Abstract
Purpose
To predict trajectories of metabolic control across adolescence from parental involvement and adolescent psychosocial maturity, and to link metabolic control trajectories to health care utilization.
Methods
252 adolescents (M age at study initiation = 12.5, SD=1.5, range 10–14 years) with type 1 diabetes (54.4% female, 92.8% Caucasian, length of diagnosis M=4.7 years, SD=3.0, range 1–12) participated in a 2-year longitudinal study. Metabolic control was gathered from medical records every three months. Adolescents completed measures of self-reliance (functional autonomy and extreme peer orientation), self-control (self-control and externalizing behavior), and parental involvement in diabetes care (acceptance, monitoring, and frequency of help). At the end of the study, mothers reported health care utilization (diabetes-related emergency room visits and hospitalizations) over the past six months.
Results
Latent class growth analyses indicated two distinct trajectories of metabolic control across adolescence: moderate control with slight deterioration (92% of the sample; average HbA1c = 8.18%) and poor control with rapid deterioration (8% of the sample; average HbA1c of 12.09%). Adolescents with poor and rapidly deteriorating metabolic control reported lower paternal monitoring and frequency of help with diabetes management, lower functional autonomy, and lower self-control than others. Those with poor and rapidly deteriorating metabolic control were 6.4 times more likely to report diabetes-related emergency room visits, and 9.3 times more likely to report diabetes-related hospitalizations near the end of the study.
Conclusions
Parental involvement and adolescents’ psychosocial maturity predict patterns of deteriorating metabolic control across adolescence and could be targeted for intervention.
Keywords: type 1 diabetes, adolescents, metabolic control, glycemic control, hba1c, health care utilization, hospitalization, longitudinal, latent class growth analysis, parental involvement
For the average adolescent with type 1 diabetes, metabolic control deteriorates over time [1, 2], increasing risks for short- and long-term health complications such as ketoacidosis, eye disease, kidney disease, and cardiovascular problems [3]. Although declines in metabolic control are typical, for some adolescents metabolic control remains relatively stable over time, and others may even experience improvements in metabolic control across adolescence [4, 5]. Varying trajectories of metabolic control may arise from differences in parental involvement and adolescents’ psychosocial maturity, two factors that have been linked with better metabolic control cross-sectionally [6–8]. In the present study, we utilized latent class growth analysis (LCGA) to identify different patterns of change in metabolic control across adolescence [4, 5]. We examined parenting behaviors and aspects of psychosocial maturity that are likely important for understanding adolescents’ metabolic control trajectories, and linked these trajectories to diabetes-related health care utilization.
Multiple facets of parental involvement have been associated with better metabolic control during adolescence [6, 8–10], including behavioral involvement in the daily management of diabetes (e.g., getting adolescents diabetes supplies), parental diabetes monitoring (e.g., being aware of daily management activities), and parents’ warmth and acceptance [6, 9]. The benefits of parental involvement have been established cross-sectionally, but parental involvement may also put adolescents on a longitudinal trajectory of faster or slower deterioration in metabolic control. Most studies have focused on the importance of mothers’ involvement, however, recent work suggests that fathers’ monitoring and emotional support may be especially important during adolescence, and both were examined in the present study [9, 10].
Adolescents’ psychosocial maturity may also contribute to trajectories of metabolic control across adolescence [11]. Developmental theorists have found that characteristics of psychosocial maturity such as self-reliance (including a sense of autonomy and a lack of extreme reliance on others) and self-control (defined as controlling impulses and inhibiting socially unacceptable behavior including aggression and rule breaking) protect adolescents from risk behaviors (e.g., substance abuse and risky sexual behavior) [12]. Psychosocial maturity may also predict adolescents’ trajectories of metabolic control given cross-sectional findings that link self-reliance and self-control (e.g., low externalizing behavior and good impulse control) with better adolescent diabetes outcomes [13, 14].
Most research linking parental involvement and psychosocial maturity to metabolic control is cross-sectional and cannot address whether such factors predict longitudinal change in metabolic control or different patterns of change across time. The present study utilized LCGA, which identifies subgroups of adolescents who display qualitatively distinct growth trajectories in metabolic control [15]. This approach can characterize subgroups of adolescents who display problematic metabolic control trajectories and who might benefit from targeted interventions. Two studies have used LCGA to model HbA1c; both identified a subgroup of adolescents who displayed sharply deteriorating metabolic over time [4, 5]. These adolescents were more likely to come from single-parent families and to report greater conflict with friends, greater negative emotion, poorer adherence, and poorer general self-concept than those with other trajectories [4, 5]. However, risk factors [16] that are particularly important during adolescence, such as low parental involvement and poor psychosocial maturity, were not examined in relation to poor metabolic control trajectories. Further, there are inconsistencies between the studies that limit the generalizability of the findings (e.g., the deteriorating group started out with good HbA1c in one study, but poor HbA1c in the other). Because LCGA is “an exploratory, data-driven technique, and thus highly subject to chance relationships existing in a particular set of data” (Grimm [17], p. 124), results must be replicated across samples. The current study builds upon previous research in two important ways: first, we examined whether key facets of adolescent development - parental involvement and psychosocial maturity - predict metabolic control trajectories; second, we examined such trajectories in a third sample, lending support for the generalizability of previous findings and addressing prior inconsistencies.
The present study examined three specific aims. First, we identified distinct trajectories of metabolic control across adolescence. Second, we examined which aspects of parental involvement and psychosocial maturity predicted these metabolic control trajectories. Based on previous LCGA studies, we expected to identify at least two trajectories, one which would display sharp deterioration in metabolic control over time. We hypothesized that lower parental involvement and psychosocial maturity would characterize adolescents who demonstrated sharper deterioration in metabolic control. Third, we examined links between trajectories of metabolic control and health care utilization (i.e. diabetes-related emergency room visits and hospitalizations), to determine the clinical significance of different trajectories. We predicted that a trajectory characterized by sharp deterioration in metabolic control (vs. mild or no deterioration) would be associated with greater health care utilization.
Methods
Participants included 252 adolescents with type 1 diabetes (54.37% female) and their mothers enrolled in a longitudinal study of families’ adaptation to diabetes. The present study includes data collected during the first two years of participation. At baseline, adolescents were between 10 and 14 years of age (M= 12.49, SD= 1.52), had been diagnosed with diabetes an average of 4.74 years (SD= 2.96, range = 1 – 12 years), and were either on an insulin pump (51.20%) or prescribed multiple daily injections (M= 4.14 injections/day, SD= 1.81). Most participants were Caucasian (92.77%) and middle class (64.78%), reporting annual household incomes of $50,000 or more. To participate, adolescents had to live with their mother; 94.9% were biological mothers, 1.2% were adoptive mothers, and 2% were step-mothers. Most participants (79.9%) were from intact families; 2.8% of mothers reported being remarried, 9.8% were divorced, 4.3% were single or living with an unmarried partner, and .4% were widowed.
Participants were recruited from two sites (a university/private partnership clinic [76%] or a community-based private practice [24%]), which prescribed similar treatment regimens and followed similar clinic procedures. At recruitment, adolescents were given a set of measures to complete at home, and were told to complete the measures individually (i.e. without talking to parents). They returned these measures and completed additional measures during a laboratory visit an average of 12 days later. For this study, we analyzed psychosocial and behavioral measures administered at baseline, and clinical outcomes measured two years later. Glycated hemoglobin (HbA1c) values were drawn from medical records, and were available approximately every three months over the two-year period. All study procedures were reviewed and approved by the appropriate IRB. Parents provided written informed consent and adolescents provided written assent.
Measures
Parental involvement
Three measures were used to assess aspects of parental involvement in diabetes management (i.e. monitoring, acceptance, and frequency of help). For each measure, adolescents reported separately on mothers’ and fathers’ involvement. All adolescents reported on their mother, and most (98.02%) reported on fathers’ involvement. To assess parental monitoring, adolescents completed a five-item measure of parents’ knowledge of diabetes care behaviors [9] (e.g., “How much does your mother/father really know what your blood sugar readings are?”). Responses ranged from 1=doesn’t know to 5=knows everything (α=.90 for reports on mothers; α=.91 for reports on fathers). Adolescents also completed the five-item acceptance subscale of the Mother-Father-Peer Scale (MFP) [18], reporting on the quality of their relationships with their parents on a scale from 1=strongly disagree to 5=strongly agree (e.g., “My mother/father enjoys being with me”; α=.73 for reports on mothers; α=.83 for reports on fathers). Finally, adolescents reported on parents’ behavioral involvement in diabetes management using a one-item measure (Frequency of Help; FOH) developed for this study: “In an average week, how often does your mother/father help you with your diabetes?” Responses ranged from 0 days (never) to daily. This single item correlated well with the Diabetes Responsibility Scale (DRS) [19], a validated measure of parental behavioral involvement (r = .51 and .33 for FOH from mother and father, respectively). The DRS was not analyzed presently because it asks adolescents to report on parents as a unit rather than on mother/father separately.
Psychosocial Maturity
Self-reliance
Two measures assessed adolescents’ self-reliance. Adolescents completed the 5-item functional autonomy subscale from the Adolescent Autonomy Questionnaire [20], which captures the extent to which adolescents set and work toward goals (e.g., “I know how to achieve my goal”). As alpha was marginally acceptable (α=.59), we assessed whether this low reliability reflected the multidimensional nature of the scale. Maximized Lambda4, which is less influenced by dimensionality than alpha (Osburn, 2000), indicated an acceptable internal consistency (Max-λ4 = .78).
Adolescents also completed the Extreme Peer Orientation scale (EPO) [21], which measures a willingness to sacrifice positive development (e.g., avoid homework, ignore personal goals) in order to win peer approval [22, 23]. Research suggests that individuals with high EPO allow their behavior to be guided by the opinions of others [23], thus high scores on this measure reflect low self-reliance. The original four-item scale was adapted by adding three diabetes items (e.g., “would you ignore your diabetes management needs in order to make someone like you?”). Responses ranged from 1=never to 5=often; α=.66.
Self-control
Two measures assessed adolescents’ self-control. Adolescents completed a 7-item self control scale [24] (e.g., “I am good at resisting temptation”). Responses ranged from 1=not at all to 5=very much; α=.70.
Adolescents also completed the Youth Self-Report (YSR) [25], reporting their externalizing behaviors. Adolescents responded to 32 items assessing aggressive behavior (e.g., “I argue a lot”) and rule-breaking (e.g., “I drink alcohol without my parents’ approval”) on a scale from 0=not true to 2=very true. Aggressive and rule-breaking subscales were combined to form an index of externalizing behavior, α=.88.
Metabolic control
Adolescents’ metabolic control was indexed by glycated hemoglobin (HbA1c) values, which were obtained from medical records approximately every three months at the clinic visit using the Bayer DCA2000.
Health-care utilization
At the 2 year assessment, mothers reported in a questionnaire the number of times in the past six months adolescents had diabetes-related emergency room visits and hospitalizations (e.g., “how many times in the past six months has your child been hospitalized because of diabetes”?) [10]. Responses were recoded as 0=none or 1=at least one. Confirmation of mother reports was not possible because these events could occur at multiple medical centers other than those at which the study was conducted.
Statistical Analyses
LCGA, conducted in Mplus version 4.21, was used to analyze HbA1c data collected approximately every 3 months over a 2-year period [26]. Similar to a cluster analysis, LCGA groups individuals into classes based on similarities in growth trajectories (i.e. intercepts and slopes). We estimated one-, two-, and three-class models, using age as our marker of time. Age was centered at 13 years, near the mean of the sample.
At time 1, we enrolled 252 participants; at 6 months, we had retained 93.25% of the original sample; 88.89% were retained at 1 year, 84.52% at 1.5 years, and 80.95% at 2 years. We were also missing data as a result of participants skipping time points or missing clinic visits. Missing data were handled using full information maximum likelihood (FIML) estimation.
We evaluated the LCGA models in several ways. We used the Bayesian Information Criterion (BIC) to determine relative model fit. Smaller BIC values indicated a better-fitting model. We also conducted the Lo-Mendell-Rubin Likelihood Ratio Test when estimating the two- and three-class models. This test compared each model to a model with one fewer latent class. A p-value less than .05 indicated that the model with more classes was a better fit. Based on their patterns of growth in metabolic control, the LCGA procedure assigned people in the sample to a particular latent class (i.e. the class with the largest posterior probabilities). To determine how accurately each model placed people into classes, we examined entropy values and average probabilities (ranging from 0 to 1, with 1 indicating perfectly accurately classification).
After selecting the best model based on indicators of model fit and classification accuracy, we conducted independent samples t-tests, logistic regression analyses, and χ2 tests for independence to identify correlates of probable group assignment and indicators of diabetes-related health care utilization.
Results
Identifying Metabolic Control Trajectories
We estimated one-, two-, and three-class growth models. Results indicated that the two-class model was a better fit than the one-class model (i.e. lower BIC [6212.65 vs. 6663.49] and significant Lo-Mendell-Rubin Likelihood Ratio Test [coefficient=473.25, p<.01]). When comparing the two- and three-class models, the Lo-Mendell-Rubin Likelihood ratio test was not significant (coefficient=53.88, p=.08), and the BIC values were similar (the three-class model was slightly better: 6181.19 vs. 6212.65). These results indicated that the two- and three-class models fit the data equally well. However, entropy and average latent class probabilities were better for the two-class versus the three-class model (e.g. entropy2-class=.92 and entropy3-class=.79), indicating that the two-class model more accurately placed people into classes.
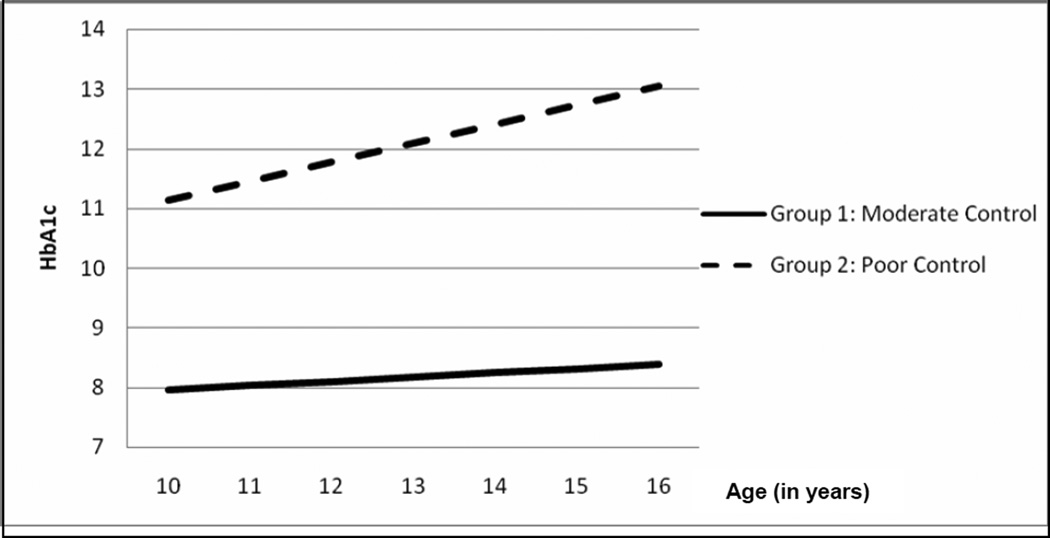
In the two-class model, the first latent class (Group 1) contained 92% of the sample (n = 231). Group 1 had moderate HbA1c values at age 13 (intercept = 8.18) and had a small, but significant, increase in HbA1c with age (slope = .07, p < .05). The second latent class (Group 2) contained 8% of the sample (n = 21). Group 2 demonstrated poor HbA1c values at age 13 (intercept = 12.09) and a sharp increase in HbA1c with age (slope = .32, p < .05). Growth trajectories are depicted in Figure 1.
Figure 1.
Longitudinal trajectories of metabolic control across adolescence.
Predicting Metabolic Control Trajectories
We conducted independent samples t-tests to compare the two latent classes across indicators of parental involvement and psychosocial maturity measured at the first time point (see Table 1). Adolescents in Group 2, characterized by poor and rapidly deteriorating metabolic control, reported significantly lower monitoring and assistance with daily diabetes management from their fathers and lower functional autonomy and lower self-control than those in Group 1. Adolescents in Group 2 had a tendency (p=.08) to report greater extreme peer orientation than those in Group 1. Results indicated that adolescents in the two groups were similar in their reported maternal involvement (acceptance, monitoring, and assistance with diabetes management) and paternal acceptance.
Table 1.
Comparing means and proportions across the latent classes
| Moderate control group | Poor control group | t | |||
|---|---|---|---|---|---|
| Variable at baseline | M | SD | M | SD | |
| Parental Involvement | |||||
| Maternal acceptance | 4.41 | .64 | 4.18 | .76 | 1.48 |
| Paternal acceptance | 4.25 | .82 | 4.09 | .75 | 0.77 |
| Maternal diabetes monitoring | 4.12 | .78 | 3.81 | .86 | 1.65 |
| Paternal diabetes monitoring | 3.09 | 1.03 | 2.34 | 1.28 | 2.75 ** |
| Maternal frequency of help | 4.58 | .88 | 4.50 | 1.04 | 0.34 |
| Paternal frequency of help | 3.52 | 1.47 | 2.76 | 1.79 | 2.01 * |
| Self-Reliance | |||||
| Functional autonomy | 3.53 | .55 | 3.15 | .46 | 2.84 ** |
| Extreme peer orientation | 2.56 | .51 | 2.79 | .53 | −1.76 † |
| Self-Control | |||||
| Self-control | 3.44 | .63 | 3.12 | .64 | 2.06 * |
| Externalizing behavior | 7.47 | 6.78 | 13.55 | 7.09 | −3.65 ** |
| Variable at 2 yearsa | % No | % Yes | % No | % Yes | c2 |
| Emergency room visit | 94.12 | 5.88 | 71.43 | 28.57 | 5.48 * |
| Hospitalization | 95.88 | 4.12 | 71.43 | 28.57 | 8.33 ** |
Reported diabetes-related clinical outcomes in the past six months.
p < .01;
p < .05;
p = .08.
To identify the unique contributions of each of our predictors to explaining metabolic control trajectories, we conducted a series of logistic regression analyses. In the first regression, we predicted trajectories (i.e. mild vs. sharp deterioration in metabolic control) from indicators of maternal and paternal involvement. After accounting for the variables’ shared variance, lower paternal monitoring remained a unique predictor of rapidly deteriorating metabolic control (p = .03), R2 =.098. In the second regression analysis, we examined associations between self-reliance and metabolic control trajectories. After controlling for extreme peer orientation, lower functional autonomy remained a unique predictor of poorer trajectories of metabolic control (p = .01); R2 = .096. Finally, in the third logistic regression, we predicted trajectories from indicators of self-control. Greater externalizing behavior continued to predict poorer trajectories of metabolic control after accounting for the effects of self-control (p = .01); R2 = .100.
Are Metabolic Control Trajectories Associated with Health Care Utilization?
χ2 tests for independence determined whether the two trajectories were associated with health care utilization measured at two years. For adolescents in Group 2 (poor and rapidly declining metabolic control), odds of having emergency room visits were 6.40 time greater (p < .05) than for adolescents in Group 1 (mild and slightly decreasing metabolic control) (28.57% vs. 5.88%). Adolescents in Group 2 were also 9.31 times more likely (p < .01) than those in Group 1 to be hospitalized due to diabetes (28.57% vs.4.12%) (see Table 1).
Discussion
Using LCGA, we identified two distinct trajectories of metabolic control across adolescence. The majority of adolescents in our sample displayed a trajectory with HbA1c values in the moderate range at age 13 (the intercept) and a small but significant increase in HbA1c over time. For these adolescents, HbA1c at age 13 (i.e. 8.18%) was above the American Diabetes Association’s recommended level of 7.5% [27], but remained below the 10% level that many have labeled “poor” HbA1c [28]. A second group, representing 8% of the sample, had a rapidly deteriorating metabolic control trajectory, with poor HbA1c at age 13 (i.e. 12.09%). Lower fathers’ involvement and lower psychosocial maturity predicted membership in the group that displayedpoor and rapidly deteriorating metabolic control, and this group also displayed greater health care utilization two years later.
It is notable that the trajectories of metabolic control identified here bear similarities to the two previous studies using LCGA to examine trajectories of adolescent metabolic control [4, 5]. In all three studies, most adolescents displayed a trajectory characterized by moderate control that slowly deteriorated over time, and a smaller subgroup displayed sharply deteriorating metabolic control. Luyckx and Seiffge-Krenke, however, found a third “optimal control” group (with good control that did not deteriorate over time) that we did not replicate, potentially because their participants were older than our adolescents.
A significant contribution of this study was the identification of lower fathers’ monitoring and behavioral involvement as predictors of severe deterioration in metabolic control across adolescence. Adolescents with poor and rapidly deteriorating metabolic control reported lower diabetes monitoring from fathers and lower paternal assistance with diabetes than those with mildly deteriorating metabolic control. In contrast, mothers’ involvement did not predict adolescents’ metabolic control trajectories. Low fathers’ involvement may be an indicator of poor overall family functioning (e.g., low cohesion or organization), which has been associated with paternal involvement and diabetes outcomes [5, 10]. However, the unique contribution of fathers’ involvement is consistent with previous cross-sectional findings [9]. This study highlights the importance of fathers’ involvement for adolescents’ diabetes outcomes, and suggests that future research should investigate why paternal involvement is so important for adolescents’ diabetes management.
Aspects of psychosocial maturity such as low self-control (especially externalizing behaviors) and low self-reliance (particularly low functional autonomy) were important predictors of metabolic control trajectories. These results are consistent with cross-sectional findings linking autonomy, impulse control, and externalizing behaviors with diabetes outcomes [2, 13], and with longitudinal findings linking general self-concept (which includes impulse control and environmental mastery) [4] and externalizing behaviors [2] with trajectories of metabolic control. Effective management of type 1 diabetes requires planning, monitoring, and controlling eating and exercise behavior, and completing daily tasks that are viewed as tedious and repetitive. Adolescents with poor self-reliance, who have difficulties setting and achieving personal goals, and adolescents with poor self-control, who have trouble resisting impulses and delaying gratification, may have a particularly difficult time with diabetes management tasks, resulting in poorer metabolic control over time.
Parental involvement and psychosocial maturity likely work together to influence diabetes outcomes during adolescence. When parents are involved in diabetes management, they may partially compensate for deficits in adolescents’ self-control and self-reliance. Further, characteristics of psychosocial maturity may increasingly predict poor metabolic control, as parents reduce their involvement and adolescents take on more responsibility for diabetes management [11]. Thus, psychosocial maturity is important to consider as parents and adolescents negotiate the transfer of diabetes responsibility. Less psychosocially mature adolescents may require parental assistance into late adolescence and early adulthood.
The results of this study have significant clinical implications. Researchers estimate approximately 15% of US health care expenditures are related to diabetes, with hospitalizations representing 40% of these costs [29]. We identified a group of adolescents with sharp deterioration in metabolic control who reported being 9.3 times more likely than others to be hospitalized due to diabetes complications. Although this was a relatively small group of adolescents (roughly 8% of the sample), the financial and health-related costs associated with poor metabolic control suggests they are a particularly important group to target for intervention. Previous cross-sectional studies of high-risk youth with poor metabolic control suggest that they have multiple risk factors across multiple systems (e.g., low SES, poor family environment, low peer support), and that they may benefit from multicomponent interventions [30]. This study adds to the growing literature on predictors of metabolic control among high-risk youth, and suggests that interventions to improve or compensate for these youth’s low paternal involvement and poor psychosocial maturity may improve their longitudinal trajectories of metabolic control across adolescence. Although paternal involvement and psychosocial maturity were important in this study for distinguishing those with very poor metabolic control trajectories from others, it is likely that these factors are also important for improving metabolic control in youth with more moderate (but still sub-optimal) metabolic control trajectories.
Results of this study should be interpreted in the context of some limitations. First, the sample was primarily Caucasian, middle class, and from intact families. Additional trajectories of metabolic control could be identified with a more diverse sample [31]. Second, our identification of important predictors of trajectories was limited by the variables that were included in the longitudinal study. In future research, other metrics of parental involvement (e.g., conflict, attachment, collaboration), or other measures of psychosocial maturity (e.g., future orientation) should be considered [32, 33]. Third, there were limitations to some of the measures used in this study. Our measure of parental monitoring reflects adolescents’ perceptions of how much parents know about adolescents’ diabetes management, and thus may reflect adolescents’ disclosure to parents, in addition to their perception of parents’ diabetes’ monitoring behavior. Future research on parental monitoring would benefit from the development and use of measures that better distinguish between adolescent disclosure, parents’ knowledge, and parents’ active attempts to monitor adolescents’ diabetes management. In this study, we used a single-item measure to assess maternal and paternal behavioral involvement in diabetes management. Although this measure exhibits moderate correlations with an existing validated measure of parental behavioral involvement [19], more information on this measure’s validity is needed. Diabetes-related hospitalizations and emergency room visits were measured in this study using mothers’ self-report. Although the validity of self-reported health-care utilization is unclear, known limitations of self-report data, such as vulnerability to recall errors or social desirability, suggest that future research may benefit from objective assessments of health care utilization.
In sum, this study provides an important contribution to the literature on trajectories of metabolic control across adolescence by highlighting the importance of targeting paternal involvement and psychosocial maturity in future interventions for adolescents with poor metabolic control.
Acknowledgments
This research was supported by Grant Number R01 DK-063044 from the National Institute of Diabetes and Digestive and Kidney Diseases, awarded to Dr. Deborah J. Wiebe and Dr. Cynthia A. Berg. We thank members of the ADAPT research group for their valuable input and assistance during the development and execution of this project. We also thank the physicians and staff at the Utah Diabetes Center and Mountain Vista Medicine, PC, as well as the adolescents and their families who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pamela S. King, University of Utah
Cynthia A. Berg, University of Utah
Jonathan Butner, University of Utah
Linda M. Drew, University of Texas Southwestern Medical Center
Carol Foster, University of Utah
David Donaldson, University of Utah
Mary Murray, University of Utah
Michael Swinyard, Mountain Vista Medicine, Salt Lake City, UT
Deborah J. Wiebe, University of Texas Southwestern Medical Center.
References
- 1.Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology. 2008;34(3):254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood. Diabetes Care. 2001;24(9):1536–1540. doi: 10.2337/diacare.24.9.1536. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 4.Luyckx K, Seiffge-Krenke I. Continuity and change in glycemic control trajectories from adolescence to emerging adulthood. Diabetes Care. 2009;32(5):797–801. doi: 10.2337/dc08-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, Siminerio L. Brief report: Trajectories of glycemic control over early to middle adolescence. Journal of Pediatric Psychology. 2010 doi: 10.1093/jpepsy/jsq011. (advanced access - still in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis DA, Podolski C-L, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007;32(8):907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- 7.Wiebe DJ, Berg CA, Korbel C, et al. Children's Appraisals of Maternal Involvement in Coping With Diabetes: Enhancing Our Understanding of Adherence, Metabolic Control, and Quality of Life Across Adolescence. Journal of Pediatric Psychology. 2005;30(2):167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- 8.Anderson BJ, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. The Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 9.Berg CA, Butler JM, Osborn P, et al. Role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008;31(4):678–683. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki T, Gavin L. Paternal Involvement in the Management of Pediatric Chronic Diseases: Associations with Adherence, Quality of Life, and Health Status. Journal of Pediatric Psychology. 2006;31(5):501–511. doi: 10.1093/jpepsy/jsj042. [DOI] [PubMed] [Google Scholar]
- 11.Wysocki T, Linschied TR, Taylor A, Yeates KO, Hough BS, Naglieri JA. Deviation from developmentally appropriate self-care autonomy. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]
- 12.Cauffman E, Steinberg L. (Im)maturity of judgment in adolescence: Why adolescents may be less culpable than adults. Behavioral Sciences & the Law. 2000;18(6):741–760. doi: 10.1002/bsl.416. [DOI] [PubMed] [Google Scholar]
- 13.Palmer DL, Berg CA, Wiebe DJ, et al. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29(1):35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- 14.Duke DC, Geffken GR, Lewin AB, Williams LB, Storch EA, Silverstein JH. Glycemic control in youth with type 1 diabetes: Family predictors and mediators. Journal of Pediatric Psychology. 2008;33(7):719–727. doi: 10.1093/jpepsy/jsn012. [DOI] [PubMed] [Google Scholar]
- 15.Nagin DS. Group-based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 16.Ellis DA, Naar-King S, Frey M, Templin T, Rowland M, Cakan N. Multisystemic treatment of poorly controlled type 1 diabetes: Effects on medical resource utilization. Journal of Pediatric Psychology. 2005;30(8):656–666. doi: 10.1093/jpepsy/jsi052. [DOI] [PubMed] [Google Scholar]
- 17.Grimm KJ, Ram N. A second-order growth mixture model for developmental research. Research in Human Development. 2009;6(2–3):121–143. [Google Scholar]
- 18.Epstein S. The Mother-Father Peer Scale. 1983 [Google Scholar]
- 19.Rubin RR, Young-Hyman D, Peyrot M. Parent-child responsibility and conflict in diabetes care. Diabetes. 1989;38(Suppl. 2):28A. [Google Scholar]
- 20.Noom MJ, Dekovic M, Meeus W. Conceptual analysis and measurement of adolescent autonomy. Journal of Youth and Adolescence. 2001;30:577–595. [Google Scholar]
- 21.Fuligni AJ, Eccles JS. Perceived parent-child relationships and early adolescents' orientation toward peers. Developmental Psychology. 1993;29:622–632. [Google Scholar]
- 22.Drew LM, Berg CA, Wiebe DJ. The mediating role of extreme peer orientation in the relationships between adolescent-parent relationship and diabetes management. Journal of Family Psychology. 2010;24(3):299–306. doi: 10.1037/a0019352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuligni AJ, Eccles JS, Barber BL, Clements P. Early adolescent peer orientation and adjustment during high school. Developmental Psychology. 2001;37:28–36. [PubMed] [Google Scholar]
- 24.Finkenauer C, Engels RCME, Baumeister RF. Parenting behaviour and adolescent behavioural and emotional problems: The role of self-control. International Journal of Behavioral Development. 2005;29(1):58–69. [Google Scholar]
- 25.Achenbach TM. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- 26.Nagin DS. Analyzing developmental trajectoris: A semiparametric group-based approach. Psychological Methods. 1999;4:139–157. [Google Scholar]
- 27.Association AD. Standards of medical care in diabetes. Diabetes Care. 2009;32(S1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wysocki T, Harris M, Greco P, et al. Randomized controlled trial of behavior therapy for families of adolescents with insulin dependent diabetes mellitus. Journal of Pediatric Psychology. 2000;25:23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- 29.Svoren BM, Butler D, Levine B-S, Anderson BJ, Laffel LMB. Reducing acute adverse outcomes in youths with Type 1 Diabetes: A randomized, controlled trial. Pediatrics. 2003;112(4):912–922. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 30.Naar-King S, Podolski C-L, Ellis DA, Frey MA, Templin T. Social ecological model of illness management in high-risk youths with type 1 diabetes. Journal of Consulting and Clinical Psychology. 2006;74(4):785–789. doi: 10.1037/0022-006X.74.4.785. [DOI] [PubMed] [Google Scholar]
- 31.Wang JT, Wiebe DJ, White PC. Developmental trajectories of metabolic control among White, Black, and Hispanic youth with type 1 diabetes. Journal of Pediatrics. 2011 doi: 10.1016/j.jpeds.2011.03.053. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Nansel TR, et al. Development and validation of the Collaborative Parent Involvement Scale for youths with type 1 diabetes. Journal of Pediatric Psychology. 2009;34(1):30–40. doi: 10.1093/jpepsy/jsn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised diabetes family conflict scale. Diabetes care. 2007;30(7):1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]