Abstract
Mineralocorticoid Receptor (MR) activation is involved in blood pressure regulation and the pathogenesis of cardiovascular diseases, such as cardiac fibrosis, vascular inflammation and arterial aging. Recent investigations suggest a role for MR activation in metabolic dysregulation.
OBJECTIVE
To test the effect of MR blockade on basal and postprandial glucose and lipid levels after a meal high in fat and glucose in healthy males.
SUBJECTS AND METHODS
A prospective, self-controlled study was performed in 13 healthy adult males aged 18–45 years. Blood was drawn before, 2h, and 4h after a high fat/high glucose meal (50g fat, 75 g glucose), followed by low-dose eplerenone treatment (50 mg daily) for 14 days. Subjects returned for a second high fat/high glucose meal after the medication period. Basal and postprandial blood glucose and lipid levels were compared before and after eplerenone treatment.
RESULTS
Eplerenone treatment affected neither basal nor postprandial glucose and lipid levels in our study population.
CONCLUSION
Our results suggest that low-dose, non-blood pressure-affecting, MR blockade does not alter postprandial lipid and glucose homeostasis in healthy adult subjects.
Keywords: aldosterone, eplerenone, metabolism, high fat/high glucose diet
INTRODUCTION
The mineralocorticoid receptor (MR) is expressed in kidney epithelium mediating water- and electrolyte balance, and, hence, blood pressure homeostasis [1]. Functional MR also has been demonstrated in a variety of non-epithelial tissues, including endothelial cells, vascular smooth muscle cells and peripheral blood mononuclear cells (PBMCs). MR activation is involved in cardiovascular diseases, such as arterial hypertension, cardiovascular inflammation and remodelling, and endothelial dysfunction. MR blockade has been shown to prevent adverse cardiovascular events in humans and animal studies [2,3].
Recent studies in humans and animals have demonstrated direct associations of the metabolic syndrome with elevated plasma levels of aldosterone, which is the natural ligand for the MR [4]. For example, a study by Goodfriend et al. showed a negative correlation between aldosterone and cardioprotective high density lipoprotein (HDL) levels [5], suggesting that these effects are, at least in part, mediated through increased MR activation. Evidence from human trials also suggests that MR activation may exacerbate alterations in glucose homeostasis, such as insulin resistance, in patients with diabetes mellitus or the metabolic syndrome [6]. However, in summary, the available data remains controversial and there is still no conclusive evidence that establishes aldosterone and MR activation as critical factors in lipid or glucose homeostasis [4]. The adverse cardiovascular effects of elevated plasma glucose levels in diabetes patients are well known [7,8]. Similarly, hyperlipidemia is a known risk factor for the onset and progression of arteriosclerosis, even in subjects without diabetes [9]. In addition, fatty acids are known to induce insulin resistance, compromise cardiac function, and promote vascular dysfunction [10]. The goal of this study was to further clarify the role of MR activation in metabolic regulation. Specifically, we tested the effect of low-dose MR blockade on basal and postprandial glucose and lipid levels in healthy males.
SUBJECTS AND METHODS
Participants
A total of 13 healthy adult males (BMI ≥ 17 and ≤ 27 kg/m2), aged 18–45 years, were included in the final analysis of our study. None of the study participants had any clinical evidence of hypertension, cardiovascular, hepatic, renal or any other organ system disease, as determined by a complete physical exam, a personal medical history, family medical history, routine blood work (complete blood count, serum glucose, sodium, creatinine, potassium, liver tests), EKG and urine analysis. The upper cut-off limit of 45 years was used to reduce possible confounding due to age-related metabolic changes. Subjects with arterial blood pressure 90/60 mmHg or less were excluded from the study. Subjects taking any prescription or herbal medications were excluded, and tobacco users were excluded. Subjects with alcohol consumption of more than 2 drinks per day were excluded, as ethanol is known to affect triglyceride levels. Subjects taking dietary supplements (e.g. high dose fish oil supplements > 1g of EPA and DHA per diem) were excluded, as these substances are known to influence lipid levels. Degree of physical activity was similar in all participants prior to enrolment (maximum weekly physical activity between 2–10h/week). All study participants were on a liberal salt diet throughout the whole study period. The institutional review board approved the protocol (2010-P-002191/1), and each subject provided written consent prior to enrolment.
A self-controlled intervention study was conducted. After a 9-12h fasting period subjects were admitted to the Outpatient Center for Clinical Investigation at the Brigham and Women's Hospital. After remaining supine for 30 min, blood was drawn for basal glucose, insulin and lipid measurement, including total, low density lipoprotein (LDL), high density lipoprotein (HDL), and very low density lipoprotein (VLDL) cholesterol using an indwelling intravenous catheter. Subsequently, subjects consumed a meal challenge consisting of oral fat (50g fat as heavy cream) and oral glucose (75 g glucose as Glucola™), followed by blood draws 2h and 4h after the meal. Study day 1 was followed by a two-week treatment with 50 mg eplerenone daily. After two weeks, subjects returned to the study center to receive the second high fat/high glucose meal (day 15) similar to day 1.
Baseline blood pressure was measured on study days 1 and 15 prior to the meal while supine, using the average of two readings from a Dinamap automated device (Critikon, Tampa, FL, USA).
Laboratory procedures
Serum glucose and lipids were analysed using standard lab techniques in a commercial laboratory, Laboratory Corporation of America (Raritan, NJ). Insulin was analysed in the Harvard Catalyst Central Laboratory (HCCL) by AccessHigh Sensitive Insulin Immunoassay (Beckman Coulter, Chaska, MN) with a detection limit of 0.03 uIU/mL.
Assessment of Insulin Resistance (IR)
IR was evaluated using the homeostatic model assessment of insulin resistance (HOMA-IR) using the following equation: fasting insulin (mU/L) × glucose (mmol/L)/22.5 [11].
Statistical analysis
Data are presented as means ± SD. Normality of the data was assessed using normal probability plots and Shapiro-Wilk test. Assuming normal distribution and equal variance of the data, significance of differences at baseline (prior to the high fat/high glucose meal) before and after eplerenone treatment, as well as differences between different time points (basal, 2h, 4h) before and after eplerenone treatment were tested by 2-way Analysis of Variance (ANOVA) with repeated measures, followed by post-hoc analysis. P<0.05 was considered significant, analyses were performed using Sigma Plot software, version 11.0.
RESULTS
Effect of MR blockade on Basal Glucose and Lipid Levels
All subjects were healthy, normotensive, and free of any metabolic, kidney or cardiovascular disease, as described in the Subjects and Methods section. Please refer to table 1 for characteristics of the study population. Table 1 also shows glucose, insulin, insulin resistance, (as assessed by homeostasis model assessment of insulin resistance [HOMA-IR]), and lipid levels after a 9–12h fasting period at the beginning of the study period (day 1). Two week low-dose (50 mg/day) eplerenone-treatment did not significantly affect fasting levels of glucose, insulin, HOMA-IR, triglycerides (TG), high density lipoproteins (HDL), low density lipoproteins (LDL), very low density lipoproteins (VLDL), and total cholesterol in healthy adult males. MR blockade did not alter blood pressure levels in the participants. Mean systolic blood pressure was 124±10 mmHg (day 1) versus 128±12 mmHg (day15), and diastolic blood pressure was 74±7 mmHg (day 1) versus 78±8 mmHg (day15).
Table 1.
Baseline fasting characteristics of the study population (n=13).
| Mean±SD | |
|---|---|
| Age (years) | 25 8 |
| BMI (kg/m2) | 22 3 |
| Systolic BP (mmHg) | 120 12 |
| Diastolic BP (mmHg) | 74 9 |
| White Blood Cell Count (103/μl) | 4.94 1.1 |
| Thyroid-stimulating hormone (μIU/ml) | 1.6 0.5 |
| Alkaline Phosphatase (IU/I) | 69 17 |
| Alanine Aminotransferase (IU/I) | 21 11 |
| Aspartate Aminotransferase (IU/I) | 23 6 |
| Blood Urea Nitrogen (mg/dl) | 13 3 |
| Creatinine (mg/dl) | 0.94 0.1 |
| Glucose (mg/dl) | 91 7 |
| Insulin (μU/ml) | 4.5 3.6 |
| HOMA-IR | 0.9 0.7 |
| Triglycerides (mg/dl) | 72 28 |
| High density lipoproteins (mg/dl) | 50 12 |
| Low density lipoproteins (mg/dl) | 84 22 |
| Very low density lipoproteins (mg/dl) | 16 8 |
| Total cholesterol (mg/dl) | 150 27 |
Effect of MR blockade on Postprandial Glucose and Lipid Levels
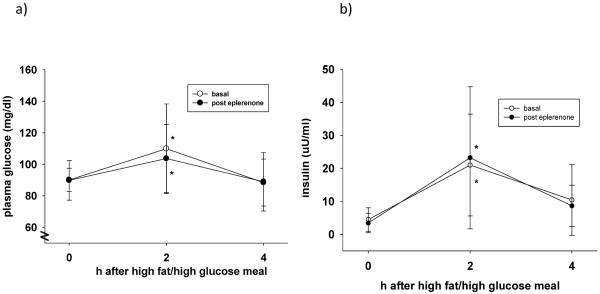
Figures 1a and b show postprandial glucose and insulin levels 2h and 4h after a high fat/high glucose meal, respectively. As expected, 2h after the meal, glucose and insulin increased significantly, returning to normal levels after 4h. MR blockade did not significantly affect the postprandial glucose and insulin profile in our study population.
Figure 1.
(a–b): Glucose and insulin levels after a meal high in fat and glucose before (open circles) and after (black circles) eplerenone treatment (50mg daily). Eplerenone did not significantly affect the postprandial glucose profile. *p<0.05 versus t=0.
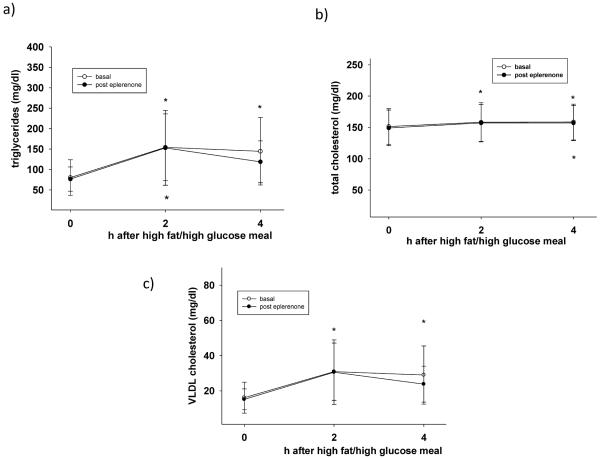
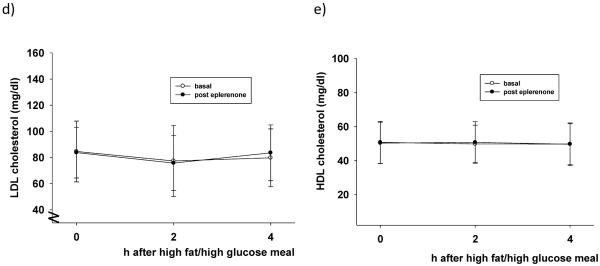
Figure 2 illustrates postprandial lipid levels 2h and 4h after a high fat/high glucose meal. Triglyceride, total cholesterol, and VLDL cholesterol plasma levels significantly increased after the meal, as expected (p<0.05 vs. t=0h). Postprandial values before and after MR blockade were not statistically different. However, after the 2 week MR blockade, VLDL (figure 2c) and triglyceride (figure 2a) levels 4h after the meal were similar to preprandial values (t=0h), suggesting a faster return to baseline in MR-treated subjects.
Figure 2.
(a–e): Lipid levels after a meal high in fat and glucose before (open circles) and after (black circles) eplerenone treatment (50mg daily). Eplerenone did not affect the postprandial profile of any of the measured lipid fractions. *p<0.05 versus t=0
DISCUSSION
Fasting and postprandial TG and glucose levels are elevated in obese patients with the metabolic syndrome and type 2 diabetes mellitus, two conditions that are directly associated with increased prevalence of arteriosclerosis [12]. Moreover, postprandial hyperglycemia and dyslipidemia are associated with increased risk for coronary artery disease (CAD), even in subjects with normal fasting TG levels and without the metabolic syndrome [13].
To date no clear conclusions can be made as to whether MR activation directly affects glucose and lipid homeostasis in healthy subjects and patients with alterations of the glucose homeostasis [4]. For example, Conn reported that >50% of a small number of patients with Primary Aldosteronism (PA) exhibited disturbed glucose tolerance [14]. Similarly, an increased incidence of altered glucose homeostasis was observed in PA patients [15], and surgical removal of the tumour normalized insulin sensitivity and other parameters of disturbed glucose metabolism [16,17]. In contrast, an earlier report showed no differences with respect to plasma glucose and insulin response to glucose challenge in patients with PA compared with essential hypertensive subjects [18]. Possible mechanisms linking MR activation with increased insulin resistance include decreased insulin receptor expression and alterations of insulin signaling [19]. In addition, other indirect effects of MR on mechanisms of insulin resistance, such as increased oxidative stress or decreased adiponectin expression, have been suggested [20].
No studies investigating the effect of MR blockade on parameters of glucose and lipid metabolism have been performed in healthy subjects. Our study failed to demonstrate a significant effect of 14 day, low-dose (50mg/day) eplerenone treatment on basal and postprandial glucose levels in normal weight, healthy males. One limitation of our study is the relatively short treatment period. A two week treatment period might be too short to affect potential long-term metabolic effects mediated by MR activation. To prevent potential confounding of our results by a decrease in blood pressure we chose a low eplerenone dose of 50mg/daily, assuming that in healthy, normotensive individuals this dose would –if at all- only moderately affect blood pressure levels [21]. Our results show that 50mg eplerenone daily for two weeks did not significantly affect blood pressure in our study population. Future studies will have to show whether higher eplerenone doses over a longer period of time would affect metabolic parameters in healthy subjects.
A study in humans by Goodfriend et al. showed that subjects with high aldosterone plasma levels had low HDL and elevated TG plasma levels [22], suggesting that these effects may be mediated through increased MR activation. In contrast, a more recent study showed decreased HDL plasma levels in metabolic syndrome patients with elevated aldosterone plasma levels, regardless of all of the other components of the metabolic syndrome [23]. Fallo et al. demonstrated that TG and HDL levels were not significantly different in patients with PA compared with those with essential hypertension, suggesting that not MR activation but arterial hypertension may affect blood lipids [24]. Accordingly, in 2891 subjects from the Framingham Heart Study no direct correlation between plasma lipids and aldosterone could be observed [25]. Little is known about the mechanisms of interaction between MR and lipid homeostasis. Wada et al. have shown that MR blockade ameliorates histological alterations in the liver of mice fed a high calorie diet. Liver expression of inflammatory markers, such as interleukin-6, and lipogenic enzymes were also significantly suppressed by MR blockade [26].
Different MR blockers, e.g. spironolactone versus eplerenone, have different pharmacokinetic profiles. Therefore, the effect of MR blockade on metabolic parameters may vary by the MR antagonist applied. For example, eplerenone has been shown to improve insulin resistance in animal models of obesity [27,28]. Similarly, in a mouse model of diet-induced diabetes mellitus MR blockade by spironolactone induced improvements in glucose and lipid metabolism [26]. In contrast, Homma et al. demonstrated that spironolactone, but not eplerenone, negatively affected parameters of glucose metabolism in a rat model of metabolic syndrome, likely mediated through increased aldosterone levels [29]. In accordance, results from the CHARM study have indicated that spironolactone use may be associated with an increased risk for the development of diabetes [30].
In our study, investigating healthy adult male subjects, no effect of MR blockade through eplerenone on basal and postprandial parameters of lipid homeostasis could be observed. Our findings, albeit on a relatively small sample size, support the view that low-dose MR blockade does not significantly affect glucose and lipid metabolism in healthy adults. However, the observation that TG and VLDL may return to baseline faster with eplerenone treatment raises the possibility that in another subject population, such as metabolic syndrome or diabetes mellitus type 2, MR blockade might have beneficial effects on the response to a high fat/high glucose meal as glucose and lipid homeostasis are already deranged, and compensatory mechanisms have failed. Therefore, the results from our study encourage us to conduct a future investigation, focusing on the effect of MR blockade on lipid and glucose homeostasis in patients with the metabolic syndrome and type 2 diabetes.
ACKNOWLEDGEMENTS
We acknowledge the helpful technical assistance of Lauren Donahue and Karen Magsipoc.
FUNDING
This manuscript was supported through grants from the National Institutes of Health: R01HL 087060 and K24HL-103845 (GKA) and UL1 RR025758-01 (Harvard Clinical and Translational Science Center, from the National Center for Research Resources).
Abbreviations
- MR
mineralocorticoid receptor
- PBMCs
peripheral blood mononuclear cells
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- VLDL
very low density lipoprotein
- TG
triglycerides
- PA
primary aldosteronism
- BMI
body mass index
- EKG
electrocardiogram
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- BUN
blood urea nitrogen
- HOMA-IR
homeostasis model assessment of insulin resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors of this manuscript have nothing to disclose.
AUTHORS' CONTRIBUTIONS
AWK design and execution of the study, subject recruitment, data collection, data analysis, manuscript writing and submission, LS data collection, data analysis, ADR data collection, data analysis, AHL review and interpretation of data, GHW review and interpretation of data, GKA design of the study, review and interpretation of data, manuscript writing.
REFERENCES
- 1.Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail.Rev. 2005;10:7–13. doi: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology. 2010;151:5098–5102. doi: 10.1210/en.2010-0465. [DOI] [PubMed] [Google Scholar]
- 3.Funder JW. Aldosterone and mineralocorticoid receptors in the cardiovascular system. Prog.Cardiovasc.Dis. 2010;52:393–400. doi: 10.1016/j.pcad.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Krug AW, Ehrhart-Bornstein M. Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51:1252–1258. doi: 10.1161/HYPERTENSIONAHA.107.109439. [DOI] [PubMed] [Google Scholar]
- 5.Goodfriend TL, Egan B, Stepniakowski K, et al. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension. 1995;25:30–36. doi: 10.1161/01.hyp.25.1.30. [DOI] [PubMed] [Google Scholar]
- 6.Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J.Clin.Endocrinol.Metab. 2006;91:3457–3463. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch.Intern.Med. 2004;164:2090–2095. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- 8.Reusch JE, Wang CC. Cardiovascular disease in diabetes: where does glucose fit in? J.Clin.Endocrinol.Metab. 2011;96:2367–2376. doi: 10.1210/jc.2010-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebenbichler CF, Kirchmair R, Egger C, et al. Postprandial state and atherosclerosis. Curr.Opin.Lipidol. 1995;6:286–290. doi: 10.1097/00041433-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Margioris AN. Fatty acids and postprandial inflammation. Curr.Opin.Clin.Nutr.Metab Care. 2009;12:129–137. doi: 10.1097/MCO.0b013e3283232a11. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 13.Ceriello A, Assaloni R, Da RR, et al. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518–2524. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- 14.Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N.Engl.J.Med. 1965;273:1135–1143. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- 15.Skrha J, Haas T, Sindelka G, et al. Comparison of the insulin action parameters from hyperinsulinemic clamps with homeostasis model assessment and QUICKI indexes in subjects with different endocrine disorders. J.Clin.Endocrinol.Metab. 2004;89:135–141. doi: 10.1210/jc.2002-030024. [DOI] [PubMed] [Google Scholar]
- 16.Giacchetti G, Ronconi V, Turchi F, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J.Hypertens. 2007;25:177–186. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 17.Sindelka G, Widimsky J, Haas T, et al. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp.Clin.Endocrinol.Diabetes. 2000;108:21–25. doi: 10.1055/s-0032-1329211. [DOI] [PubMed] [Google Scholar]
- 18.Strauch B, Widimsky J, Sindelka G, et al. Does the treatment of primary hyperaldosteronism influence glucose tolerance? Physiol Res. 2003;52:503–506. [PubMed] [Google Scholar]
- 19.Hitomi H, Kiyomoto H, Nishiyama A, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 20.Garg R, Adler GK. Role of mineralocorticoid receptor in insulin resistance. Curr.Opin.Endocrinol.Diabetes Obes. 2012;19:168–175. doi: 10.1097/MED.0b013e3283533955. [DOI] [PubMed] [Google Scholar]
- 21.Craft J. Eplerenone (Inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure. Proc.(Bayl.Univ Med.Cent.) 2004;17:217–220. doi: 10.1080/08998280.2004.11927973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot.Essent.Fatty Acids. 1999;60:401–405. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 23.Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 24.Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J.Clin.Endocrinol.Metab. 2006;91:454–459. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 25.Kathiresan S, Larson MG, Benjamin EJ, et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am.J.Hypertens. 2005;18:657–665. doi: 10.1016/j.amjhyper.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151:2040–2049. doi: 10.1210/en.2009-0869. [DOI] [PubMed] [Google Scholar]
- 27.Guo C, Ricchiuti V, Lian BQ, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata A, Maeda N, Hiuge A, et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc.Res. 2009;84:164–172. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 29.Homma T, Fujisawa M, Arai K, et al. Spironolactone, but not Eplerenone, Impairs Glucose Tolerance in a Rat Model of Metabolic Syndrome. J.Vet.Med.Sci. 2012 doi: 10.1292/jvms.12-0007. [DOI] [PubMed] [Google Scholar]
- 30.Preiss D, Zetterstrand S, McMurray JJ, et al. Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care. 2009;32:915–920. doi: 10.2337/dc08-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]