher permittivity of the extracellular matrix in the tumor tissue.65 Hyperthermia from electromagnetic waves generated by radiofrequency or microwaves has been reported to increase the permeability of the BBB in vivo.66
Nowadays, radiofrequency is frequently used in oncology as a treatment for glioma, either alone or in combination with chemotherapy and/or radiotherapy. Gong et al67 reported a higher uptake of adriamycin in isotransplanted C6 glioma rats treated with radiofrequency-induced hyperthermia. Furthermore, other investigators have reported that a high concentration of drug can be achieved using local radiofrequency hyperthermia chemotherapy.68
Laser
Kiessling et al69 were the first to introduce the notion that laser light could be used to induce a localized disruption of the BBB by focally applying a Nd:YAG laser pulse. This has now been demonstrated to be a minimally invasive approach for the treatment of glioma. Well-defined, laser-induced membranous defects in the capillary endothelium lead to transient disruption of the BBB and allow molecules to permeate into the brain parenchyma.70 During the last decade, laser-induced hyperthermia has been used as a component of photodynamic therapy, which consists of treatment with a tumor-localizing photosensitizer and subsequent laser light activation.71 Among the four photosensitizers currently available that have received approval for use in photodynamic therapy, the prodrug 5-aminolevulinic acid (5-ALA) appears to be particularly appealing for the treatment of glioma due to its characteristics of tumor specificity and rapid systemic clearance. An 5-ALA–laser combination was demonstrated to disrupt the BBB rapidly via formation and enlargement of endothelial gaps following treatment and to leave a time window that is significantly longer than that with FUS. An exciting application of laser in glioma treatment is the combination of laser and nanotechnologies for target drug delivery. Choi et al72 have reported a study in which they delivered large molecules using a near-infrared ultrashort pulsed laser, and found that a variety of peripherally administered molecules, including nanoparticles and, even more interesting, genetically engineered viruses, could be induced to penetrate the BBB.
Magnetic hyperthermia
Magnetic hyperthermia is a strategy used to attract a drug to the region of a tumor by application of drug-free or drug-loaded MNPs subjected to an external alternating magnetic field (AMF).73 The locally administered MNPs interact with the applied external AMF and increase retention of MNPs at the tumor site.74 The externally imposed AMF would allow both target-specific intravascular accumulation and facilitated penetration of MNPs across the BBB.75 Another distinct advantage of magnetic hyperthermia relates to the stability of MNPs over a long period of time, which allows multiple treatments without reinjection.
Magnetic hyperthermia has been evaluated for its ability to facilitate the delivery of MNPs across the BBB in animal models. Using fluorescent MNPs, Kong et al76 demonstrated that an external AMF increased the efficacy of delivery of MNPs across the normal BBB. Chertok et al77 reported that, although MNPs can be delivered passively to the glioma vasculature without an AMF, the addition of an AMF allowed prolonged retention of the MNPs within the glioma lesion, resulting in a fivefold increase in exposure of the tumor to MNPs when compared with animals treated without an AMF. Similar results were reported for MNPs loaded with 3′-azido-3′-deoxythymidine-5′-triphosphate,78 paclitaxel,79 and/or Tat peptide.80,81
It is notable that some researchers have combined two different hyperthermia techniques to increase drug delivery to gliomas. For example, Chu et al82 used FUS followed by an AMF to make the BBB transiently permeable and to enhance the localization of bis-chloroethylnitrosourea immobilized on MNPs. Combination of these two techniques improved the delivery of bis-chloroethylnitrosourea by 26-fold compared with the MNPs alone.
Realistically, hyperthermia has the potential to be an effective and easily feasible adjunct to established therapies used for glioma. On the other hand, it must be emphasized that application of hyperthermia for drug delivery in the treatment of infiltrative glioma is still in its infancy. Serious side effects, including necrosis and elevated intracranial pressure, have been described in human trials. The efficacy and mechanism of action are still not clearly understood. Further investigations will be necessary to achieve translational elucidation.
Receptor-mediated transport
Taking advantage of endogenous influx BBB transporters for the delivery of target agents from the circulation into the brain parenchyma is thought to be a good strategy for the treatment of glioma, especially when using hydrophilic molecules or macromolecules that would otherwise have minimal ability to cross the BBB.83 This process involves ferrying molecules across the BBB via substrate–transporter interactions, among which the two major mechanisms are carrier-mediated transport (CMT) and receptor-mediated transport (RMT).84
CMT provides a facilitated mechanism for certain small molecules, nutrients, and hormones to passively cross the BBB with the aid of various specific substrate transporters located on the BBB.85 Although some water-soluble molecules, such as catecholamines and L-DOPA, were reported to penetrate the BBB at pharmacologically significant rates via CMT, no successful application of CMT for the treatment of glioma are available to date.
Whereas CMT can transport small molecules from the blood to the brain, RMT systems are expressed on the BBB and handle the physiological transport of large endogenous molecules necessary for brain function.86 During the process of RMT, macromolecules are able to move across the membrane of an endothelial cell into the brain, owing to expression of several peptide-specific receptors on the BBB, among which the neonatal Fc receptor,87 low-density lipoprotein receptor-related protein receptor, transferrin receptor,88 lactoferrin receptor,89 and insulin receptor90 are the best characterized.91 The mechanisms of RMT are still not well understood, but binding of a specific ligand to its corresponding receptor is believed to induce an endocytic event that triggers the formation of endocytic vesicles.92 Some of the aforementioned receptors have been functionalized into drug delivery vectors to act as a molecular Trojan horse upregulating the permeability of the BBB to proteins, peptides, gene materials, or drug-loaded colloidal carriers, such as nanoparticles. For instance, Shilo et al93 qualitatively demonstrated that insulin-targeted gold nano-particles can cross the BBB after systemic administration. Gao et al94 conjugated folate and transferrin to doxorubicin-loaded liposomes to form dual-targeted (transferrin–folate) doxorubicin-loaded liposomes. The amount of doxorubicin that was transported across BBB in the transferrin–folate doxorubicin-loaded liposome group was about sevenfold higher than that in the doxorubicin-loaded liposome group, implying that dual-targeting significantly improved the transport of doxorubicin across the BBB. Boado et al95 fused iduronate 2-sulfatase, a recombinant lysosomal enzyme, to the human insulin receptor monoclonal antibody and found that this strategy could deliver the fusion protein across the BBB at therapeutic levels, while iduronate 2-sulfatase did not cross the BBB at all. Yang et al96 designed dual peptide-modified liposomes loaded with vascular endothelial growth factor, siRNA, and docetaxel by attaching two receptor-specific peptides, ie, low-density lipoprotein receptor-related protein receptor (Angiopep-2) and neuropilin-1 receptor for glioma targeting and BBB penetration. The dual peptide-modified liposomes persisted the binding ability to glioma cells, showed in four types of glioma cells line the highest uptake compared with those single modified or non-modified liposomes. All these investigations yielded encouraging results for drug delivery and treatment of glioma by traversing the BBB via RMT.
A first-in-human Phase I study of GRN1005, a paclitaxel-Angiopep-2 peptide-drug conjugate that binds to the low-density lipoprotein receptor-related protein-1 receptor, has been performed in patients with recurrent glioma.97 The clinical data show that GRN1005 greatly facilitated the penetration of paclitaxel into tumor tissue, while paclitaxel alone showed no benefit in recurrent glioblastoma multiforme.98
RMT has been widely investigated for the delivery of macromolecular pharmaceuticals in the treatment of glioma. Nevertheless, there are still some limitations to be noted. Widespread expression of these receptors in tissues other than the BBB offsets the selectiveness and efficiency of drug delivery. Other limitations includes rapid degradation of cargo, a small dissociation rate, and potential toxicity after repeated treatments.99
Cell-penetrating peptides
Cell-penetrating peptides (CPPs), also known as protein transduction domains or membrane translocation sequences, represent one of the most promising molecular mechanisms for passive delivery of biologically active molecules into cells.100 CPPs are cationic or amphipathic peptide sequences that can traverse mammalian plasma membranes and penetrate the BBB.101 They are attractive for use as molecular delivery vehicles to ferry various therapeutic cargoes into brain tissue in the setting of CNS disease, including glioma.83
Since the seminal descriptions of the Tat peptide102 and penetratin peptide,103 an increased number of CPPs have been identified from natural proteins104 or generated as chimeric CPPs105 and synthetic CPPs.106–108 Although these CPPs have been intensively investigated for delivery of different cargoes across biological membranes, the exact mechanism of cellular uptake remains controversial. The endocytic and nonendocytic pathways have both been reported to be responsible for the translocation of CPPs,104 depending on the CPP used, its concentration, the cell type, and the cargo involved.109 To those CPPs that have high affinity for membranes, the non-endocytic pathways occur in a direct penetrating manner without consuming energy.84 The endocytic pathway begins with adsorption of CPPs at the cell surface, followed by endocytosis at the cell membrane, vesicle formation, endosome formation, and endosomal release.110
CPPs show good penetrating ability when carrying molecules. At the same time, they have low cellular toxicity, high efficiency, and an almost unlimited ability to attach to the cell surface.111 In addition, their capacity for subcellular localization further improves the intracellular trafficking of transported substances, making CPPs ideal for delivery of biologically active molecules, especially molecules with high interest and low permeability, including small molecules,112 proteins,113 nucleic acids,114 and nanoparticles,115,116 both in vitro and in vivo.117 For example, Fu et al113 demonstrated rapid and specific delivery of Luc into the CNS by RDP, a CPP with 39 amino acid residues derived from the rabies virus glycoprotein. Sharma et al116 reported that the presence of penetratin significantly promoted the cellular transport of transferrin liposomes encapsulating doxorubicin (approximately 15% crossed the BBB in vitro and 4% crossed it in vivo). Youn et al114 prepared a myristic acid-conjugated transportan equipped with a transferrin receptor-targeting peptide to stabilize encapsulation of siRNA and target delivery of siRNA into brain cells by overcoming the BBB. The results in murine brain endothelioma and human glioma cell lines clearly indicated both successful siRNA uptake and a functional gene silencing effect. Balzeau et al115 reported a 6- to 13-fold increase in internalization of lipid nanocapsules by glioblastoma multiforme cells when they bound a tubulin-interacting CPP to lipid nanocapsules loaded with paclitaxel. These studies not only highlight the importance of CPPs for transport of pharmaceuticals across the BBB, but also bring us a step closer to the therapeutic application of CPPs for treating various CNS-related diseases, including glioma.
In spite of the encouraging application of CPPs in drug delivery, there are some underlying limitations. The lack of specific target may disperse the drug transportation into the brain and increase peripheral side effects. The heterogeneity of the various CPPs involved hampers the elucidation of the exact mechanisms involved in the delivery of these peptide molecules.105 Moreover, the large size of the CPP complex may initiate an immune response. In vivo applications of CPPs can also be limited by certain enzymes that can easily break down these peptides.47
Cell-mediated delivery
Use of cells that traffic to sites of tumor pathology as a delivery vehicle for therapeutic agents represents a novel strategy to combat a broad spectrum of diseases, including glioma, and is one of the most exciting frontiers in drug delivery research.118 This cell-mediated delivery systems emerged with several inherited advantages such as targeted transport, controlled drug release, decreased drug immunogenicity, and an improved cytotoxicity profile.119,120 Recent investigations have focused on use of two cells types, ie, immunocytes and stem cells, as cellular Trojan horses to carry concealed therapeutic cargoes across the impermeable BBB.121 In particular, two types of stem cells with distinct origins, ie, neural stem cells122 and mesenchymal stem cells,123–125 are especially attractive owing to their excellent tropism toward invasive malignancies within the brain irrespective of the BBB.126,127 In addition, these cells are inherently easy to cultivate and transplant, and are innocuous in a variety of applications.128 To date, cell-mediated delivery systems have been used to deliver a plethora of therapeutic cargoes, including cytokines,129 enzyme/prodrug combinations,130 viral particles,131 nanoparticles,132 and genes.133 Aboody et al130 used an enzyme/prodrug-targeted delivery system involving a human neural stem cell line to target glioma in mice. Their system locally converts the prodrug 5-fluorocytosine to the active chemotherapeutic 5-fluorouracil, enabling delivery of a high concentration of 5-fluorouracil directly in and around the site of the glioma. Lee et al133 demonstrated in an animal model that mesenchymal stem cells can deliver synthetic exogenous miRNA mimics to glioma cells. Further, Ahmed et al134 showed that both neural stem cells and mesenchymal stem cells can successfully ferry oncolytic adenovirus (CRAd-S-pk7) across the BBB. These encouraging findings provide compelling evidence of the effectiveness of this type of cell-mediated delivery across the BBB.
Despite the advantages and exciting clinical potential of target cell-mediated systems, some limitations need be considered. The loaded cytotoxic drugs themselves can sometimes damage the cell carriers,120 which will definitely offset any therapeutic effectiveness. In addition, the limited ability of cells to efficiently load, disintegrate, and release the entrapped therapeutic agents could not be ignored during delivery. Another concern arises from the possibility of gene insertion and the ensuing dysregulation of normal cell function when genetic modification of stem cells is used.135 Furthermore, the substantial quantities of cells needed has hampered the translation of these promising delivery systems to use in humans. Further research is still necessary to optimize the surface characteristics, multivalent attachment, controlled release, and biocompatibility.
Current clinical situation and perspective
Each of the strategies outlines above has its own distinct advantages and disadvantages, as summarized in Table 1. To date, these strategies have yielded exciting results in preclinical animal models of glioma, which has encouraged the approval of clinical studies using such regimens. Nevertheless, despite the success seen in some animal models, progress in the clinical setting is still modest when compared with the application of these strategies in other types of tumor, such as ovarian tumors, mammary adenocarcinoma, and oral carcinoma; only marginal effects can be observed, with survival measured in terms of months instead of years.
Table 1.
Summary of strategies to enhance the permeability of the blood–brain barrier for treatment of glioma
| Strategy | Principal advantages | Potential problems | Clinical trial |
|---|---|---|---|
| Nanotechnology | Sustained release of payload Uptake by the tumor parenchyma |
Rapid removal | Phase II |
| Hyperthermia | Easy to execute Compatible with drugs |
Necrosis and higher intracranial pressure Possible tissue damage | Phase II |
| RMT | Site-specific | Potential toxicity Rapid degradation of the cargo Low dissociation rate |
Phase III |
| CPPs | Great penetrating ability Low cellular toxicity |
Rapid removal Lack of specificity Initiate immune response |
Phase II |
| Cell-mediated delivery | Targeted transport Controlled drug release Low cytotoxicity |
Cell damage Larger quantity of cells needed |
Phase I |
Abbreviations: CPPs, cell-penetrating peptides; RMT, receptor-mediated transport.
There are at least five clinical trials currently ongoing which involved the application of nanoparticles (see ClinicalTrials.gov). As a promising way to elicit the specific delivery of drugs, nanoparticles are expected to become part of the next generation of treatments for glioma, although many questions still require extensive investigation.136 It is notable that several research groups are actively investigating to combine different functions with nanoparticles as platforms for targeting glioma cells. For example, induction of nanoparticles via local hyperthermia is already under Phase II or III study for the treatment of glioma.137,138 The data available demonstrate the safety and efficacy of this technique in humans, with an increase in overall survival as compared with a reference population.
Currently, Phase I/II clinical trials of “stem cell therapy for cancer” are underway, including in glioma. Based on encouraging preclinical results,139 Aboody et al130 have received approval to conduct the first clinical study of genetically modified neural stem cells in patients with recurrent high-grade glioma.
However, at the same time, the clinical trials of some strategies have yielded unsatisfactory outcomes. Some strategies have failed to have significant benefits, suggesting an urgent need to further optimize these regimens. As a paradigm for RMT systems, GRN1005 showed promising Phase I data (see the Receptor-mediated transport section). However, interim analysis of the Phase II trial showed no CNS responses, so the development of GRN1005 was discontinued.140 Similarly, Tf-CRM107 demonstrated an increased median survival time in Phase I and II clinical trials, but an early Phase III clinical trial was terminated due to disappointing preliminary results.141
There are several possible explanations for the confusing discrepancy, for example, the difference between the actual microenvironment of tumor cells in patients and that provided by the serum and medium used for cell lines in the laboratory, and the wide heterogeneity of patient tumors, which are classified simply as gliomas in clinical trials. Another important reason relates to the challenges of delivering therapeutic agents not only across the BBB but also into infiltrating tumor cells nestled within normal brain tissue. Further basic and translational research, as well as enrollment of patients in clinical trials, are still necessary to understand and make progress from bench to beside against this lethal tumor.
Safety profile
Undesirable side effects were not uncommon in either the animal studies or the human trials. For instance, a nanoparticle-related inflammatory response, including substantial neutrophil influx and mortality, was reported at high dose.136 Transient disruption of the BBB by hyperthermia caused unwanted delivery of anticancer agents to normal brain tissue and also increased intracranial pressure.138 Encephalomalacia was reported after injection of Tf-CRM107, although systemic toxicity was minimal and transient.141 Indiscriminate cellular uptake of CPPs limited their applications because systemic injection led to their uptake beyond the target tissue, increasing the risk of toxicity and off-target effects.142 Current stem cell experiments involve millions of stem cells, among which only few can fulfill their designated purpose of migrating across the BBB and surviving at the tumor site. The rest non-migratory stem cells are extraordinary detrimental to the recipient because of the induction of heterogeneous tumor and inflammation.139
Conclusion
There is no controversy about the importance of improving drug delivery across the BBB, considering the failure of effective treatments to cure the invasive glioma cells, which are shielded by the BBB. The prognosis in patients with glioma will remain dismal until we identify the “golden finger” to ensure effective delivery of anticancer treatment across the BBB to the tumor cells. The recently introduced delivery strategies described in this review share the ability to enhance the permeability of the BBB in a less invasive or even noninvasive manner, and to deliver therapeutics across the BBB to reach the brain parenchyma. Indeed, no single strategy is powerful enough to offer substantial breakthrough for glioma treatment, so the future application of combined efforts and therapeutic agents might lead to a successful resolution.
Acknowledgments
FZ thanks Robert Gutteridge for his generous help and Muthanna Al-Baldawi for invaluable suggestions on the structure of the manuscript. This work was supported by the Natural Science Foundation of People’s Republic of China 2013 (81102899) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jovčevska I, Kočevar N, Komel R. Glioma and glioblastoma – how much do we (not) know? Mol Clin Oncol. 2013;1:935–941. doi: 10.3892/mco.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bicker J, Alves G, Fortuna A, Falcão A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm. 2014;87:409–432. doi: 10.1016/j.ejpb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Ni D, Zhang J, Bu W, et al. Dual-targeting upconversion nanoprobes across the blood–brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastoma. ACS Nano. 2014;8:1231–1242. doi: 10.1021/nn406197c. [DOI] [PubMed] [Google Scholar]
- 4.Shively KM, Langheinrich BW, Keucher T, Cockerill D. Glioblastoma, brain metastases, spine metastases. Oncol Care News. 2012;III:1–8. [Google Scholar]
- 5.Stuplich M, Hadizadeh DR, Kuchelmeister K, et al. Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol. 2012;30:2012–2015. doi: 10.1200/JCO.2011.40.9565. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson D, Mischel PS. Charting the course across the blood–brain barrier. J Clin Invest. 2014;121:31–33. doi: 10.1172/JCI45758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Mrugala MM. Advances and challenges in the treatment of glioblastoma: a clinician’s perspective. Discov Med. 2013;15:221–230. [PubMed] [Google Scholar]
- 9.Lemasson B, Chenevert TL, Lawrence TS, et al. Impact of perfusion map analysis on early survival prediction accuracy in glioma patients. Transl Oncol. 2013;6:766–774. doi: 10.1593/tlo.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother. 2013;13:389–403. doi: 10.1586/ern.13.7. [DOI] [PubMed] [Google Scholar]
- 11.David LS, Daniel L, Tyler EM. Brain tumor stem cells: molecular characteristics and their impact on therapy. Mol Aspects Med. 2014;39C:82–101. doi: 10.1016/j.mam.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kniesel U, Wolburg H. Tight junctions of the blood–brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/a:1006995910836. [DOI] [PubMed] [Google Scholar]
- 13.Lehnus KS, Donovan LK, Huang X, et al. CD133 glycosylation is enhanced by hypoxia in cultured glioma stem cells. Int J Oncol. 2013;42:1011–1017. doi: 10.3892/ijo.2013.1787. [DOI] [PubMed] [Google Scholar]
- 14.Auvergne RM, Sim FJ, Wang S, et al. Transcriptional differences between normal and glioma-derived glial progenitor cells identify a core set of dysregulated genes. Cell Rep. 2013;3:2127–2141. doi: 10.1016/j.celrep.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caruso G, Caffo M, Alafaci C, et al. Could nanoparticle systems have a role in the treatment of cerebral gliomas? Nanomedicine. 2011;7:744–752. doi: 10.1016/j.nano.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Modrek AS, Bayin NS, Placantonakis DG. Brain stem cells as the cell of origin in glioma. World J Stem Cells. 2014;6:43–52. doi: 10.4252/wjsc.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novakova I, Subileau E, Toegel S, et al. Transport rankings of non-steroidal antiinflammatory drugs across blood–brain barrier in vitro models. PLoS One. 2014;9:e86806. doi: 10.1371/journal.pone.0086806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauptman JS. From the bench to the bedside: sleeping when you’re awake, lasers and the blood–brain barrier, neurons with a taste for lactate, and more. Surg Neurol Int. 2014;2:100–102. doi: 10.4103/2152-7806.83024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen SJ, Hirschberg H. Site-specific opening of the blood–brain barrier. J Biophotonics. 2010;3:356–367. doi: 10.1002/jbio.200900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Fan C, Ting C, Yeh C. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432–444. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiken R. Molecular neuro-oncology and the challenge of the blood-brain barrier. Semin Oncol. 2014;41(4):438–445. doi: 10.1053/j.seminoncol.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the blood–brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;4:33–39. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 24.Linda M, Neagu M, Demoulin J, Constantinescu SN. Therapy targets in glioblastoma and cancer stem cells: lessons from haematopoietic neoplasms. J Cell Mol Med. 2013;17:1218–1235. doi: 10.1111/jcmm.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois LG, Campanati L, Righy C, et al. Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci. 2014;12:418. doi: 10.3389/fncel.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yutaka M, Tomoya T, Kazuko T, et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano. 2013;7:8583–8592. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- 27.Wolburg H, Noell S, Fallier-Becker P, Mack AF, Wolburg-Buchholz K. The disturbed blood–brain barrier in human glioblastoma. Mol Aspects Med. 2012;33:579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLos One. 2013;8:e62425. doi: 10.1371/journal.pone.0062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veringa SJ, Biesmans D, van Vuurden DG, et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLos One. 2013;8:e61512. doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan C, Lu W. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13(12):2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Zhao X, Mao H, et al. Intravenous injection of oncolytic picornavirus SVV-001 prolongs animal survival in a panel mouse models of pediatric glioma. Neuro Oncol. 2013;15:1173–1185. doi: 10.1093/neuonc/not065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhujbal SV, Vos PD, Niclou SP. Drug and cell encapsulation: alternative delivery options for the treatment of malignant brain tumors. Adv Drug Deliv Rev. 2014:67–68. 142–153. doi: 10.1016/j.addr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Barua NU, Gill SS, Love S. Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathol. 2014;24:117–127. doi: 10.1111/bpa.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Woensel M, Wauthoz N, Rosière R, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers (Basel) 2013;5:1020–1048. doi: 10.3390/cancers5031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeken JF, Löscher W. The blood–brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 36.Auffinger B, Thaci B, Nigam P, Rincon E, Cheng Y, Lesniak MS. New therapeutic approaches for malignant glioma: in search of the Rosetta stone. F1000 Med Rep. 2012;4:18–23. doi: 10.3410/M4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J, Shen S, Wang D, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Yoon T, Cho Y. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013;2013:782041. doi: 10.1155/2013/782041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilella A, Tosi G, Grabrucker AM, et al. Insight on the fate of CNS-targeted nanoparticles. Part I: Rab5-dependent cell-specific uptake and distribution. J Control Release. 2014;174:195–201. doi: 10.1016/j.jconrel.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Hernández-Pedro NY, Rangel-López E, Magaña-Maldonado R, et al. Application of nanoparticles on diagnosis and therapy in gliomas. Biomed Res Int. 2013;2013:351031. doi: 10.1155/2013/351031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int J Nanomedicine. 2014;9:795–811. doi: 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malatesta M, Galimberti V, Cisterna B, Costanzo M, Biggiogera M, Zancanaro C. Chitosan nanoparticles are efficient carriers for delivering biodegradable drugs to neuronal cells. Histochem Cell Biol. 2014;141:551–558. doi: 10.1007/s00418-013-1175-9. [DOI] [PubMed] [Google Scholar]
- 43.Gromnicova R, Davies HA, Sreekanthreddy P, et al. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS One. 2013;8:e81043. doi: 10.1371/journal.pone.0081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen SA, Day ES, Ko CH, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krol S. Challenges in drug delivery to the brain: nature is against us. J Control Release. 2012;164:145–155. doi: 10.1016/j.jconrel.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 46.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Stockwell J, Abdi N, Lu X, Maheshwari O. Novel central nervous system drug delivery systems. Chem Biol Drug Des. 2014;87:507–520. doi: 10.1111/cbdd.12268. [DOI] [PubMed] [Google Scholar]
- 48.Sabel M, Rommel F, Kondakcim M, Gorol M, Willers R, Bilzer T. Laser induced thermotherapy and blood–brain barrier changes: a review. Med Laser Appl. 2002;169:164–169. [Google Scholar]
- 49.Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takagi H, Azuma K, Tsuka T. Antitumor effects of high-temperature hyperthermia on a glioma rat model. Oncol Lett. 2014;7:1007–1010. doi: 10.3892/ol.2014.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res. 2014;34:565–574. [PubMed] [Google Scholar]
- 52.Liu HL, Hua MY, Chen PY, et al. Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Wang H, Lin G, Lin H, Wong T. Evaluation of the increase in permeability of the blood–brain barrier during tumor progression after pulsed focused ultrasound. Int J Nanomedicine. 2012;7:723–730. doi: 10.2147/IJN.S28503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang FY, Lin GL, Horng SC, et al. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:964–970. doi: 10.1109/TUFFC.2011.1897. [DOI] [PubMed] [Google Scholar]
- 55.Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood–brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao L, Song Q, Bai W, et al. Facilitated brain delivery of poly(ethylene glycol) e poly(lactic acid) nanoparticles by microbubble-enhanced unfocused ultrasound. Biomaterials. 2014;35:3384–3395. doi: 10.1016/j.biomaterials.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 57.Xie F, Boska MD, Lof J, Ubertimg MG, Tsutsui JM, Porter TR. Effects of transcranial ultrasound and intravenous microbubbles on blood–brain barrier permeability in a large animal model. Ultrasound Med Biol. 2008;34:2028–2034. doi: 10.1016/j.ultrasmedbio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Diaz RJ, McVeigh PZ, Reilly MA, et al. Focused ultrasound delivery of Raman nanoparticles across the blood–brain barrier: potential for targeting experimental brain tumors. Nanomedicine. 2014;10:1075–1087. doi: 10.1016/j.nano.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–907. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 60.Wei KC, Chu PC, Wang HJ, et al. Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblas-toma treatment: a preclinical study. PLos One. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Chen P, Yang H, et al. In vivo MR quantification of superparamagnetic iron oxide nanoparticle leakage during blood–brain barrier opening in swine. J Magn Reson Imaging. 2011;34:1313–1324. doi: 10.1002/jmri.22697. [DOI] [PubMed] [Google Scholar]
- 63.Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163:125–129. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moriyama E, Salcman M, Broadwell RD. Blood–brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: I. Temperature and power measurements. Surg Neurol. 1991;35:177–182. doi: 10.1016/0090-3019(91)90068-k. [DOI] [PubMed] [Google Scholar]
- 66.Sirav B, Seyhan N. Blood–brain barrier disruption by continuous-wave radio frequency radiation. Electromagn Biol Med. 2009;28:215–222. doi: 10.1080/15368370802608738. [DOI] [PubMed] [Google Scholar]
- 67.Gong W, Wang Z, Liu N, et al. Improving efficiency of adriamycin crossing blood–brain barrier by combination of thermosensitive liposomes and hyperthermia. Biol Pharm Bull. 2011;34:1058–1064. doi: 10.1248/bpb.34.1058. [DOI] [PubMed] [Google Scholar]
- 68.Wang D, Zhang Y, Chen H, et al. Hyperthermia promotes apoptosis and suppresses invasion in C6 rat glioma cells. Asian Pacific J Cancer Prev. 2012;13:3239–3245. doi: 10.7314/apjcp.2012.13.7.3239. [DOI] [PubMed] [Google Scholar]
- 69.Kiessling M, Herchenhan E, Eggert HR. Cerebrovascular and metabolic effects on the rat brain of focal Nd:YAG laser irradiation. J Neurosurg. 1990;73:909–917. doi: 10.3171/jns.1990.73.6.0909. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez T, Pozo F. Induction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorods. Int J Nanomedicine. 2012;7:1511–1523. doi: 10.2147/IJN.S28470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tetard MC, Vermandel M, Mordon S, Lejeune J-P, Reyns N. Experimental use of photodynamic therapy in high grade gliomas: a review focused on 5-aminolevulinic acid. Photodiagnosis Photodyn Ther. 2014;11:319–330. doi: 10.1016/j.pdpdt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Choi M, Ku T, Chong K, Yoon J, Choi C. Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability. Proc Natl Acad Sci U S A. 2011;108:9256–9261. doi: 10.1073/pnas.1018790108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112:5818–5978. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 74.Shevtsov MA, Nikolaev BP, Yakovleva LY, et al. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int J Nanomedicine. 2014;9:273–287. doi: 10.2147/IJN.S55118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stepp P, Thomas F, Lockman PR, Chen H, Rosengart AJ. In vivo interactions of magnetic nanoparticles with the blood–brain barrier. J Magn Magn Mater. 2009;321:1591–1593. [Google Scholar]
- 76.Kong SD, Lee J, Ramachandran S, et al. Magnetic targeting of nanoparticles across the intact blood–brain barrier. J Control Release. 2012;164:49–57. doi: 10.1016/j.jconrel.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chertok B, David AE, Yang VC. Brain tumor targeting of magnetic nanoparticles for potential drug delivery: effect of administration route and magnetic field topography. J Control Release. 2011;155:393–399. doi: 10.1016/j.jconrel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saiyed ZM, Gandhi NH, Nair MP. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood–brain barrier. Int J Nanomedicine. 2010;5:157–166. doi: 10.2147/ijn.s8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dilnawaz F, Singh A, Mewar S, Sharma U, Jagannathan NR, Sahoo SK. The transport of non-surfactant based paclitaxel loaded magnetic nanoparticles across the blood–brain barrier in a rat model. Biomaterials. 2012;33:2936–2951. doi: 10.1016/j.biomaterials.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 80.Han L, Zhang A, Wang H, Pu P, Kang C, Chang J. Construction of novel brain-targeting gene delivery system by natural magnetic nanoparticles. J Appl Polym Sci. 2011;121:3446–3454. [Google Scholar]
- 81.Frank KM, Dirk U. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen PY, Liu HL, Hua MY, et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol. 2010;12(10):1050–1060. doi: 10.1093/neuonc/noq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenehjem DD, Hartz AM, Bauer B, Anderson GW. Novel and emerging strategies in drug delivery for overcoming the blood–brain barrier. Future Med Chem. 2009;1:1623–1641. doi: 10.4155/fmc.09.137. [DOI] [PubMed] [Google Scholar]
- 84.Parrish K, Sarkaria J, Elmquist W. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin Pharmacol Ther. 2015 Jan 12; doi: 10.1002/cpt.71. Epub. [DOI] [PubMed] [Google Scholar]
- 85.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 86.Allhenn D, Boushehri MA, Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int J Pharm. 2012;436:299–310. doi: 10.1016/j.ijpharm.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 87.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNF a decoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012;32:1933–1938. doi: 10.1038/jcbfm.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247:291–307. doi: 10.1007/s00232-014-9637-0. [DOI] [PubMed] [Google Scholar]
- 89.Miao D, Jiang M, Liu Z, et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol Pharm. 2014;11:90–101. doi: 10.1021/mp400189j. [DOI] [PubMed] [Google Scholar]
- 90.Kuo YC, Shih-Huang CY. Solid lipid nanoparticles carrying chemotherapeutic drug across the blood–brain barrier through insulin receptor-mediated pathway. J Drug Target. 2013;21:730–738. doi: 10.3109/1061186X.2013.812094. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Guo Y, Kuang Y, An S, Ma H, Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34:9142–9148. doi: 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 92.Khawli LA, Prabhu S. Drug delivery across the blood–brain barrier. Mol Pharm. 2013;10:1471–1472. doi: 10.1021/mp400170b. [DOI] [PubMed] [Google Scholar]
- 93.Shilo M, Motiei M, Hana P, Popovtzer R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6:2146–2152. doi: 10.1039/c3nr04878k. [DOI] [PubMed] [Google Scholar]
- 94.Gao JQ, Lv Q, Li LM, et al. Glioma targeting and blood brain barrier penetration by dual-targeting doxorubicin liposomes. Biomaterials. 2013;34:5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 95.Boado RJ, Hui EK, Lu JZ, Sumbria RK, Pardridge WM. Blood–brain barrier molecular Trojan horse enables imaging of brain uptake of radioiodinated recombinant protein in the rhesus monkey. Bioconjug Chem. 2013;24:1741–1749. doi: 10.1021/bc400319d. [DOI] [PubMed] [Google Scholar]
- 96.Yang ZZ, Li JQ, Wang ZZ, Dong DW, Qi XR. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35:5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 97.Drappatz J, Brenner A, Wong ET, et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- 98.Fine RL, Chen J, Balmaceda C, et al. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12:5770–5776. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 99.Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Trans-membrane proteins of the tight junctions at the blood–brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2014 Nov 26; doi: 10.1016/j.semcdb.2014.11.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 100.Sebbage V. Cell-penetrating peptides and their therapeutic applications. Bioscience Horizons. 2009;2:64–72. [Google Scholar]
- 101.Zou LL, Ma JL, Wang T, Yang TB, Liu CB. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hauber J, Perkins A, Heimer EP, Cullen BR. Transactivation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987;84:6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vivés E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 104.Harreither E, Rydberg HA, Amand HL, et al. Characterization of a novel cell penetrating peptide derived from human Oct4. Cell Regen (Lond) 2014;3:1–14. doi: 10.1186/2045-9769-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trabulo S, Cardoso AL, Mano M, Pedroso De Lima MC. Cell-penetrating peptides – mechanisms of cellular uptake and generation of delivery systems. Pharmaceuticals (Basel) 2010;3:961–993. doi: 10.3390/ph3040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Ran R, Chen J, et al. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014;35:4835–4847. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 107.Gangoso E, Thirant C, Chneiweiss H, Medina JM, Tabernero A. A cell-penetrating peptide based on the interaction between c-Src and connexin43 reverses glioma stem cell phenotype. Cell Death Dis. 2014;5:e1023. doi: 10.1038/cddis.2013.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eriste E, Kurrikoff K, Suhorutšenko J, et al. Peptide-based gliomatargeted drug delivery vector gHoPe2. Bioconjug Chem. 2013;24:305–313. doi: 10.1021/bc300370w. [DOI] [PubMed] [Google Scholar]
- 109.Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 110.Xiao G, Gan LS. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int J Cell Biol. 2013;2013:703545. doi: 10.1155/2013/703545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simon MJ, Kang WH, Gao S, Banta S, Morrison B. Evaluation of the cell-penetrating peptide TAT as a trans-blood–brain barrier delivery vehicle; Proceedings of the 2010 IEEE 36th Annual Northeast Bioengineering Conference; March 26–28, 2010; Columbia, NY, USA. [Google Scholar]
- 112.Michiue H, Sakurai Y, Kondo N, et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials. 2014;35:3396–3405. doi: 10.1016/j.biomaterials.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 113.Fu A, Wang Y, Zhan L, Zhou R. Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide. Pharm Res. 2012;29:1562–1569. doi: 10.1007/s11095-012-0667-y. [DOI] [PubMed] [Google Scholar]
- 114.Youn P, Chen Y, Furgeson DY. A myristoylated cell-penetrating peptide bearing a transferrin receptor-targeting sequence for neuro-targeted siRNA delivery. Mol Pharm. 2014;11:486–495. doi: 10.1021/mp400446v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Balzeau J, Pinier M, Berges R, Saulnier P, Benoit JP, Eyer J. The effect of functionalizing lipid nanocapsules with NFL-TBS. 40–63 peptide on their uptake by glioblastoma cells. Biomaterials. 2013;34:3381–3389. doi: 10.1016/j.biomaterials.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 116.Sharma G, Modgil A, Zhong T, Sun C, Singh J. Influence of short-chain cell-penetrating peptides on transport of doxorubicin encapsulating receptor-targeted liposomes across brain endothelial barrier. Pharm Res. 2013;31:1194–1209. doi: 10.1007/s11095-013-1242-x. [DOI] [PubMed] [Google Scholar]
- 117.Sharma G, Modgil A, Layek B, et al. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: biodistribution and transfection. J Control Release. 2013;167:1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 118.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 119.Choi MR, Bardhan R, Stanton-Maxey KJ, et al. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol. 2012;3:47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drugs delivery. Expert Opin Drug Deliv. 2011;8:415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bovenberg MS, Degeling MH, Tannous BA. Advances in stem cell therapy against gliomas. Trends Mol Med. 2013;19:281–291. doi: 10.1016/j.molmed.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 122.Ahmed AU, Lesniak MS. Glioblastoma multiforme: can neural stem cells deliver the therapeutic payload and fulfill the clinical promise? Expert Rev Neurother. 2011;11:775–777. doi: 10.1586/ern.11.65. [DOI] [PubMed] [Google Scholar]
- 123.Roger M, Clavreul A, Venier-Julienne MC, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 124.Fan C, Wang D, Zhang Q, Zhou J. Migration capacity of human umbilical cord mesenchymal stem cells towards glioma in vivo. Neural Regen Res. 2013;8:2093–2102. doi: 10.3969/j.issn.1673-5374.2013.22.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebrahimi A, Lalvand N. Drug delivery using genetically modified mesenchymal stem cells: a promising targeted-delivery method. Hygeia Journal for Drugs and Medicines. 2013;5:90–104. [Google Scholar]
- 126.Binello E, Germano IM. Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro Oncol. 2012;14:256–265. doi: 10.1093/neuonc/nor204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elhami E, Dietz B, Xiang B, et al. Assessment of three techniques for delivering stem cells to the heart using PET and MR imaging. EJNMMI Res. 2013;3:72. doi: 10.1186/2191-219X-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Najbauer J, Huszthy PC, Barish ME, et al. Cellular host responses to gliomas. PLoS One. 2012;7:e35150. doi: 10.1371/journal.pone.0035150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmed AU, Alexiades NG, Lesniak MS. The use of neural stem cells in cancer gene therapy: predicting the path to the clinic. Curr Opin Mol Ther. 2010;12:546–552. [PMC free article] [PubMed] [Google Scholar]
- 130.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;59:184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Castleton A, Dey A, Beaton B, et al. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood. 2014;123:1327–1335. doi: 10.1182/blood-2013-09-528851. [DOI] [PubMed] [Google Scholar]
- 132.El-Sadik AO, El-Ansary A, Sabry SM. Nanoparticle-labeled stem cells: a novel therapeutic vehicle. Clin Pharmacol. 2010;2:9–16. doi: 10.2147/CPAA.S8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm. 2012;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Costa PM, Pedrosa De Lima MC. Genetic syndromes and gene therapy viral and non-viral gene therapy for glioblastoma: new insights into the treatment of malignant brain tumors. J Genet Syndr Gene Ther. 2013;4:1000161. [Google Scholar]
- 136.Hernández-Pedro NY, Rangel-López E, Magaña-Maldonado R, et al. Application of nanoparticles on diagnosis and therapy in gliomas. Biomed Res Int. 2013;2013:351031. doi: 10.1155/2013/351031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun J, Guo M, Pang H, Qi J, Zhang J, Ge Y. Treatment of malignant glioma using hyperthermia. Neural Regen Res. 2013;8:2775–2782. doi: 10.3969/j.issn.1673-5374.2013.29.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kant R. Drug delivery systems, CNS protection, and the blood–brain barrier. Biomed Res Int. 2014;2014:869269. doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li SC, Kabeer MH, Vu LT, et al. Training stem cells for treatment of malignant brain tumors. World J Stem Cells. 2014;6:432–440. doi: 10.4252/wjsc.v6.i4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arko L, Katsyv I, Park GE, Luan WP, Park JK. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2011;128:1–36. doi: 10.1016/j.pharmthera.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.MacEwan SR, Chilkoti A. Harnessing the power of cell-penetrating peptides: activatable carriers for targeting systemic delivery of cancer therapeutics and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:31–48. doi: 10.1002/wnan.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]