ABSTRACT
Misincorporation of d-tyrosine (d-Tyr) into cellular proteins due to mischarging of tRNATyr with d-Tyr by tyrosyl-tRNA synthetase inhibits growth and biofilm formation of Bacillus subtilis. Furthermore, many B. subtilis strains lack a functional gene encoding d-aminoacyl-tRNA deacylase, which prevents misincorporation of d-Tyr in most organisms. B. subtilis has two genes that encode tyrosyl-tRNA synthetase: tyrS is expressed under normal growth conditions, and tyrZ is known to be expressed only when tyrS is inactivated by mutation. We hypothesized that tyrZ encodes an alternate tyrosyl-tRNA synthetase, expression of which allows the cell to grow when d-Tyr is present. We show that TyrZ is more selective for l-Tyr over d-Tyr than is TyrS; however, TyrZ is less efficient overall. We also show that expression of tyrZ is required for growth and biofilm formation in the presence of d-Tyr. Both tyrS and tyrZ are preceded by a T box riboswitch, but tyrZ is found in an operon with ywaE, which is predicted to encode a MarR family transcriptional regulator. Expression of tyrZ is repressed by YwaE and also is regulated at the level of transcription attenuation by the T box riboswitch. We conclude that expression of tyrZ may allow growth when excess d-Tyr is present.
IMPORTANCE Accurate protein synthesis requires correct aminoacylation of each tRNA with the cognate amino acid and discrimination against related compounds. Bacillus subtilis produces d-Tyr, an analog of l-Tyr that is toxic when incorporated into protein, during stationary phase. Most organisms utilize a d-aminoacyl-tRNA deacylase to prevent misincorporation of d-Tyr. This work demonstrates that the increased selectivity of the TyrZ form of tyrosyl-tRNA synthetase may provide a mechanism by which B. subtilis prevents misincorporation of d-Tyr in the absence of a functional d-aminoacyl-tRNA deacylase gene.
INTRODUCTION
Bacteria must appropriately aminoacylate each tRNA with the correct amino acid in order to maintain accurate translation (1). It was shown previously that growth of Bacillus subtilis is inhibited in the presence of d-tyrosine (d-Tyr) as a result of mischarging of tRNATyr with d-Tyr by tyrosyl-tRNA synthetase and subsequent incorporation into proteins (2). Misincorporation of d-Tyr into proteins also inhibits biofilm formation, as a result of growth inhibition, in undomesticated strains of B. subtilis (3; see also the accompanying paper by Leiman et al. [4]). d-Tyr competitively inhibits prephenate dehydrogenase, which is involved in biosynthesis of l-tyrosine (l-Tyr) (5). Therefore, the cellular concentration of l-Tyr decreases in the presence of d-Tyr, which increases the probability that tRNATyr will be mischarged with d-Tyr by tyrosyl-tRNA synthetase (6).
d-Tyr is produced by B. subtilis during stationary phase, which influences peptidoglycan biosynthesis (7). Furthermore, Bacillus species produce antibiotic peptides such as iturin and bacillomycin that contain d-Tyr (8). Many organisms prevent misincorporation of d-Tyr during protein synthesis by expressing the dtd gene, which encodes d-aminoacyl-tRNA deacylase, an enzyme that removes d-Tyr from tRNATyr (2, 9, 10). However, many laboratory strains of B. subtilis contain mutations in the dtd gene (3).
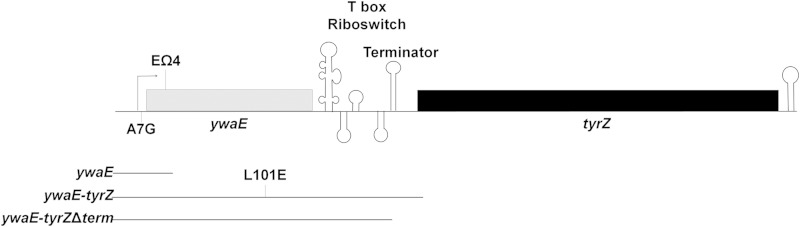
B. subtilis has two tyrosyl-tRNA synthetase-encoding genes: tyrS is expressed during vegetative growth, and tyrZ is known to be expressed only when tyrS is inactivated by mutation (11, 12). We hypothesized that TyrZ may allow the cell to maintain accurate translation when d-Tyr is present. The tyrZ gene is located downstream of the ywaE gene, which is predicted to encode a MarR family transcriptional regulator (13), and tyrZ is predicted to be cotranscribed with ywaE (Fig. 1).
FIG 1.
ywaE-tyrZ operon organization and mutations. The ywaE gene is depicted by a gray box, and the tyrZ gene is depicted by a black box. The T box riboswitch is depicted by a series of stem-loops between the ywaE and tyrZ genes, with the intrinsic transcriptional terminator labeled. The promoter is depicted by an arrow, and a terminator helix for the operon is depicted by a stem-loop. The locations of the spontaneous mutations in the ywaE gene used in the study (EΩ4 and EopA7G) are indicated. The DNAs included in the lacZ reporter fusions are indicated below the operon. The location of the L101E mutation is indicated in the fusion for the ywaEL101E-tyrZ-lacZ fusion. All fusions were constructed in the absence (wild type) or presence of the A7G mutation.
Members of the MarR family of transcriptional regulators regulate many different genes involved in metabolic pathways, stress responses, virulence, and transport of harmful compounds (13–17). These regulators typically repress transcription initiation by binding to a palindromic (or pseudopalindromic) sequence that overlaps the promoter. DNA binding by the transcriptional regulator is often inhibited by binding of an anionic phenolic compound to the protein (18–20). The predicted cotranscription of ywaE and tyrZ raised the possibility that YwaE could regulate tyrZ transcription initiation.
Both tyrS and tyrZ are preceded by a T box riboswitch (12), which is located in the leader region of the monocistronic tyrS gene and between ywaE and tyrZ, respectively. Expression of genes regulated by a T box riboswitch is dependent on direct interaction between the riboswitch and the cognate uncharged tRNA, which determines whether the RNA folds into the helix of an intrinsic transcriptional terminator or a competing antiterminator (21). A triplet sequence in the riboswitch, termed the specifier sequence, which corresponds to the codon for the amino acid related to the downstream gene, pairs with the anticodon of either the charged or uncharged forms of the cognate tRNA (21–23). The free acceptor end of the uncharged tRNA binds to and stabilizes the antiterminator structure, which prevents terminator formation (22). Charged tRNA is unable to stabilize the antiterminator, which allows formation of the more stable intrinsic transcriptional terminator, resulting in premature termination of transcription (22).
The goal of the present study was to investigate the physiological role of the B. subtilis tyrZ gene. We hypothesized that TyrS and TyrZ might have different kinetic properties with respect to incorporation of d-Tyr and l-Tyr. We tested whether the ability to express tyrZ affects inhibition of growth and biofilm formation by d-Tyr. In addition, genetic analysis of the ywaE-tyrZ operon was conducted to investigate the regulation of tyrZ expression.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in the study are listed in Table S1 in the supplemental material. B. subtilis strains were grown in 2× YT broth (24), tryptose blood agar base (TBAB; Difco), or Spizizen minimal medium with glucose as a carbon source (25). Biofilm-forming strains of B. subtilis were grown in modified MSgg minimal medium with glycerol as a carbon source (3, 26). Escherichia coli strains used for plasmid propagation and protein purification were grown in LB medium (24). Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml; kanamycin, 30 μg/ml; neomycin, 5 μg/ml; erythromycin, 1 μg/ml; and chloramphenicol, 5 μg/ml for selection or 0.1 μg/ml for induction. Required amino acids were supplied at 50 μg/ml. Growth was carried out at 37°C, unless otherwise stated.
Genetic techniques.
PCRs were carried out using Taq DNA polymerase (Invitrogen). Chromosomal DNA from strain BR151 or mutant derivatives was used as the template for PCR, unless otherwise stated. All restriction enzymes and T4 DNA ligase were supplied by New England BioLabs and used according to the manufacturer's recommendations. Plasmid DNA was purified using Wizard columns (Promega).
All lacZ reporter fusions were constructed using plasmid pFG328 (21) and PCR to generate insert DNA fragments. Oligonucleotide primers (Integrated DNA Technologies) used for PCR are listed in Table S2 in the supplemental material. Constructs were inserted into the BamHI and XbaI sites of pFG328 and verified by DNA sequencing (Genewiz). The ywaEL101E-tyrZ-lacZ fusion was generated by site-directed mutagenesis using plasmid pFG328-ywaE-tyrZ as the template. Plasmids were introduced into XL2-Blue ultracompetent cells (Stratagene) by transformation per the manufacturer's recommendations, with selection for ampicillin resistance.
B. subtilis strains were complemented with a functional dtd gene in which the mutant AAG lysine codon was replaced with the AUG start codon. Upstream and downstream fragments, generated by PCR, were used as the PCR template of the final dtd gene product (see Table S2 in the supplemental material), which was inserted into the BamHI and XbaI sites of plasmid pFG328 to generate plasmid pFG328-dtd.
All lacZ reporter gene fusions and plasmid pFG328-dtd were introduced into the SPβ genome by transformation of strain ZB370A (27). Transducing phage was purified by passage through strain ZB449 and introduced into the appropriate background strain by transduction, with selection for chloramphenicol or erythromycin resistance.
The coding regions of B. subtilis tyrS and tyrZ were amplified by PCR (see Table S2 in the supplemental material) and inserted into the NcoI and XhoI sites of the expression vector pET33b+ (Novagen), which adds a C-terminal His6 tag to each enzyme for purification. Plasmids were inserted into BL21(DE3) cells by transformation with selection for kanamycin resistance.
Overexpression and purification of TyrS and TyrZ.
BL21(DE3) cells harboring the appropriate plasmid were grown overnight statically at 37°C and diluted 170-fold with fresh LB medium containing the appropriate antibiotic. Cells were grown at 37°C with agitation until the A600 reached ∼0.6. Isopropyl-β-d-thiogalactopyranoside (1 mM) was added, and cells were harvested after 3 h of growth at 37°C. His6-tagged proteins were purified according to the Qiagen QIAexpressionist high-stringency protocol. Purification of TyrZ required 600 mM NaCl in the lysis and wash buffers. Purified proteins were dialyzed and stored in 50 mM Tris-HCl (pH 7.5), 140 mM KCl, 10 mM MgCl2, 5 mM 2-mercaptoethanol, and 0.2 μg/ml bovine serum albumin (BSA). Protein concentration was determined using the Bio-Rad protein assay and was 840 μg/ml for TyrS and 1.2 mg/ml for TyrZ. Denaturing protein gel analysis revealed that the proteins exhibited the molecular weight expected of monomers and that purity was >80%.
Active site titration assay.
The concentration of active TyrS and TyrZ was determined by incubating TyrS (4.2 μg) and TyrZ (6.0 μg) at 37°C for 10 min with 50 μM [14C]tyrosine (482 mCi/mmol; PerkinElmer) in the presence of 100 mM HEPES (pH 7.2), 30 mM KCl, 10 mM MgCl2, 5 mM ATP, and 1.1 μl pyrophosphatase (200 U/ml; Roche). The reaction mixture was passed through a nylon filter (Whatman Protran BA85; 0.45-μm pore size, 25-mm diameter) under vacuum and then washed with 3 ml wash buffer (50 mM HEPES [pH 7.2], 15 mM KCl, 5 mM MgCl2). The filter was dried at 85°C followed by liquid scintillation counting. The active concentrations of TyrS and TyrZ were 3.7 μM and 8.0 μM, respectively.
ATP-32PPi exchange assay.
TyrS (50 nM) and TyrZ (75 to 100 nM) were incubated with the amino acid indicated in 100 mM HEPES (pH 7.5), 140 mM KCl, 10 mM MgCl2, 2 mM NaF, 10 mM dithiothreitol (DTT), 2 mM ATP, and 2 mM 32PPi (3 to 5 cpm/pmol; PerkinElmer) in a total volume of 110 μl. The concentration of l-Tyr ranged from 0.3 to 250 μM for TyrS and 1 to 3,000 μM for TyrZ, and d-Tyr ranged from 1 to 2,000 μM for TyrS and 300 to 3,500 μM for TyrZ. At 0.5- to 1-min intervals, samples (25 μl) of the reaction mixture were removed and added to 970 μl of a quenching solution (1% charcoal, 5.6% perchloric acid, 75 mM NaPPi). The reaction mixture was filtered through 3-mm Whatman cellulose chromatography filter paper discs (2.4-cm diameter) under vacuum and then washed with 15 ml of water and 5 ml of 95% ethanol. The filters were dried at 85°C followed by liquid scintillation counting. Kinetic parameters were determined by fitting the data to Michaelis-Menten kinetics by nonlinear regression (Kaleidagraph; Synergy Software) and reported as averages ± standard errors of three independent repeats.
Insertional inactivation of tyrS and tyrZ.
Plasmid pBLG1Neo, a derivative of plasmid pMMN13 (28), contains a fragment of tyrS extending from 327 to 984 bp downstream of the AUG start codon. The neomycin resistance cassette from pBEST501 (29) was inserted at the BclI site 617 bp downstream of the AUG in tyrS. The plasmid was introduced into strain BR151 by transformation. Transformants were selected for neomycin resistance and screened for chloramphenicol sensitivity to generate BR151-SKO strains. A similar strategy was used to construct plasmid tyrZ-pMMNneo to generate strain BR151-ZKO (30). Strains NCIB 3610-ZKO and NCIB 3610-SKO were constructed by SPP1 phage transduction and BR151-ZKO or BR151-SKO-EΩ4 as donor strain, respectively, with selection for neomycin resistance (31). To restore the tyrS gene and generate strains BR151-EopA7G and BR151-EΩ4, chromosomal DNA from strain TyrSupX, in which the cat resistance cassette is inserted into the acsA gene upstream of tyrS (32), was introduced into strains BR151-SKO-EopA7G and BR151-SKO-EΩ4 by transformation. Transformants were selected for chloramphenicol resistance and screened for neomycin sensitivity. Strain 3610-EV164E was constructed by SPP1 transduction using strain TyrSupX (32) as the donor strain and 3610-SKO as the recipient strain with selection for chloramphenicol resistance. Expression of tyrZ was confirmed by reverse transcription-PCR (RT-PCR) (see Fig. S1 in the supplemental material).
β-Galactosidase assays.
B. subtilis cells harboring lacZ fusions were grown until early exponential phase in Spizizen minimal medium (25). Cells were harvested and resuspended in fresh medium in the presence or absence of 2 μg/ml 4-amino-l-phenylalanine (4-ALP), to induce tyrosine starvation. Samples were collected after 3 h of incubation, permeabilized by toluene, and assayed as described by Miller (24). Growth and β-galactosidase assays were repeated at least 3 times.
Zone-of-growth-inhibition assays.
Strains were grown in Spizizen minimal medium to exponential phase and then diluted 30-fold. A lawn of bacterial growth was generated by combining 0.1 ml of diluted culture with 3 ml of minimal medium solidified with 1% Bacto agar (Difco), and the mixture was poured onto the surface of a minimal medium plate. Filter discs (1.2 cm) were placed onto the surface, and d-Tyr (0, 2.5, 5, 12.5, and 25 μg) was applied to each disc. Bacterial growth near the filter disc was monitored after 24 and 48 h. Assays were repeated at least 3 times.
Biofilm formation assay.
Strains were grown at 37°C with agitation overnight and then diluted 1,000-fold in modified MSgg broth or spotted (5 μl) onto modified MSgg plates solidified with 1.5% Bacto agar in the presence or absence of 6 μM d-Tyr. The cultures were grown at 25°C without agitation for 1 to 5 days. Biofilms were photographed after 3 days at ×10 magnification using a dissecting microscope (Cambridge Instruments model no. Z30L) and a Samsung Galaxy S5 camera.
RESULTS
TyrZ is more selective than TyrS for l-Tyr.
The presence of two genes, tyrS and tyrZ, that encode tyrosyl-tRNA synthetase led us to determine the kinetic properties of each enzyme. The ability of B. subtilis TyrS and TyrZ to activate l-Tyr or d-Tyr was assayed by ATP-PPi exchange (Table 1). This assay measures the first step of aminoacylation in which the amino acid and ATP condense to form the enzyme-bound aminoacyl-adenylate. This step is reversible, and incubation in the presence of radiolabeled pyrophosphate permits the rate of formation of radiolabeled ATP to serve as a readout of activation of l- or d-Tyr. TyrS activated both l- and d-Tyr. However, activation of d-Tyr showed a 2.7-fold increase in the Km and a 9-fold decrease in the kcat compared to the activation of l-Tyr. Comparison of the respective kcat/Km values revealed that TyrS is 22-fold more specific for the activation of l-Tyr over d-Tyr. In contrast, TyrZ activated l-Tyr but was inhibited by concentrations of l-Tyr of >0.3 mM. The individual Km and kcat values could not be determined for d-Tyr activation by TyrZ due to the limit of solubility of d-Tyr, but extrapolation to determine kcat/Km indicated that TyrZ is 510-fold more specific for the activation of l-Tyr than d-Tyr. Comparison of the kcat/Km for each enzyme showed that TyrZ is 7-fold less efficient than TyrS at activating l-Tyr, whereas TyrZ is 170-fold less efficient than TyrS during the activation of d-Tyr. These results indicate that TyrZ has higher selectivity than TyrS for l-Tyr over d-Tyr but has a lower overall catalytic efficiency.
TABLE 1.
Kinetic analysis of TyrS and TyrZ
| Enzyme |
l-Tyr |
d-Tyr |
Specificitya | ||||
|---|---|---|---|---|---|---|---|
| Km (μM) | kcat (s−1) | kcat/Km (s−1 μM−1) | Km (μM) | kcat (s−1) | kcat/Km (s−1 μM−1) | ||
| TyrS | 33 ± 4 | 21 ± 1 | 6.4 × 10−1 ± 5 × 10−2 | 89 ± 3 | 2.3 ± 0.3 | 2.9 × 10−2 ± 1 × 10−2 | 22 |
| TyrZ | 36 ± 4b | 6.7 ± 0.3b | 8.7 × 10−2 ± 3 × 10−3 | >300 | >0.7 | 1.7 × 10−4 ± 3 × 10−5c | 510 |
Specificity was determined as follows: (kcat/Km) l-Tyr/(kcat/Km) d-Tyr.
Values were extrapolated ignoring substrate inhibition at concentrations of l-Tyr of >0.3 mM.
Value extrapolated in the absence of saturating concentrations of d-Tyr due to solubility limits of d-Tyr.
Compensatory mutations occur in ywaE when tyrS is inactivated.
The different selectivity of TyrS and TyrZ led us to determine how expression of tyrS and tyrZ affected growth in the presence of d-Tyr. Each gene was inactivated by insertion of a neomycin resistance cassette, followed by selection for neomycin resistance in the absence of d-Tyr. Disruption of either gene did not significantly affect the growth rate in rich or minimal medium (data not shown). Mutants in which tyrS and tyrZ were inactivated simultaneously could not be recovered (T. M. Henkin and F. J. Grundy, unpublished results). When tyrZ was inactivated in strain BR151, colonies were isolated after 24 h of growth, yielding strains designated ZKO. In contrast, after inactivation of tyrS, colonies appeared only after 48 to 72 h of incubation, yielding strains designated SKO. We hypothesized that a second mutation might have occurred in the ywaE gene based on its location upstream of tyrZ and the prediction that ywaE encodes a MarR regulator. Furthermore, introduction of a wild-type copy of the ywaE gene carried on an SPβ prophage resulted in loss of growth of SKO strains, presumably because of repression of tyrZ expression. We therefore sequenced the ywaE gene of multiple independent SKO isolates and identified nine independent mutations in the ywaE gene. Three different mutations were identified in the coding region, including a 4-nucleotide (nt) insertion 32 nt downstream of the start codon (designated EΩ4) predicted to cause a frameshift, G264A, which creates an in-frame stop codon, and G232A, which converts an alanine to threonine at a position that corresponds to the helix-turn-helix domain in other MarR homologs (13, 20). Five independent mutations were identified in the Shine-Dalgarno (SD) region of ywaE that are predicted to disrupt translation initiation. A separate A7G mutation was identified between the predicted −10 region of the promoter and the SD sequence (designated EopA7G). Inactivation of tyrS in strain NCIB 3610 (3610) did not affect the growth rate in rich or minimal medium and resulted in a single ywaE T491A mutation (3610-SKO-EV164E) that converts valine to glutamic acid at a position corresponding to the dimerization domain of other MarR homologs (13, 20). These mutations are predicted to either disrupt the function of YwaE or decrease expression of ywaE.
YwaE represses transcription of the ywaE-tyrZ operon.
TyrS, which is expressed during vegetative growth (12), is more efficient than TyrZ at aminoacylation of tRNATyr with l-Tyr. This suggested that expression of tyrZ might be regulated, to enable primary utilization of TyrS. The identification of mutations in ywaE in strains in which tyrZ was expressed suggested that YwaE represses transcription of the ywaE-tyrZ operon. Expression of a ywaE-lacZ fusion was low in strains that have a functional ywaE gene (BR151, ZKO, and SKO-EopA7G) (Table 2). However, expression of the fusion increased 51-fold when the tyrS and ywaE genes were inactivated (SKO-EΩ4) and 110-fold when only the ywaE gene was inactivated (EΩ4), in comparison to expression of this fusion in BR151. These results are consistent with the prediction that YwaE represses transcription of the ywaE-tyrZ operon and are further supported by RT-PCR analysis of tyrZ transcript levels (see Fig. S1 in the supplemental material).
TABLE 2.
Expression of ywaE-lacZ and ywaEopA7G-lacZ fusions
| Strain | ywaEb | β-Galactosidase activitya |
Fold changec | |
|---|---|---|---|---|
| ywaE-lacZ | ywaEopA7G-lacZ | |||
| BR151 | + | 0.73 ± 0.15 | 92 ± 16 | 130 |
| ZKO | + | 0.83 ± 0.23 | 71 ± 3.2 | 86 |
| SKO-EopA7G | + | 1.0 ± 0.20 | 90 ± 19 | 90 |
| SKO-EΩ4 | − | 37 ± 6.2 | 310 ± 43 | 8.4 |
| EΩ4 | − | 82 ± 5.2 | 260 ± 9.3 | 3.2 |
Fusions were integrated in single copy into B. subtilis strains. Cells were grown in minimal medium until late exponential phase and then assayed for β-galactosidase activity (Miller units). The values reported are averages of three repeats ± standard errors.
Indicates if the strain has a functional ywaE gene.
Fold change is the ratio of β-galactosidase activity for ywaEopA7G-lacZ to ywaE-lacZ.
Identification of an operator sequence important for YwaE-mediated repression.
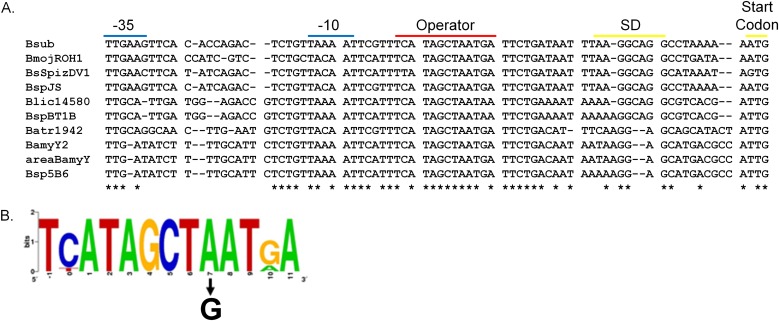
Since YwaE represses transcription of ywaE-tyrZ, and MarR family proteins recognize palindromic operator sites in the promoter regions of their target genes, we compared the promoter regions of ywaE-tyrZ operons in organisms related to B. subtilis (Fig. 2A). A conserved, pseudopalindromic 13-nt sequence (TCATAGCTAATGA) was identified between the putative −10 element and SD sequence (Fig. 2B). A role for this sequence in YwaE-mediated repression was supported by identification of the A7G mutation within this sequence as an allele that permitted inactivation of tyrS (presumably by promoting expression of tyrZ, which is required in the absence of tyrS).
FIG 2.
Identification of a putative operator sequence. The promoter regions of ywaE genes found upstream of tyrZ genes in organisms related to B. subtilis were aligned. (A) Sequences were identified using BLASTp and aligned using the ClustalW algorithm. The putative −35 and −10 sequences, the SD sequence and start codons, and the predicted operator sequence are indicated. Bsub, Bacillus subtilis subsp. subtilis strain 168; BmojROH1, Bacillus mojavensis ROH1; BsSpizDV1, Bacillus subtilis subsp. spizizenii DV1; BspJS, Bacillus sp. strain JS; Blic14580, Bacillus licheniformis DSM 13; BspBT1B, Bacillus sp. strain BT1B; Batr1942, Bacillus atrophaeus 1942; BamyY2, Bacillus amyloliquefaciens Y2; areaBamyY, Bacillus amyloliquefaciens subsp. plantarum YAU B9601-Y2; Bsp5B6, Bacillus sp. strain 5B6. (B) A WebLogo of the predicted operator sequence was created using the WebLogo sequence generator program (42, 43). An arrow depicts the location of the A7G mutation identified.
We verified the importance of this sequence for repression by YwaE by monitoring expression of a ywaEopA7G-lacZ fusion that included the A7G mutation (Table 2). Activity from this fusion increased at least 86-fold relative to expression of the wild-type fusion in strains that contain a functional ywaE gene. These results indicate that the operator sequence is important for repression by YwaE. Activity of this fusion was 3-fold higher in strains lacking a functional ywaE than in strain BR151, which suggests that the A7G mutation does not completely inactivate the operator sequence. Expression of the ywaEopA7G-lacZ fusion was at least 3-fold higher than that of the ywaE-lacZ fusion in strains that have the EΩ4 mutation, indicating that this mutation does not completely inactivate ywaE or that another regulatory protein may interact with the operator sequence. The EΩ4 mutation results in a frameshift that causes a premature stop codon 61 nt downstream of the AUG. However, there is another start codon downstream of the EΩ4 mutation. If translation initiated at this codon (albeit with low efficiency because of the absence of an obvious SD sequence), the resulting protein would contain a 23-amino-acid truncation at the N terminus, which is not highly conserved in other MarR homologs.
The tyrZ T box riboswitch is functional.
The tyrZ gene is preceded by a T box riboswitch element that is predicted to respond to the aminoacylation status of tRNATyr, such that readthrough of an intrinsic transcriptional terminator is promoted by uncharged tRNATyr (12, 21). We predicted that tRNATyr-mediated regulation of tyrZ is superimposed upon repression of transcription by YwaE. 4-ALP is a tyrosine analog that inhibits biosynthesis of l-Tyr but does not get charged onto tRNATyr, resulting in decreased aminoacylation of tRNATyr (32). Expression of the tyrS gene increases in the presence of uncharged tRNATyr (21), and a tyrS-lacZ fusion was induced 6-fold by 4-ALP (Table 3).
TABLE 3.
Expression of ywaE-tyrZ-lacZ fusions
| Fusiona | Strain | ywaEb | β-Galactosidase activity |
Induction (fold)c | |
|---|---|---|---|---|---|
| − 4-ALP | + 4-ALP | ||||
| tyrS-lacZ | BR151 | + | 24 ± 4 | 140 ± 20 | 5.8 |
| ywaE-tyrZ-lacZ | BR151 | + | 0.18 ± 0.02 | 1.3 ± 0.39 | 7.2 |
| EΩ4 | − | 0.27 ± 0.01 | 4.2 ± 0.15 | 15 | |
| ywaEL101E-tyrZ-lacZ | BR151 | + | 0.17 ± 0.02 | 1.5 ± 0.31 | 8.5 |
| SKO-EΩ4d | − | 11 ± 2.0 | 49 ± 5.5 | 4.4 | |
| EΩ4 | − | 3.9 ± 1.3 | 55 ± 3.8 | 14 | |
| ywaE-tyrZΔterm-lacZ | BR151 | + | 1.1 ± 0.17 | 1.2 ± 0.19 | 1.1 |
| EΩ4 | − | 1.8 ± 0.06 | 1.3 ± 0.22 | 0.72 | |
Cells harboring each fusion were grown in the presence or absence of 4-amino-l-phenylalanine (4-ALP) until late exponential phase and then assayed for β-galactosidase activity (Miller units). The values reported are averages of three repeats ± standard errors.
Indicates if the strain has a functional ywaE gene.
Induction by 4-ALP was determined by the ratio of β-galactosidase activity in the presence of 4-ALP to activity in the absence of 4-ALP.
This strain expresses only tyrZ.
A ywaE-tyrZ-lacZ fusion that fused the ywaE gene and the tyrZ leader region to the lacZ gene was inserted into an SPβ prophage. Expression was induced 7- and 15-fold by 4-ALP in strains BR151 and EΩ4, respectively, but expression levels were very low. Introduction of this fusion, which contains the entire ywaE gene, into strains BR151, ZKO, and SKO-EopA7G results in two functional copies of ywaE and in strain EΩ4 results in one functional copy. Repression of transcription by YwaE was relieved by construction of a ywaEL101E-tyrZ-lacZ fusion in which a conserved leucine in the putative DNA recognition domain was mutated to glutamic acid (Table 3). Since strain SKO-EΩ4 is dependent on tyrZ expression (and relief of repression by YwaE), we were unable to introduce the ywaE-tyrZ-lacZ fusion into this strain. However, the ability to introduce the ywaEL101E-tyrZ-lacZ fusion into strain SKO-EΩ4 indicated that the ywaEL101E allele disrupts YwaE function.
Regulation of the ywaEL101E-tyrZ-lacZ fusion in strains lacking ywaE is predicted to be dependent solely on the tyrZ T box riboswitch. Induction of expression of this fusion by 4-ALP was similar to that of the ywaE-tyrZ-lacZ fusion in all strains. The basal levels of activity increased 14-fold in strain EΩ4, which further confirms that YwaE encoded by the fusion is inactive. These data suggest that readthrough of the tyrZ T box terminator occurs under conditions that are predicted to increase the concentration of uncharged tRNATyr in the cell. Expression of the ywaEL101E-tyrZ-lacZ fusion in the absence of 4-ALP was 3-fold lower in strain EΩ4 than in strain SKO-EΩ4; this is likely to be due to the activity of TyrS in strain EΩ4, which increases the concentration of charged tRNATyr and decreases readthrough of the tyrZ terminator.
Expression of a ywaE-tyrZΔterm-lacZ fusion, in which the 3′ half of the T box terminator helix was deleted, was independent of 4-ALP, consistent with the role of the terminator in the response to tRNATyr (Table 3). In addition, basal activity was 20-fold higher in a ywaEopA7G-lacZ fusion compared to expression of the ywaE-tyrZΔterm-lacZ fusion (see Table S3 in the supplemental material). Together, these data demonstrate that both the T box transcriptional terminator and YwaE contribute to regulation of tyrZ expression.
Expression of tyrZ is required for resistance to d-Tyr.
Misincorporation of d-Tyr into proteins inhibits growth (2). Since TyrZ is more selective for l-Tyr in vitro, we predicted that expression of tyrZ might reduce sensitivity to growth inhibition by d-Tyr. Zone-of-growth-inhibition assays were used to assess growth inhibition by d-Tyr. Growth of strain BR151 was inhibited by d-Tyr in a concentration-dependent manner (data not shown). Inactivation of tyrZ (resulting in expression of only tyrS) in strain ZKO resulted in growth inhibition similar to that of strain BR151 (Table 4), which is consistent with the observation that strain BR151 does not express tyrZ at detectable levels (see Fig. S1A in the supplemental material). Strains SKO-EopA7G and SKO-EΩ4, which express only tyrZ (see Fig. S1A), showed a reduction in sensitivity to d-Tyr compared to strain BR151. Restoration of the tyrS gene in strains EΩ4 and EopA7G resulted in sensitivity to d-Tyr similar to that of the wild-type strain, even though these strains express tyrZ (see Fig. S1A), indicating that the presence of TyrS causes sensitivity even in the presence of TyrZ. Growth of the biofilm-forming strain 3610 was sensitive to d-Tyr but was more resistant than that of strain BR151. Inactivation of tyrZ in strain 3610-ZKO did not result in increased sensitivity to d-Tyr, which is consistent with observations made for strain BR151. Inactivation of tyrS in strain 3610-SKO-EV164E resulted in expression of tyrZ (see Fig. S1B) and increased resistance to d-Tyr. These data suggest that expression of tyrZ is required for decreased sensitivity to growth inhibition by d-Tyr.
TABLE 4.
Growth inhibition in the presence of d-Tyr
| Strain | tyrS/tyrZb | Radius of zone of growth inhibition (mm)a |
|
|---|---|---|---|
| − dtd | + dtd | ||
| BR151 | Both | 14 ± 0.3 | 5.7 ± 0.7 |
| ZKO | tyrS | 14 ± 0.3 | 6.0 ± 0.6 |
| SKO-EopA7G | tyrZ | 7.7 ± 0.3 | 0 |
| SKO-EΩ4 | tyrZ | 5.3 ± 0.3 | 0 |
| EopA7G | Both | 13 ± 1.2 | 4.3 ± 0.3 |
| EΩ4 | Both | 12 ± 0.3 | 4.7 ± 0.7 |
| 3610 | Both | 3.7 ± 0.3 | 0 |
| 3610-ZKO | tyrS | 3.7 ± 0.3 | 0 |
| 3610-SKO-EV164E | tyrZ | 0 | 0 |
Bacterial lawns were grown on minimal medium in the presence of filter discs that were soaked with d-Tyr (25 μg). The absence of growth near the filter disc was measured after incubation at 37°C for 24 h. The radius of zone of growth inhibition indicates the size of the clear zone measured from the edge of the filter disc in mm. Values are reported as averages ± standard errors.
Indicates which gene encoding tyrosyl-tRNA synthetase is present in the cell.
d-Aminoacyl-tRNA deacylase, encoded by the dtd gene, removes d-Tyr that has been mischarged onto tRNATyr. The dtd gene is expressed by most organisms to prevent misincorporation of d-Tyr (2, 9, 10). However, most B. subtilis strains contain an inactivated dtd gene (3). Since TyrZ is more selective for l-Tyr over d-Tyr, we tested whether tyrZ expression provides resistance to growth inhibition by d-Tyr in the presence of a functional dtd gene (generated by mutation of the AUU start codon to AUG and inserted into an SPβ prophage for stable introduction into each strain background) (Table 4). Comparison of growth inhibition in the absence of an active dtd gene to that in the presence of an active dtd gene demonstrated that the dtd gene reduced sensitivity to d-Tyr in all strains. Derivatives of strain BR151 that express tyrS were still sensitive to d-Tyr. These results suggest that the deacylase reduced but did not eliminate misincorporation of d-Tyr. Growth of strains complemented with the repaired dtd gene and expressing only tyrZ was not inhibited by d-Tyr at any concentration tested; growth of all strains complemented with the dtd gene was not inhibited by d-Tyr at 48 h (data not shown). These data indicate that the tyrZ and dtd gene products function independently to prevent misincorporation of d-Tyr, consistent with their roles in amino acid selectivity and proofreading, respectively.
The tyrZ gene is required for biofilm formation in the presence of d-Tyr.
Based on previously published results demonstrating that misincorporation of d-Tyr into cellular proteins indirectly inhibits biofilm formation (3), we tested whether the tyrZ gene is important for biofilm formation in the presence of d-Tyr (Fig. 3). Strains 3610, 3610-ZKO, and 3610-SKO-EV164E were grown on biofilm-inducing medium in the presence or absence of d-Tyr. All three strains formed the complex architecture characteristic of pellicle (data not shown) and colony biofilms in the absence of d-Tyr. However, only strain 3610-SKO-EV164E, which expresses tyrZ but not tyrS (see Fig. S1B in the supplemental material), formed a biofilm in the presence of d-Tyr. The tyrS gene was restored in strain 3610-EV164E, in which only the ywaE gene was inactivated. This strain was unable to form a biofilm in the presence of d-Tyr (data not shown), indicating that the ywaE mutation does not confer biofilm formation in the presence of d-Tyr. These results indicate that expression of tyrZ protects against d-Tyr during biofilm formation. Papillae emerged during growth of strains 3610 and 3610-ZKO (in which tyrZ has been inactivated) in the presence of d-Tyr, which indicates that these growth conditions result in selection for resistant mutants. The number of papillae was higher in strain 3610-ZKO than in strain 3610 (Fig. 3), suggesting that the presence of the tyrZ gene in strain 3610 is partially protective. Complementation with a functional dtd gene restored biofilm formation in the presence of d-Tyr, which indicates that expression of the dtd gene is sufficient for biofilm formation in the presence of d-Tyr. This is consistent with growth inhibition results since biofilm formation required 72 h and growth of strains complemented with a functional dtd gene was not inhibited by d-Tyr after 48 h (data not shown). Biofilm mutant strains 3610ΔepsH and 3610ΔtapA-sipW-tasA were unable to form a colony or pellicle biofilm in the presence or absence of d-Tyr, as expected (26, 33).
FIG 3.
Biofilm formation in the presence or absence of d-Tyr. Overnight cultures (5 μl) were spotted on modified MSgg plates in the presence or absence of d-Tyr (6 μM) and grown at 25°C for 3 days.
DISCUSSION
The B. subtilis genome contains two genes, tyrS and tyrZ, which encode isoforms of tyrosyl-tRNA synthetase. We found that although TyrZ is less efficient than TyrS at aminoacylation of tRNATyr with l-Tyr, it is more selective for l-Tyr over d-Tyr in vitro. B. subtilis cells that express tyrZ alone exhibited decreased sensitivity to d-Tyr in vivo, consistent with the selectivity observed in vitro. Strains in which both tyrS and tyrZ are expressed were sensitive to d-Tyr, presumably due to misincorporation of d-Tyr into proteins because of tyrS expression. Furthermore, the activity of TyrS is likely to result in reduced transcription of tyrZ via the T box mechanism. Therefore, the d-Tyr-sensitive phenotype is dominant to the d-Tyr-resistant phenotype in strains containing both genes.
There are two bacterial subfamilies of tyrosyl-tRNA synthetase (34), represented by TyrS and TyrZ, which in B. subtilis show only 30% amino acid identity. Genes in the tyrZ class are found in several bacterial phyla, including green sulfur bacteria, CFB group bacteria, Gammaproteobacteria, Deltaproteobacteria, Betaproteobacteria, Thermotogales, Fusobacteria, Enterobacteria, Aquificales, and Firmicutes. The tyrZ gene is the only tyrosyl-tRNA synthetase-encoding gene in Thermotoga maritima, Aquifex aeolicus, Thiobacillus ferrooxidans, and several Proteobacteria (e.g., Burkholderia pseudomallei and Pelobacter carbinolicus). However, there are several organisms in addition to B. subtilis that have both tyrS and tyrZ (e.g., Pseudomonas aeruginosa, Clostridium acetobutylicum, and Clostridium botulinum). The tyrZ gene seems to be scattered around the phylogenetic tree, and the presence of tyrS and tyrZ genes in the same genus makes determining the origin of the tyrZ gene unclear.
In Bacillus species that have both tyrS and tyrZ genes, the ywaE gene and T box element are also found upstream of tyrZ. However, there are instances (e.g., P. aeruginosa) where tyrS and tyrZ are present in a single organism but a transcriptional regulator is not present. There are also examples (e.g., C. acetobutylicum and C. botulinum) where the tyrZ gene contains a T box element but the tyrS gene does not.
Our studies clearly demonstrate that TyrZ protects the cell against exogenous d-Tyr during growth and biofilm formation. While it is known that B. subtilis produces d-amino acids, including d-Tyr (7, 35, 36), the observation that expression of tyrZ did not influence biofilm formation in the absence of d-Tyr suggests that endogenous d-Tyr production is insufficient to inhibit biofilm formation, under the conditions tested.
tyrZ may also protect the cell against misincorporation of other tyrosine analogs. We observed that TyrZ is more selective than TyrS for the activation of meta-tyrosine (m-Tyr), phenylalanine, and 3,4-dihydroxyphenylalanine (DOPA)–tyrosine (M. Raina and M. Ibba, unpublished results). However, m-Tyr was shown previously to be misincorporated at phenylalanine codons (37), and expression of tyrZ did not significantly reduce sensitivity to m-Tyr in vivo (R. N. Williams-Wagner and T. M. Henkin, unpublished results).
The ywaE gene in B. subtilis was previously predicted to encode a MarR family transcriptional regulator (13). We show that YwaE represses expression of the ywaE-tyrZ operon, probably through interaction with the identified operator sequence. Based on the role of tyrZ in resistance to d-Tyr, we propose renaming the ywaE gene as dtrR, for d-Tyr resistance regulator.
The tyrZ gene is also regulated at the level of transcription attenuation by a tRNATyr-responsive T box riboswitch, so that tyrZ is expressed when the concentration of uncharged tRNATyr increases. Expression of tyrS also may influence expression of tyrZ, presumably by the T box mechanism. Support for this prediction comes from the observation that expression of the ywaEL101E-tyrZ-lacZ fusion decreased 3-fold in strain EΩ4, which expresses both tyrS and tyrZ, compared to strain SKO-EΩ4, which expresses only tyrZ. The basis for this hierarchical gene regulation is not clear but may be related to differential binding of the tRNATyr ligand. Furthermore, TyrZ enzyme activity is inhibited by concentrations of l-Tyr greater than 0.3 mM in vitro, which provides a mechanism to inhibit TyrZ when conditions are favorable for TyrS activity. However, the concentration of free l-Tyr in B. subtilis cells grown under nitrogen-limiting conditions was reported to be less than 0.1 mM (38), which suggests that cellular concentrations of l-Tyr may not be sufficient to inhibit TyrZ.
For the current study, expression of tyrZ required mutations in dtrR. However, environmental conditions under which tyrZ is expressed in the presence of a functional DtrR have not been identified. MarR regulators often respond to anionic, phenolic compounds (18, 19), and tyrZ protected against growth inhibition by d-Tyr. Therefore, it is tempting to speculate that DtrR responds to d-Tyr. We were unable to observe derepression of dtrR in the presence of d-Tyr (R. N. Williams-Wagner, F. J. Grundy, and T. M. Henkin, unpublished results), possibly because of the growth conditions tested. It has been proposed previously that MarR in E. coli senses copper ions released from damaged membrane proteins (39), which suggests that the signal molecule may not always be a phenolic compound. Furthermore, expression of tyrZ but not dtrR increases when levels of the extracytoplasmic function sigma factor σY are elevated (40, 41). σY may direct transcription of a gene involved in either transport or biosynthesis of a signal molecule that results in derepression of tyrZ. Further investigation of the regulation of tyrZ expression will provide additional insight into the physiological role of TyrZ.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. A. Leiman and R. Losick for discussions and strains and C. Woltjen for technical assistance.
This work was supported by NIH grant GM047323 to T.M.H., NSF grant MCB-1412611 to M.I., and a Center for RNA Biology Fellowship to R.N.W.-W.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00008-15.
REFERENCES
- 1.Ling J, Reynolds N, Ibba M. 2009. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol 63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]
- 2.Calendar R, Berg P. 1967. d-Tyrosyl RNA: formation, hydrolysis and utilization for protein synthesis. J Mol Biol 26:39–54. doi: 10.1016/0022-2836(67)90259-8. [DOI] [PubMed] [Google Scholar]
- 3.Leiman SA, May JM, Lebar MD, Kahne D, Kolter R, Losick R. 2013. d-Amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J Bacteriol 195:5391–5395. doi: 10.1128/JB.00975-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leiman SA, Richardson C, Foulston L, Elsholz AKW, First EA, Losick R. 2015. Identification and characterization of mutations conferring resistance to d-amino acids in Bacillus subtilis. J Bacteriol 197:1632–1639. doi: 10.1128/JB.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champney WS, Jensen RA. 1970. The enzymology of prephenate dehydrogenase in Bacillus subtilis. J Biol Chem 245:3763–3770. [PubMed] [Google Scholar]
- 6.Champney WS, Jensen RA. 1969. d-Tyrosine as a metabolic inhibitor of Bacillus subtilis. J Bacteriol 98:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam H, Oh D, Cava F, Takacs CN, Clardy J, de Pedro MA, Waldor MK. 2009. d-Amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555. doi: 10.1126/science.1178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein T. 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 9.Soutourina J, Blanquet S, Plateau P. 2000. d-Tyrosyl-tRNATyr metabolism in Saccharomyces cerevisiae. J Biol Chem 275:11626–11630. doi: 10.1074/jbc.275.16.11626. [DOI] [PubMed] [Google Scholar]
- 10.Soutourina O, Soutourina J, Blanquet S, Plateau P. 2004. Formation of d-tyrosyl-tRNATyr accounts for the toxicity of d-tyrosine toward Escherichia coli. J Biol Chem 279:42560–42565. doi: 10.1074/jbc.M402931200. [DOI] [PubMed] [Google Scholar]
- 11.Glaser PA, Kunst F, Debarbouille M, Vertes A, Danchin A, Dedonder R. 1991. A gene encoding a tyrosine tRNA synthetase is located near sacS in Bacillus subtilis. DNA Seq 1:251–261. [DOI] [PubMed] [Google Scholar]
- 12.Henkin TM, Glass BL, Grundy FJ. 1992. Analysis of the Bacillus subtilis tyrS gene: conservation of a regulatory sequence in multiple tRNA synthetase genes. J Bacteriol 174:1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulavik MC, Gambino LF, Miller PF. 1995. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol Med 1:436–446. [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha ER, Herren CD, Smalley DJ, Smith CJ. 2003. The complex oxidative stress response of Bacteroides fragilis: the role of OxyR in control of gene expression. Anaerobe 9:165–173. doi: 10.1016/S1075-9964(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Inaoka T, Hirooka K, Matsuoka H, Murata M, Ohki R, Adachi Y, Fujita Y, Ochi K. 2009. Identification and characterization of a novel multidrug resistance operon, mdtRP (yusOP), of Bacillus subtilis. J Bacteriol 191:3273–3281. doi: 10.1128/JB.00151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escudero JA, Millan AS, Montero N, Gutierrez B, Ovejero CM, Carrilero L, González-Zorn B. 2013. SatR is a repressor of fluoroquinolone efflux pump SatAB. Antimicrob Agents Chemother 57:3430–3433. doi: 10.1128/AAC.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira FL, dos Santos Silva DN, Pauer H, Ferreira LQ, Ferreira E, Domingues RMCP, Lobo LA. 2013. The role of BmoR, a MarR family regulator, in the survival of Bacteroides fragilis during oxidative stress. Int J Med Micriobiol 303:443–448. doi: 10.1016/j.ijmm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Martin RG, Rosner JL. 1995. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci U S A 92:5456–5460. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson SP, Grove A. 2004. HucR, a novel uric acid-responsive member of the MarR family of transcriptional regulators from Deinococcus radiodurans. J Biol Chem 279:51442–51450. doi: 10.1074/jbc.M405586200. [DOI] [PubMed] [Google Scholar]
- 20.Perera IC, Grove A. 2010. Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. J Mol Cell Biol 2:243–254. doi: 10.1093/jmcb/mjq021. [DOI] [PubMed] [Google Scholar]
- 21.Grundy FJ, Henkin TM. 1993. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74:475–482. doi: 10.1016/0092-8674(93)80049-K. [DOI] [PubMed] [Google Scholar]
- 22.Grundy FJ, Rollins SM, Henkin TM. 1994. Interaction between the acceptor end of tRNA and the T box stimulates antitermination in the Bacillus subtilis tyrS gene: a new role for the discriminator base. J Bacteriol 176:4518–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousef MR, Grundy FJ, Henkin TM. 2003. tRNA requirements for glyQS antitermination: a new twist on tRNA. RNA 9:1148–1156. doi: 10.1261/rna.5540203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 25.Anagnostopoulos C, Spizizen J. 1961. Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuber P, Losick R. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol 169:2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano MM, Zuber P. 1989. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol 171:5347–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itaya M, Kondo K, Tanaka T. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res 17:4410. doi: 10.1093/nar/17.11.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundy FJ, Henkin TM. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol Microbiol 30:737–749. doi: 10.1046/j.1365-2958.1998.01105.x. [DOI] [PubMed] [Google Scholar]
- 31.Yasbin RE, Young FE. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol 14:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler WC. 2002. Ph.D. thesis The Ohio State University, Columbus, OH. [Google Scholar]
- 33.Branda SS, Gonza E, Dervyn E, Ehrlich SD, Losick R, Kolter R. 2004. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol 186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woese CR, Olsen GJ, Ibba M, Söll D. 2000. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol Mol Biol Rev 64:202–236. doi: 10.1128/MMBR.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. 2010. d-Amino acids trigger biofilm disassembly. Science 328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cava F, Lam H, de Pedro MA, Waldor MK. 2011. Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell Mol Life Sci 68:817–831. doi: 10.1007/s00018-010-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aronson JN, Wermus GR. 1965. Effects of m-tyrosine on growth and sporulation of Bacillus species. J Bacteriol 90:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tempest DW, Meers JL, Brown CW. 1970. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol 64:171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- 39.Hao Z, Lou H, Zhu R, Zhu J, Zhang D, Zhao BS, Zeng S, Chen X, Chan J, He C, Chen PR. 2014. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat Chem Biol 10:21–28. doi: 10.1038/nchembio.1380. [DOI] [PubMed] [Google Scholar]
- 40.Asai K, Yamaguchi H, Kang C, Yoshida K, Fujita Y, Sadaie Y. 2003. DNA microarray analysis of Bacillus subtilis sigma factors of extracytoplasmic function family. FEMS Microbiol Lett 220:155–160. doi: 10.1016/S0378-1097(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 41.Cao M, Salzberg L, Tsai CS, Masher T, Bonilla C, Wang T, Ye RW, Márquez-Magaña L, Helmann JD. 2003. Regulation of the Bacillus subtilis extracytoplasmic function protein σY and its target promoters. J Bacteriol 185:4883–4890. doi: 10.1128/JB.185.16.4883-4890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider TD, Stephens RM. 1990. Sequence Logos: a new way to displace consensus sequences. Nucleic Acids Res 18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.