Abstract
Airway mucus in cystic fibrosis (CF) is highly elastic, but the mechanism behind this pathology is unclear. We hypothesized that the biophysical properties of CF mucus are altered because of neutrophilic oxidative stress. Using confocal imaging, rheology, and biochemical measures of inflammation and oxidation, we found that CF airway mucus gels have a molecular architecture characterized by a core of mucin covered by a web of DNA and a rheological profile characterized by high elasticity that can be normalized by chemical reduction. We also found that high levels of reactive oxygen species in CF mucus correlated positively and significantly with high concentrations of the oxidized products of cysteine (disulfide cross-links). To directly determine whether oxidation can cross-link mucins to increase mucus elasticity, we exposed induced sputum from healthy subjects to oxidizing stimuli and found a marked and thiol-dependent increase in sputum elasticity. Targeting mucin disulfide cross-links using current thiol-amino structures such as N-acetylcysteine (NAC) requires high drug concentrations to have mucolytic effects. We therefore synthesized a thiol-carbohydrate structure (methyl 6-thio-6-deoxy-α-D-galactopyranoside) and found that it had stronger reducing activity than NAC and more potent and fast-acting mucolytic activity in CF sputum. Thus, oxidation arising from airway inflammation or environmental exposure contributes to pathologic mucus gel formation in the lung, which suggests that it can be targeted by thiol-modified carbohydrates.
INTRODUCTION
Mucin polymers are the principal gel-forming proteins in mucus in the healthy lung (1, 2). The low elastic modulus (G′) of healthy airway mucus gels indicates a low density of mucin cross-links (3). Lightly cross-linked mucus gels are easily transported by the mucociliary escalator, but pathologic mucus in lung disease is not easily transported and accumulates to cause airflow obstruction, atelectasis, and lung infection (4, 5). Pathologic mucus is typically highly elastic and thought to occur as a downstream consequence of airway inflammation (4). Current concepts for how airway inflammation and airway mucus pathology are linked emphasize changes in mucin or DNA concentration as a mechanism of increased mucus elasticity. There has been little emphasis on how these polymers interact in mucus or how they might be modified extracellularly to alter the elastic behavior of mucus.
It is well known that naturally occurring polymers can be modified by oxidation and cross-linking, but surprisingly, little attention has been paid to whether mucins may be modified in this way in lung diseases such as cystic fibrosis (CF). Mucins have cysteine-rich domains in their N and C termini that mediate polymer extension by end-to-end di-sulfide linkage of mucin monomers, but cysteine-rich regions are also abundant as internal domains (1, 6, 7). These internal cysteine thiols may contribute to antioxidant effects of mucins, but we considered the possibility that oxidation could modify the biophysical properties of mucins by generating disulfide cross-links between internal cysteine domains. We reasoned that lightly cross-linked mucus gel in health may be maintained by the high levels of glutathione (GSH) and the low levels of oxidative stress in normal airway secretions (8, 9). We further reasoned that high levels of oxidative stress in CF mucus (10–12) would increase the oxidative cross linkage of mucins through disulfide bonds, thereby forming highly cross-linked and elastic mucus gels.
RESULTS
CF airway mucus gels have a molecular architecture characterized by a core of mucin covered by a web of DNA
We used confocal imaging to explore molecular architecture of DNA and mucin polymers in pathologic mucus gels in CF. Whereas mucins in induced sputum from healthy subjects form a discontinuous sheet of loosely networked polymers, mucins in sputum from CF patients form a continuous sheet of densely networked polymers that frequently form thick rope-like structures (Fig. 1A). DNA polymers are not abundant in healthy airway mucus where DNA is mainly confined to cells, but DNA polymers are highly abundant in CF, where they form a web-like structure that takes its shape from the underlying mucin core (Fig. 1A and video S1). Three-dimensional (3D) rendering of mucus in CF highlights how mucins form a densely compacted core in CF mucus gels (Fig. 1, B and C) and how DNA polymers create web-like structures that drape over the mucin core (Fig. 1, D to F, and video S2).
Fig. 1. Mucin and DNA biopolymers in mucus and effect of mucolytics.
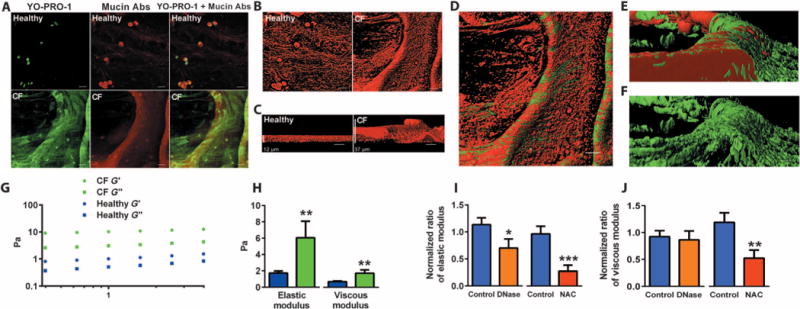
(A) Confocal image of sputum from healthy and CF subjects. Mucins (red) are present in healthy and CF sputum, whereas DNA (green) is more abundant in CF. Abs, antibodies. (B) 3D rendering of XY images of mucins. (C) Z-stack images of mucins. (D) Relationship between mucin and DNA polymers in CF mucus gel. (E) 3D rendering shows a dense mucin core with overlying DNA polymers. (F) DNA web with mucin core subtracted. (G) Typical frequency sweep of the elastic (G′) and viscous modulus (G″) of healthy (open symbols) and CF sputum (closed symbols), showing that G′ predominates over the G″ across a broad range of frequencies, indicating a viscoelastic gel. (H) Summary of average G′ and G″ (at a frequency of 1.0 Hz) in induced sputum from 15 healthy and 14 CF subjects. (I) Effects of treatment with mucolytics on G′ of induced sputum from 10 CF patients over a 4-hour test period. rhDNase (0.1 mg/ml) significantly reduced G′, but NAC (61 mM) had a larger effect (74 ± 10% reduction versus 34 ± 16%, P = 0.04). (J) Effects of treatment with mucolytics on the G″ of induced sputum from 10 patients with CF. rhDNase had little effect on G″, whereas NAC had a significant effect. The normalized ratio of elastic modulus refers to the posttreatment G′ divided by pretreatment G′. The effects of mucolytics on G′ and G″ are compared to normal saline. Scale bars, 20 mm (unless otherwise indicated). Data in (H) to (J) are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
The high elasticity of CF sputum can be markedly decreased by a reducing agent
Using rheology, we found that the elastic and viscous moduli of CF sputum were significantly higher than normal (Fig. 1, G and H), a finding consistent with other studies (13). The predominant rheological abnormality in CF sputum was a marked increase in its elastic response, indicative of increased cross-linking of the mucin polymers. The much smaller increase in viscous response argues against high mucin concentrations as the basis for the rheological signature. To determine the mechanism of mucus gel cross-linking in CF mucus, we measured the G′ of CF sputum before and after addition of N-acetylcysteine (NAC; a thiol-reducing agent) and recombinant human deoxyribonuclease (rhDNase). We found that both NAC and rhDNase decrease the G′ of CF sputum, but NAC is more effective than rhDNase at doses used clinically (Fig. 1, I and J).
CF sputum contains high concentrations of oxidized disulfide cross-links
Neutrophil oxidants are prominent in CF airways (10–12), and we considered that oxidative stress from neutrophilic inflammation could be the mechanism of excessive disulfide linkage of mucins in CF mucus. In detailed cellular and biochemical analyses of CF sputum, we confirmed marked neutrophilia and relative absence of eosinophilia (Fig. 2A), as well as high levels of myeloperoxidase (MPO) protein (Fig. 2B) and MPO activity (Fig. 2C). We also measured reactive oxygen species (ROS) in the sputum sol phase by incubating sputum with 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA), an oxidative stress indicator that is oxidized and rendered fluorescent in the presence of free radicals. We found that carboxy-H2DCFDA is readily converted to its oxidized form in CF sputum, indicating high levels of ROS (Fig. 2D). These ROS most likely arose from the high levels of MPO present in the sputum. Indeed, in mass spectrometry (MS)–based analyses of the CF sputum, we found more posttranslational modification of protein tyrosine residues through reactive halogen species (for example, chlorotyrosine and bromotyrosine) and tyrosyl radical (dityrosine) (Fig. 2E). This pattern of posttranslational oxidative modification in CF sputum is consistent with the activity of MPO, because MPO is the only known mammalian enzyme capable of generating reactive chlorinating species, and also with eosinophil peroxidase (EPO), these two enzymes uniquely contribute to the generation of brominating oxidants (14, 15). However, EPO is not relevant here because eosinophil numbers were low or absent in the CF sputum (Fig. 2A). Moreover, activated human neutrophils are known to use MPO to generate protein oxidative crosslinks through tyrosyl radical-dependent pathways forming protein-bound dityrosine (16). Therefore, the pattern of posttranslational oxidative modifications to the mucins that we describe is indicative of oxidative injury by the MPO–hydrogen peroxide system of activated neutrophils.
Fig. 2. Measurements of oxidative stress in CF mucus.
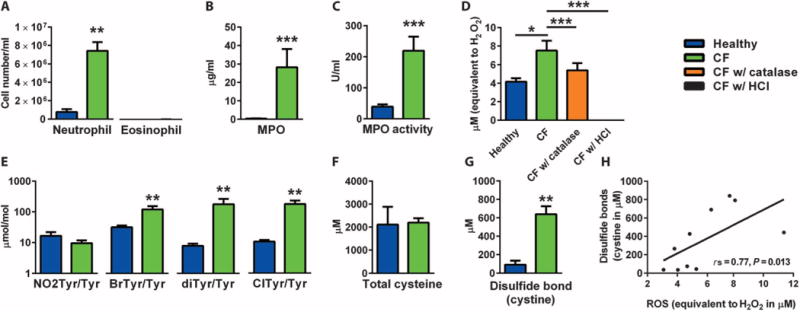
(A) Neutrophil cell number is significantly higher in induced sputum from patients with CF (n = 13) than in induced sputum from healthy subjects (n = 14). (B) MPO concentration measurements are higher in induced sputum from CF patients (n = 13) than in induced sputum from healthy controls (n = 15). (C) MPO activity measurements are higher in induced sputum from CF patients (n = 13) than in induced sputum from healthy controls (n = 15). (D) ROS, as detected by conversion of carboxy-H2DCFDA to its green fluorescent form, are markedly higher in spontaneously expectorated supernatant from CF patients (n = 5) than in induced sputum from healthy controls (n = 5). Hydrogen peroxide contributes significantly to the ROS signal, as evidenced by the reduction in ROS with catalase. The elimination of the ROS signal by HCl is included as a negative control of the enzyme-based assay. (E) Oxidized protein products measured using MS are higher in spontaneously expectorated supernatant from CF patients (n = 5) than in induced sputum from healthy controls (n = 5). (F) A fluorescent assay using dithiothreitol (DTT) and monobromobimane (mBBr) shows that the amount of total accessible cysteines is not significantly different between the spontaneously expectorated CF sputum (n = 5) and the induced healthy sputum (n = 5). (G) With iodoacetamide pre-treatment, the same assay shows that the concentration of cystines (disulfide bonds) is markedly higher in the CF sputum (n= 5) than in the healthy sputum (n = 5). (H) The concentration of disulfide bonds correlates positively with ROS. rs = 0.77, P = 0.013 [data from (D) and (F)]. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine whether the excess of ROS could convert cysteines in mucus to their oxidized disulfide products (cystines), we measured cysteine and cystine (disulfide bond) concentrations in CF sputum. Whereas cysteine concentration was not abnormal in CF (Fig. 2F), disulfide bond concentration in CF sputum was markedly higher than normal (Fig. 2G) and correlated with the levels of ROS in the same samples (Fig. 2H).
Oxidation increases the elasticity of healthy airway mucus
To directly establish a relationship between oxidation and disulfide-bonded mucin polymers, we exposed porcine gastric mucin (PGM) to dimethyl sulfoxide (DMSO). Gastric mucins in humans and pigs are encoded by MUC5AC and MUC6 genes (17, 18), whereas airway mucins are encoded by MUC5AC and MUC5B genes (1). MUC5AC, MUC5B, and MUC6 all share sequence similarities and common macromolecular characteristics, and all have regions rich in cysteine residues (Cys domains) (7). DMSO induces mild oxidation (19–21), and we found that it promoted a large increase in the elastic modulus of PGM (Fig. 3A) with a much smaller effect on its viscous modulus (Fig. 3B). By comparison, a fivefold increase in the concentration of PGM was required to similarly increase its elastic modulus (Fig. 3, A and B). Thus, exposure of mucin to an oxidation stimulus causes the same change in elasticity as large increases in mucin concentration. To confirm the oxidation effect of DMSO in human airway mucus, we exposed induced sputum from healthy subjects to DMSO and found that sputum elasticity increased significantly. This effect was caused by disulfide bridge formation, because it was prevented by iodoacetamide (Fig. 3C). These data indicate that oxidation of mucins generates disulfide bonds that alter the elasticity of mucus gels through mucin cross-linkage. Inflammation is not the only possible mechanism of oxidation of mucins in the lung. Inhaled oxidants such as ozone, cigarette smoke, and oxygen are another mechanism. The partial pressure of oxygen in air at sea level is 160 mmHg, but it is administered at partial pressures as high as 760 mmHg (100% oxygen at sea level) when used as a treatment for hypoxia. In these clinical situations, patients may experience poor mucus clearance and regional lung collapse (22–24). The mechanism of these mucus-related complications of oxygen is unknown. To explore whether oxygen provides an oxidizing stimulus to increase the elasticity of airway mucus, we modified a rheometer to permit exposure of mucus to oxygen or nitrogen in a closed system that controls humidity, temperature, and gas concentration (Fig. 3E). Using this system, we found that exposure of airway mucus to 100% oxygen gas caused an increase in the elastic modulus of induced sputum (Fig. 3F). In contrast, exposure of sputum to nitrogen gas had no significant effect. These data, coupled with those for DMSO above, lead us to propose that oxidation can cause disulfides to form between terminal cysteines to extend mucins or between internal cysteines to cross-link them (Fig. 3G).
Fig. 3. Oxidative stress causing excessive disulfide bond formation in mucus.
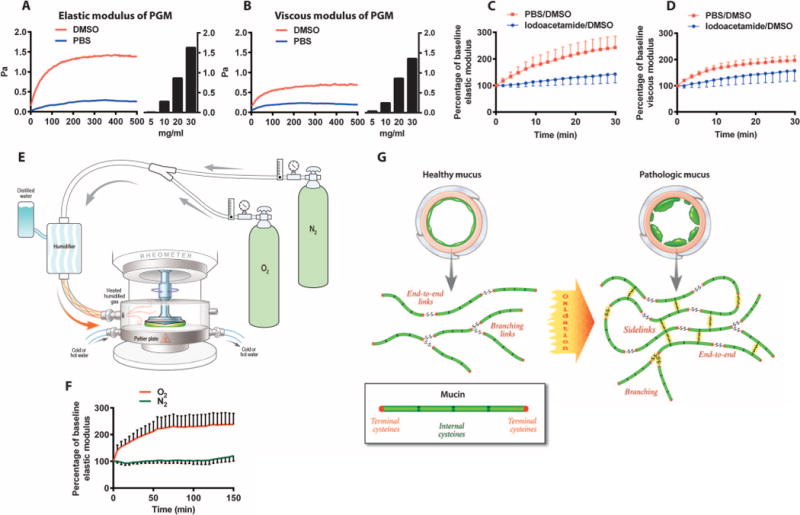
(A) Addition of DMSO [20% (v/v)] to PGM (5 mg/ml) rapidly increases its elastic modulus. An increase in the concentration of PGM from 5 to >20 mg/ml was required to have the same effect on G. (B) The effect of DMSO on the viscous modulus (G″) of PGM is smaller than that on G′ (C) Addition of DMSO [20% (v/v)] to induced sputum from healthy subjects causes a doubling in elastic modulus, an effect caused by disulfide bridge formation, because it is prevented by addition of iodoacetamide (50 mM). The area under the curve (AUC) of G′ for DMSO/phosphate-buffered saline (PBS) was significantly larger than that for DMSO/Iodoacetamide (5679 ± 974 versus 3619 ±589, P = 0.01). (D) DMSO had little effect on the viscous modulus of the induced sputum. (E) Schematic showing a cone and plate rheometer modified to permit exposure of mucus to oxygen or nitrogen in a closed system that controls humidity, temperature, and gas concentration. (F) Exposure of induced sputum samples from healthy subjects (n = 5) to 100% oxygen caused a time-dependent significant increase in elastic modulus, whereas exposure to nitrogen gas has no significant effect. The AUC of G′ for oxygen was significantly larger than that for nitrogen (31804 ±5507 versus 15391 ± 1539, P = 0.04). (G) Schematic representation for how healthy mucus can transition to pathologic mucus when oxidation promotes mucin chain extension through end-to-end disulfides and side-to-side cross-links of internal cysteines.
A thiol-saccharide is a stronger reducing agent than N-acetylcysteine and has more potent mucolytic activity
NAC is a reducing agent whose mucolytic efficacy is limited by relatively low potency, a “rotten egg” smell, and airway irritant effects (25, 26). We therefore considered the possibility that thiol-modified carbohydrates (“thiol-saccharides”) might be better reducing agents than NAC and candidates as novel mucolytic drugs. Carbohydrate scaffolds are polar, cheap, natural, and often crystalline, and offer easy access to analogs for structure-activity relationship (SAR) studies (27). The abundance of hydroxyl groups and chiral centers on carbohydrate scaffolds allows many possibilities for the introduction of a thiol group. We specifically synthesized a galactose modified with a thiol in the 6-position [methyl 6-thio-6-deoxy-α-D-galactopyranoside (TDG)] (Fig. 4A). To explore the potency of TDG as a reducing agent, we characterized its oxidation-reduction potential (ORP). ORP quantifies the tendency of a compound in solution to either gain or lose electrons to a new species. Specifically, the reduction potentials of aqueous solutions can be measured by quantifying the potential difference between an inert sensing electrode in contact with the solution and a stable reference electrode connected to the solution by a salt bridge. Compounds with lower (more negative) reduction potentials tend to reduce new species by donating electrons. We found that the ORP of TDG was more negative than NAC or the parent sugar for TDG [methyl α-D-galactopyranoside (MDG)] (Fig. 4B). We next compared the relative effects of high concentrations (61 mM) of TDG, NAC, and MDG on the elastic properties of CF sputum over a 12-min test period. We found that TDG has much larger mucolytic effects than NAC at 2 min and similar effects at 12 min (Fig. 4, C and D). The faster onset of action of TDG reflects its stronger reducing activity, and the similar effects of TDG and NAC at 12 min likely reflect the high doses of both compounds used in this experiment. Consistent with this interpretation, we found that a lower concentration of TDG (10 mM) had larger mucolytic effects than NAC in CF sputum at both 2 and 12 min (Fig. 4, E and F).
Fig. 4. A thiol-modified galactose is a novel and potent reducing agent with superior mucolytic activity.
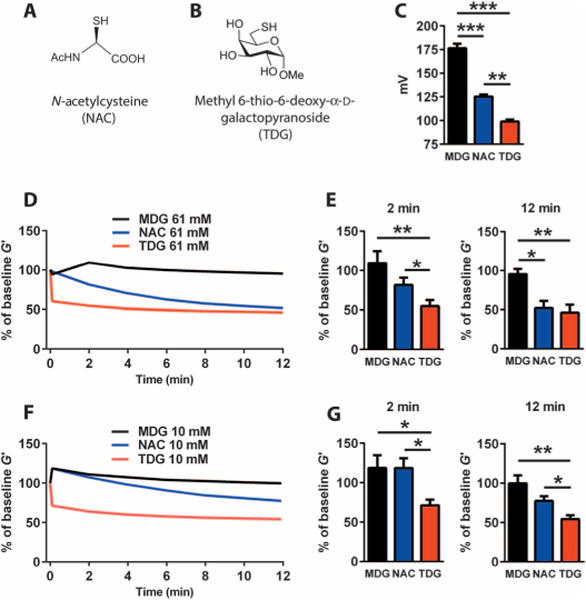
(A) Chemical structure of NAC. (B) Chemical structure of TDG. (C) The ORP of TDG is lower than that of NAC and of the parent sugar (MDG). (D) Effects of high concentrations (61 mM) of TDG, NAC, and MDG on the elastic properties of CF sputum (n = 5 donors) over a 12-min test period. (E) The mucolytic effect of TDG at 2 min is significantly larger than NAC and MDG; the mucolytic effects of TDG and NAC at 12 min are similar. (F) Effects of low concentrations (10 mM) of TDG, MDG, and NAC on the elastic properties of CF sputum (n = 5 donors) over a 12-min test period. (G) The mucolytic effects of TDG at 2 and 12 min are significantly larger than those of NAC and MDG. Data in (C) to (G) are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
To begin to assess the safety of TDG, we conducted exposure studies in mice. Specifically, we exposed mice intranasally to two concentrations of TDG or MDG daily for 5 days. Outcome measures of safety included body weight, renal function tests, measures of lung injury in bronchoalveolar lavage (BAL), and histologic appearance of tissue sections from formalin-fixed and paraffin-embedded lungs. Compared to MDG, TDG administration to the mice did not cause any discernible systemic or lung toxicity at either dose, as evidenced by significant differences from control in body weight, renal function tests, BAL cell counts or cell differential, BAL hemoglobin (Hb) concentration, or histologic appearance of lung sections from formalin-fixed and paraffin-embedded lungs (Table 1).
Table 1.
Effects of TDG on safety outcomes in mice.
| Variable [median (range)] | Control (MDG) | TDG (5 μg) | TDG (63 μg) | P (three-group comparison) |
|---|---|---|---|---|
| Body weight | 18.5 (17.4–19.6) | 18 (17.5–19) | 18.5 (17.6–19) | 0.666 |
| BAL data | ||||
| Total cell count | 30 (14–44) | 30 (25–50) | 38 (25–40) | 0.68 |
| Macrophage % | 99 (71.7–99.7) | 98.7 (98–98.7) | 99.7 (98–100) | 0.13 |
| Neutrophil % | 0 (0–9.3) | 0 (0–0) | 0 (0–0) | 1 |
| Lymphocyte % | 1 (0.3–19) | 1.3 (1.3–2) | 0.3 (0–2) | 0.13 |
| Eosinophil % | 0 (0–0) | 0 (0–0) | 0 (0–0) | 1 |
| Renal function tests | ||||
| Blood urea nitrogen | 24 (22–27) | 25 (23–29) | 21 (21–25) | 0.046 |
| Creatinine | 0.26 (0.24–0.29) | 0.26 (0.25–0.28) | 0.26 (0.25–0.26) | 0.71 |
DISCUSSION
Despite the clinical importance of pathologic mucus gels in a wide range of lung diseases, the mechanism for why these abnormal gels form so frequently is not well understood. To date, research has focused almost exclusively on mechanisms of hypersecretion of mucin polymers from mucin-secreting cells in the airway. There has been little focus on qualitative changes in mucin cross-linking and the mechanism of such changes. Here, we uncover a link between the oxidative stress accompanying marked airway inflammation in CF and the highly elastic mucus that is a central clinical problem in this disease. We provide direct experimental evidence for this link by showing that oxidative stimuli (either a mild oxidizing chemical or oxygen at high partial pressure) can markedly increase the elasticity of airway mucus from healthy subjects. We propose that this occurs because mucin chains are extended through end-to-end disulfides between terminal cysteines or are cross-linked through side-to-side disulfides between internal cysteines (Fig. 3G).
Our imaging and rheology data show that pathologic mucus in CF comprises a dense core of mucins and an outer envelope of DNA polymers. 3D rendering of confocal images of the mucus gel allowed us to clearly show separation of structures formed by mucin and DNA biopolymers within the gel. Specifically, mucins form the central core of mucus, with DNA biopolymers taking their separate shape and structure from the core mucin structure. We provide several lines of evidence that the densely compacted core of mucins visible in the CF mucus gels reflects increased mucin disulfide cross-links. First, the mucus is susceptible to mucolysis with a reducing agent that breaks disulfide bonds. In addition, whereas the concentration of disulfide cross-links (oxidized cysteine product, cystine) is very low in healthy sputum, it is markedly increased in CF sputum and correlates with measures of ROS in the same samples. Because mucins are the abundant source of cysteines, we conclude that oxidation of mucins in CF sputum causes an increase of disulfide cross-links as a mechanism for its high elasticity. Notably, oxidation of PGM using DMSO caused the same increase in elasticity as large increases in mucin concentration, emphasizing the importance of qualitative changes in mucins for their biomechanical properties. Such qualitative changes are also relevant in human airway mucus, because DMSO increased the elasticity of induced sputum from healthy subjects. We could prove that this effect was caused by disulfide bond formation in the sputum, because it was prevented by a chemical (iodoacetamide) that caps free thiols to prevent them from forming disulfide bonds.
To extend our findings from endogenous oxidants associated with lung inflammation to oxidants that occur in inhaled air, we examined the effect of oxygen on airway mucus elasticity. The partial pressure of oxygen in air at sea level is 160 mmHg, but it is administered at partial pressures as high as 760 mmHg (100% oxygen at sea level) when used as a treatment for hypoxia. Notably, in these clinical situations, it has been observed that patients experience poor mucus clearance and regional lung collapse. The mechanism of these mucus-related complications of oxygen is unknown. Here, we show that induced sputum exposed to oxygen becomes more elastic and that this effect does not occur with exposure to nitrogen gas. These data suggest that breathing oxygen at elevated partial pressures could alter the biophysical properties of airway mucus. Our findings draw attention to the importance of qualitative changes in mucin polymers, and specifically to excessive disulfide linkage of these polymers, in increasing the elasticity of the mucus gel. Although mucins are normally protected from oxidation by high airway levels of GSH relative to the oxidative stress levels, our work suggest that perturbation of this redox balance in inflammatory lung diseases has adverse implications for optimal cross-linking density of mucins and optimal elasticity of the mucus gel. One caveat to this conclusion is that continued input of host-derived GSH in vivo could lessen the oxidizing effects of environmental oxidants that we observed in our ex vivo system.
Recent studies in newborn CF piglets designed to explore the initiating mechanisms of decreased mucociliary transport (MCT) in the earliest phases of CF show that MCT is slowed after cholinergic stimulation and that CF submucosal glands secrete mucus that sometimes remains tethered to the gland ducts, hindering MCT (28). This MCT defect is not attributable to periciliary liquid depletion, because it persists when the airway surface is submerged in saline. These findings link impaired MCT to loss of CF transmembrane conductance regulator (CFTR) anion transport and suggest that defective MCT is a primary abnormality not dependent on infection, inflammation, or remodeling. These data do not preclude that inflammatory events that occur later in childhood and into adulthood (including progressive infection and inflammation) can also alter mucus gel elasticity to further disrupt MCT. Our data in established adult CF deal with the mechanisms of mucus gel pathology that occur in adults with established disease and compliment the findings about initiating mechanisms in abnormalities of MCT in CF, rather than challenging them.
Our data provide a strong argument for targeting disulfide linkages as a rational mucolytic strategy for mucus-associated diseases of the lung. Although we have focused our studies here on CF, it is known that asthma and chronic obstructive pulmonary disease are also associated with high levels of oxidative stress (29–32) and that these lung diseases are also associated with prominent airway mucus pathology. The occurrence of both oxidative stress and mucus pathology in multiple lung diseases suggests that reducing agents should be a broadly effective mucolytic strategy. NAC is a thiol-amino structure and reducing agent that is used clinically as a mucolytic therapy, but its clinical application is limited by multiple factors, including relatively low potency and requirement for high-dose delivery by nebulizer. In addition, it has a rotten egg smell and airway irritant effects (25, 26). Analyses of the efficacy of NAC in lung disease have shown limited mucolytic efficacy attributable to mucolysis (33–35) including in CF (34), but it is important to note that nearly all of these clinical trials of NAC have used an oral formulation. For example, a recently published comparison of oral NAC versus placebo administered three times daily in patients with CF showed no significant effect on outcomes of airway inflammation and only modest effects on lung function (36). Notably, the effects of NAC as a mucolytic was not assessed in this paper, and mucolysis would have been difficult to demonstrate because there is no penetration of orally administered NAC into airway secretions (37). Mucolysis requires that thiol-based mucolytics be administered by the inhaled route.
NAC is an acetylated sulfur-containing amino acid (Fig. 4A), and it has relatively weak reducing activity (38) that may reflect the intrinsic tendency of cysteine to retain electrons. Thus, targeting mucin disulfide cross-links using thiol-modified amino acid scaffolds is a limited strategy for achieving mucolytic effects. Because carbohydrate scaffolds have an abundance of hydroxyl groups for introduction of a thiol group, we considered the possibility that a thiol-carbohydrate structure might be a stronger reducing agent than NAC and might be more potent as a mucolytic. We synthesized a galactose structure modified with a thiol in the 6-position (TDG) and found that it has stronger reducing activity than NAC and more potent and fast-acting mucolytic activity in CF sputum. In addition, initial safety studies of TDG delivered to the airways of mice do not suggest any safety concerns, so that we propose thiol-modified carbohydrates as novel mucolytic treatments for mucus pathology in CF and other lung diseases characterized by oxidative stress and mucus pathology.
Together, our data should change current concepts for how airway inflammation and airway mucus pathology are linked. Mucin hypersecretion is currently viewed as the mechanism of increased mucus elasticity in inflammatory lung diseases, and this has driven treatment strategies that target the mucin hypersecretion pathway (39). This treatment approach is challenging because down-regulating mucin-based airway defense systems is both difficult to do and subject to adverse consequences. Our data show that modification of mucin polymers by oxidation can markedly increase their elastic behavior, and they support a relatively simple mucolytic strategy using thiol-modified carbohydrates as a novel and better approach than current approaches that rely on thiol-modified amino acids.
MATERIALS AND METHODS
Study design
The purpose of this study was to investigate the mechanism of highly elastic airway mucus gels in CF with a view to developing a rationally designed mucolytic treatment. Sputum samples were collected from CF subjects and healthy control subjects and compared using rheology, confocal microscopy, and detailed cellular and biochemical analysis, including MS. For comparison of sputum elasticity and viscosity in health and CF, the sample size was based on preliminary data from our laboratory, which indicated that sputum from 15 subjects per group would provide >90% power to detect significant increases in both elasticity and viscosity. For comparison of other outcomes of sputum pathology, we made measures in sputum from at least five healthy and five CF donors. Ex vivo experiments on sputum involved treatment with oxidizing stimuli and mucolytic compounds, including a thiol-modified saccharide synthesized by regioselective 6-O-tosylation of MDG followed by acetylation and displacement of the 6-O-tosylate with thioacetate to ultimately yield TDG. In vivo evaluation of the safety of TDG was done in mice administered drug intranasally in two doses versus MDG control. The sample size for the studies in mice was chosen to provide a preliminary guide about toxicity.
Study participants
Subjects with CF who met the CF Foundation criteria for the diagnosis of CF were recruited from the UCSF (University of California, San Francisco) adult CF center. Nonsmoking adult subjects with no history of lung disease were recruited as healthy controls using community advertising. Clinical details about these subjects are provided in table S1.
Sputum induction
A description of methods of sputum induction and sputum processing is provided in the Supplementary Materials. Total and differential cell counts in sputum were measured using methods previously described (40).
Rheology
Rheological measurements were made with AR2000 cone-and-plate rheometer (TA Instruments) using methods we have described previously (3). All airway mucus samples equilibrated to the testing temperature (4°C for all experiments except the humidified gas experiments in Fig. 3F and the comparison of mucolytics in Fig. 4, which were done at 37°C on the Peltier plate). We first performed strain sweeps at an oscillatory frequency of 1 Hz, followed by frequency sweeps from 0.1 to 50 Hz at 1 to 5% strain. These strains correspond to angular displacements of ~10−3 to 10−4 rads, well within the linear viscoelastic range for airway mucus. Testing samples within this range is crucial for probing the microstructure of the mucus gel at equilibrium to gently stress but not disrupt the gel cross-links and entanglements. Elastic (G′) and viscous (G″) moduli were calculated from the samples’ measured response to the oscillating angular displacement. All the frequency sweep data were collected at a strain of 5% and between 0.4 and 4 Hz. All summary data were from frequency sweeps performed at a strain of 5% at an angular frequency of 1 Hz (6.28 rads/s). All data from the time sweep data were collected at a strain of 5% at an angular frequency of 1 Hz during the time course. Normalized ratio was calculated by dividing the post-treatment measurement of G′/G″ by the pretreatment measurement of G′/G″. Percentage of baseline was calculated by comparing the current measurement to the initial baseline measurement at time 0.
Mucolytic treatments on sputum
In Fig. 1 (I and J), the effects of NAC and rhDNase on the G′/G″ of sputum from CF patients (compared with normal saline) were determined by treating whole sputum with 10% of the mucolytic by volume at 4°C and then incubating at 37°C for 4 hours. The measurements were made at the baseline and after the treatment/incubation. The chosen dose of NAC and rhDNase reflects those used clinically [NAC (100 mg/ml; Hospira) and rhDNase (1 mg/ml; Genentech)]. In Fig. 4 (C to F), the effects of the mucolytics on the G of sputum from CF patients were also determined by treating the whole sputum with 10% of the mucolytic by volume and monitored on the rheometer at 37°C for 12 min with humidified nitrogen protection. The measurements were made at the baseline and every 2 min after the treatment. The final concentration of 61 mM was chosen to match the clinically used NAC dose [10% (v/v) of 100 mg/ml].
Immunofluorescence
Details of sputum processing for imaging studies are described in the Supplementary Materials. Sputum slides were briefly rehydrated in water. Antigen retrieval was first performed in a warm citrate buffer for 5 min, followed by immersion in a glycine solution for further epitope retrieval for an additional 10 min. Sputum slides were blocked in a 10% normal goat serum (JR Scientific) and 2% immunoglobulin G (IgG)-free bovine serum albumin (BSA) (Sigma-Aldrich) solution for 1 hour at room temperature. Slides were incubated in a cocktail of MUC5AC/5B mouse anti-human IgG1 antibodies (table S2) at a final concentration of 0.5 μg/ml overnight at 4°C. After a series of brief washes, slides were incubated in a 2% IgG-free BSA solution with secondary antibody goat anti-mouse Cy3-conjugated F(ab′)2 fragments (Jackson ImmunoResearch) at a final concentration of 1.5 μg/ml and DNA stain YO-PRO-1 Iodide (Life Technologies) at a final concentration of 2 μM for 1 hour at room temperature. After a series of brief washes, slides were mounted with Fluoromount-G (SouthernBiotech), coverslipped, sealed with nail polish, and left to dry before imaging. Slides were imaged using an Olympus FluoView FV10i laser scanning confocal microscope. Z-stack images were collected at 0.75-μm intervals at 1024 × 1024 pixels using a 60× phase-contrast oil immersion objective numerical aperture 1.35 and 473-nm (12.5 mW)/559-nm lasers using a variable barrier filter mechanism to set the fluorescence channels to the appropriate detection wavelengths for fluorophores used. A 3D rendering of the confocal image was generated by the FluoView Navigator software. Additional processing of the image was performed in Imaris 7.6.0 (Bitplane) to reconstruct the 3D volumes and molecular architecture of both mucins and DNA in the sputum.
Measurement of ROS in sputum
Five healthy and five CF airway mucus samples were ultracentrifuged at 32,000 rpm at 4°C for 1 hour to separate sol phase and gel phase. The sol phase was collected and incubated with 10% (v/v) of the treatment [PBS for healthy and CF control, catalase (100 U/ml), 1 N HCl] at 37°C for 30 min. The samples were then incubated with 1 mM carboxy-H2DCFDA (Life Technologies) and esterase (10 U/ml) (Sigma-Aldrich) at 37°C for 15 min. Serial dilutions of 10 mM hydrogen peroxide (Sigma-Aldrich) in PBS were used as standards. The plate was read at 495-nm excitation/527-nm emission. A standard curve was plotted from the average relative fluorescence unit (RFU) representing the concentrations of the standards. The concentrations of ROS in samples were calculated against the standard curve. Stock solutions of hydrogen peroxide were calibrated by measuring the absorbance at 240 nm. Dividing this absorbance by 43.6 gives the stock concentration in molar units.
Measurement of MPO
MPO protein concentrations of sputum were measured by MPO DuoSet ELISA (R&D Systems). MPO activity was measured by using the NWLSS Myeloperoxidase Activity Assay (Northwest Life Sciences Specialties).
Measurement of total cysteines and cystines in sputum
Five healthy and five CF airway mucus samples were ultracentrifuged at 32,000 rpm at 4°C for 1 hour to separate sol phase and gel phase. The gel phase from the ultracentrifugation process was resuspended in 8 M guanidine-HCl (Sigma-Aldrich) to 10 times the original mucus volume. To measure cross-linked cysteine, the samples were pretreated with 10% (v/v) 500 mM iodoacetamide solution for 1 hour at room temperature. The samples were then incubated with 10% (v/v) of 1 M DTT (Sigma-Aldrich) at 37°C for 2 hours to quench the excessive iodoacetamide and reduce all the preexisting disulfide bonds. 7K MWCO Zeba desalting columns (Thermo Scientific) were used to remove all the small molecules, including quenched iodoacetamide, DTT, and GSH. To measure total accessible cysteine, the same samples were incubated with 10% (v/v) of 1 M DTT at 37°C for 2 hours to reduce all the disulfide bonds. 7K MWCO Zeba desalting columns were used to remove the DTT and any GSH. Samples were buffer-exchanged into 50 mM tris-HCl (pH 8.0) at the same time. Serial dilutions of standard from 5 mM L-cysteine (Sigma-Aldrich) were made in 50 mM tris-HCl (pH 8.0). One hundred microliters of samples and standards was put into black MaxiSorp microplate (Thermo Scientific). mBBr (2 mM) (Life Technologies) was diluted in 100 ml of the same buffer and added into samples and standards for a final concentration of 1 mM. After 15-min incubation at room temperature, the plate was read at 395-nm excitation/490-nm emission on a Synergy H1 Multi-Mode Microplate Reader (BioTek). A standard curve was plotted from the average RFU representing the concentrations of the standards. The thiol (cysteine) concentrations of samples were calculated against the standard curve. The concentration of total accessible cysteine and cystine (disulfide cross-linked cysteines) was calculated.
Mass spectrometry
For profiling of oxidized protein products in ultracentrifuged sputum samples (not treated with DTT), the methods were previously described (41). The content of protein-bound 3-nitrotyrosine, 3-chlorotyrosine, 3-bromotyrosine, and o,o′-dityrosine was determined by stable isotope dilution liquid chromatography-MS/MS analyses as previously described (42). Briefly, upon thawing, synthetic [13C6]-3-chlorotyrosine, [13C6]-3-bromotyrosine, [13C6]-3-nitrotyrosine, [13C12]-o,o′-dityrosine, and [13C9,15 N] tyrosine were added to samples and used as internal standards for quantification of 3-chlorotyrosine, 3-bromotyrosine, 3-nitrotyrosine, o,o′-dityrosine, and tyrosine, respectively. Simultaneous monitoring for the potential formation of artifactual 3-chlorotyrosine, 3-bromotyrosine, 3-nitrotyrosine, or o,o′-dityrosine (detected as corresponding [13C9,15 N] isotopologues) during sample preparation showed no detectable intrapreparative chlorination, bromination, nitration, or dityrosine cross-linking under the assay conditions used. MS studies were performed using a triple quadrupole API 5000 mass spectrometer (AB SCIEX) interfaced to an Aria LX Series HPLC (Cohesive Technologies).
Synthesis of TDG
Regioselective 6-O-tosylation of MDG followed by acetylation and displacement of the 6-O-tosylate with thioacetate gave a 6-SAc intermediate, which was deacetylated using sodium methoxide to yield TDG (for full experimental details, see Supplementary Materials and Methods).
Oxidation-reduction potential
ORP was measured using an S400 Seven Excellence meter with an InLab Redox Pro electrode (Mettler Toledo). NAC (Sigma-Aldrich), TDG, and MDG were dissolved in ultrapure distilled water (Life Technologies) at the concentration of 10 mM.
In vivo toxicology studies in mice
Fifteen 7-week-old C57BL/6 mice were purchased directly from The Jackson Laboratory. TDG was administered intranasally in two doses to separate groups of mice (n = 5 each). Dose #1 was based on the equivalent dose that would be delivered to humans as a 10 mM dose in 2.5 ml of nebulizer volume (adjusted for mouse/human differences in body weight, this equates to 5 μg in 30-μl volume). Dose #2 (high dose) was based on the same concentration that would be delivered to humans (63 μg in 30 μl) without correction for human/mouse body weight differences. Control mice (n = 5) were treated with a single dose of parent sugar compound (MDG)—5 μg in 30 μl of saline. Body weights were recorded on days 1, 5, and 8. On day 8, BAL was performed with 1 ml of ice-cold PBS + 2% fetal bovine serum. BAL (10 μl) was used to measure the Hb concentration using a Plasma/Low Hb Photometer (HemoCue AB). Fifty microliters was used to generate data for total and differential cell count. Mice were euthanized on day 8, and whole lung was fixed in 10% formalin and subsequently embedded into paraffin, sectioned, and stained using hematoxylin and eosin stain. Sera were obtained from blood collected by cardiac puncture from MDG-and TDG-treated mice and tested for renal function outcomes at the San Francisco General Hospital Clinical Laboratory. Animal experiments followed protocols approved by the UCSF Institutional Animal Care and Use Committee.
Statistics
Methods used for statistical analysis are detailed in the Supplementary Materials.
Supplementary Material
Table S1. Clinical characteristics of the healthy and CF subjects.
Table S2. Mucin antibodies.
Video S1. 3D architecture of CF sputum.
Video S2. 3D rendering of the architecture of CF sputum.
Acknowledgments
We thank Y. M. Chung for performing the MS profiling of oxidized protein products in CF sputum, K. Norsworthy for assistance with sputum collections, Z. Mekonnen for assistance with sputum cytology analysis, H. MacLeod for assistance with sputum preparation for immunofluorescence studies, M. Ongpin for statistical help, and C. Gralapp for illustrations.
Funding: Supported by R01HL080414, P50HL107191, and P01HL103453 from NIH and in part by an investigator-initiated grant from Genentech.
Footnotes
Author contributions: M.E.L.-S. performed the imaging studies of the airway mucus gels. S.C.K., S.O., and B.W. helped design the experiments. E.M.D. managed collection of sputum samples and characterization of study subjects. B.M.D. customized the rheometer to permit controlled gas exposures. S.O. and M.H. designed and synthesized the thiol-modified carbohydrate compound. S.G. assisted in sputum sample preparation. S.C.E. and S.D.C. assisted in the design of the study and edited the manuscript. S.L.H. directed the MS analysis and edited the manuscript. S.Y. performed the rheological analyses, measures of oxidative stress, and measures of cysteine oxidation. X.H. did the toxicity studies of TDG in mice. S.Y., S.O., and J.V.F. designed the study, analyzed the data, and wrote the manuscript.
Competing interests: S.Y., S.D.C., S.O., and J.V.F. are inventors on a patent for thiol-saccharides as novel mucolytic drugs. The other authors declare no competing interests.
Materials and Methods
REFERENCES AND NOTES
- 1.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 2.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innes AL, Carrington SD, Thornton DJ, Kirkham S, Rousseau K, Dougherty RH, Raymond WW, Caughey GH, Muller SJ, Fahy JV. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–210. doi: 10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King M. Physiology of mucus clearance. Paediatr Respir Rev. 2006;7(Suppl. 1):S212–S214. doi: 10.1016/j.prrv.2006.04.199. [DOI] [PubMed] [Google Scholar]
- 6.Ambort D, van der Post S, Johansson ME, Mackenzie J, Thomsson E, Krengel U, Hansson GC. Function of the CysD domain of the gel-forming MUC2 mucin. Biochem J. 2011;436:61–70. doi: 10.1042/BJ20102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 9.Cross CE, van der Vliet A, O’Neill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ Health Perspect. 1994;102(Suppl. 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hays SR, Fahy JV. Characterizing mucous cell remodeling in cystic fibrosis: Relationship to neutrophils. Am J Respir Crit Care Med. 2006;174:1018–1024. doi: 10.1164/rccm.200603-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Vliet A, Cross CE. Phagocyte oxidants and nitric oxide in cystic fibrosis: New therapeutic targets? Curr Opin Pulm Med. 2000;6:533–539. doi: 10.1097/00063198-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 12.van der Vliet A, Nguyen MN, Shigenaga MK, Eiserich JP, Marelich GP, Cross CE. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen H, Hvidt S, Sheils CA, Janmey PA. Elastic contributions dominate the visco-elastic properties of sputum from cystic fibrosis patients. Biophys Chem. 2004;112:193–200. doi: 10.1016/j.bpc.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidasegenerated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 16.Jacob JS, Cistola DP, Hsu FF, Muzaffar S, Mueller DM, Hazen SL, Heinecke JW. Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J Biol Chem. 1996;271:19950–19956. doi: 10.1074/jbc.271.33.19950. [DOI] [PubMed] [Google Scholar]
- 17.Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 18.Sturmer R, Muller S, Hanisch FG, Hoffmann W. Porcine gastric TFF2isamucus constituent and differs from pancreatic TFF2. Cell Physiol Biochem. 2014;33:895–904. doi: 10.1159/000358662. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Zhou L, Chen L, Dastidar SG, Verma C, Li J, Tan D, Beuerman R. Effect of structural parameters of peptides on dimer formation and highly oxidized side products in the oxidation of thiols of linear analogues of human β-defensin 3 by DMSO. J Pept Sci. 2009;15:95–106. doi: 10.1002/psc.1100. [DOI] [PubMed] [Google Scholar]
- 20.Snow JT, Finley JW, Friedman M. Oxidation of sulfhydryl groups to disulfides by sulfoxides. Biochem. Biophys Res Commun. 1975;64:441–447. doi: 10.1016/0006-291x(75)90272-7. [DOI] [PubMed] [Google Scholar]
- 21.Tam J, Wu C, Liu W, Zhang J. Disulfide bind formation in peptides by dimethyl sulfoxide. Scope and applications. J Am Chem Soc. 1991;113:6657–6662. [Google Scholar]
- 22.Edmark L, Auner U, Enlund M, Ostberg E, Hedenstierna G. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia. Acta Anaesthesiol Scand. 2011;55:75–81. doi: 10.1111/j.1399-6576.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 23.Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care. 2011;15:305. doi: 10.1186/cc10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reber A, Engberg G, Wegenius G, Hedenstierna G. Lung aeration. The effect of pre-oxygenation and hyperoxygenation during total intravenous anaesthesia. Anaesthesia. 1996;51:733–737. doi: 10.1111/j.1365-2044.1996.tb07885.x. [DOI] [PubMed] [Google Scholar]
- 25.Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur Respir Rev. 2010;19:127–133. doi: 10.1183/09059180.00003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuta A, Baraniuk JN. Therapeutic approaches to mucus hypersecretion. Curr Allergy Asthma Rep. 2005;5:243–251. doi: 10.1007/s11882-005-0044-6. [DOI] [PubMed] [Google Scholar]
- 27.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50:1209–1227. [PubMed] [Google Scholar]
- 28.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA, Welsh MJ. Cystic fibrosis. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comhair SA, Erzurum SC. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. quiz 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacNee W. Oxidants/antioxidants and COPD. Chest. 2000;117:303S–317S. doi: 10.1378/chest.117.5_suppl_1.303s-a. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson M, Sugumar K, Milan SJ, Hart A, Crockett A, Crossingham I. Mucolytics for bronchiectasis. Cochrane Database Syst Rev. 2014;5:CD001289. doi: 10.1002/14651858.CD001289.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;7:CD007168. doi: 10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y, Cai W, Lei S, Zhang Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD. 2014;11:351–358. doi: 10.3109/15412555.2013.858315. [DOI] [PubMed] [Google Scholar]
- 36.Conrad C, Lymp J, Thompson V, Dunn C, Davies Z, Chatfield B, Nichols D, Clancy J, Vender R, Egan ME, Quittell L, Michelson P, Antony V, Spahr J, Rubenstein RC, Moss RB, Herzenberg LA, Goss CH, Tirouvanziam R. Long-term treatment with oral N-acetylcysteine: Affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. J Cyst Fibros. 2014 doi: 10.1016/j.jcf.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Cotgreave IA, Eklund A, Larsson K, Moldeus PW. No penetration of orally administered N-acetylcysteine into bronchoalveolar lavage fluid. Eur J Respir Dis. 1987;70:73–77. [PubMed] [Google Scholar]
- 38.Gibson KR, Neilson IL, Barrett F, Winterburn TJ, Sharma S, MacRury SM, Megson IL. Evaluation of the antioxidant properties of N-acetylcysteine in human platelets: Pre-requisite for bioconversion to glutathione for antioxidant and antiplatelet activity. J Cardiovasc Pharmacol. 2009;54:319–326. doi: 10.1097/FJC.0b013e3181b6e77b. [DOI] [PubMed] [Google Scholar]
- 39.Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, Criner GJ, Kim V, Prasse A, Nivens MC, Tetzlaff K, Heilker R, Fahy JV. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/rccm.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 40.Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9:2448–2453. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 41.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Citardi MJ, Song W, Batra PS, Lanza DC, Hazen SL. Characterization of oxidative pathways in chronic rhinosinusitis and sinonasal polyposis. Am J Rhinol. 2006;20:353–359. doi: 10.2500/ajr.2006.20.2858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics of the healthy and CF subjects.
Table S2. Mucin antibodies.
Video S1. 3D architecture of CF sputum.
Video S2. 3D rendering of the architecture of CF sputum.


