ABSTRACT
Many rhizobial species use complex N-acyl-homoserine lactone (AHL)-based quorum sensing (QS) systems to monitor their population density and regulate their symbiotic interactions with their plant hosts. There are at least three LuxRI-type regulatory systems in Rhizobium etli CFN42: CinRI, RaiRI, and TraRI. In this study, we show that CinI, RaiI, and TraI are responsible for synthesizing all AHLs under the tested conditions. The activation of these AHL synthase genes requires their corresponding LuxR-type counterparts. We further demonstrate that CinRI is at the top of the regulatory cascade that activates RaiRI and TraRI QS systems. Moreover, we discovered that CinR possesses a specific affinity to bind cinI promoter in the absence of its cognate AHL ligand, thereby activating cinI transcription. Addition of AHLs leads to improved binding to the cinI promoter and enhanced cinI expression. Furthermore, we found that compared to the wild type, the cinR mutation displayed reduced nodule formation, and cinR, raiR, and traI mutants show significantly lower levels of nitrogen fixation activity than the wild type. These results suggest that the complex QS regulatory systems in R. etli play an important role in its symbiosis with legume hosts.
IMPORTANCE Many bacteria use quorum sensing (QS) to monitor their cell densities and coordinately regulate a number of physiological functions. Rhizobia often have diverse and complex LuxR/LuxI-type quorum sensing systems that may be involved in symbiosis and N2 fixation. In this study, we identified three LuxR/LuxI-type QS systems in Rhizobium etli CFN42: CinRI, RaiRI, and TraRI. We established a complex network of regulation between these QS components and found that these QS systems played important roles in symbiosis processes.
INTRODUCTION
Establishment of symbiosis between rhizobia and their legume hosts is a complex process requiring multiple intricate signal exchanges. Many plant-associated bacteria, such as species of legume-nodulating rhizobia, use a set of diffusible N-acyl homoserine lactones (AHLs) involved in quorum sensing (QS) (1) systems to communicate with each other and optimize their interactions with plant hosts. In a typical complete LuxRI QS system, AHLs are synthesized by an LuxI-type protein and are accumulated to threshold levels as cell density increases. An LuxR-type transcriptional activator is activated by threshold levels of AHLs and induces expression of specific target genes to regulate multiple physiological functions. Quorum sensing has been implicated in various aspects of legume symbioses, including exopolysaccharide production, which is important for infection, plasmid transfer, competitiveness, nodule formation, and nitrogen fixation (2).
Quorum sensing in rhizobia is very diverse, and two strains from the same species of rhizobia often do not have the same quorum sensing components (3). For example, in Sinorhizobium meliloti, SinR/SinI and ExpR in strain Rm1021 regulate exopolysaccharide production and swarming (4–6), TraR/TraI in Rm41 controls plasmid transfer (2, 6), and VisN/VisR in RU10/406 regulates motility (7). In the Rhizobium etli strain CNPAF512, two QS systems have been identified, RaiRI and CinRI, both of which control symbiosis (8, 9). The raiI mutants cause a slight increase in the number of nodules formed per plant (9), whereas mutations in cinI exhibit a 60 to 70% reduction in nitrogen fixation efficiency (8). Another R. etli strain, CFN42, has a complex but less well characterized QS system (10, 11). There are three different LuxI-type AHL synthases (CinI, RaiI, and TraI) and four cognate LuxR-type regulators (CinR, RaiR, TraR1, and TraR2) in the genome sequence (Fig. 1A). Only one N-acyl-homoserine lactone, N-(3-oxooctanoyl)-l-homoserine lactone (3-oxo-C8-HSL), synthesized by TraI, has been identified. Together with its two activators, TraR and the adjacent CinR, 3-oxo-C8-HSL has been found to be involved in self-conjugative plasmid transfer (12). In order to fully understand the quorum sensing regulation in R. etli CFN42, we constructed mutations in each of the aforementioned QS regulatory components and studied the effects of these mutants on transcription of QS regulatory genes as well as AHL production. Interestingly, we found that CinR can activate cinI expression in the absence of its ligand, thereby demonstrating the complexity of the QS regulatory pathways. The relationship between QS and symbiosis is also investigated.
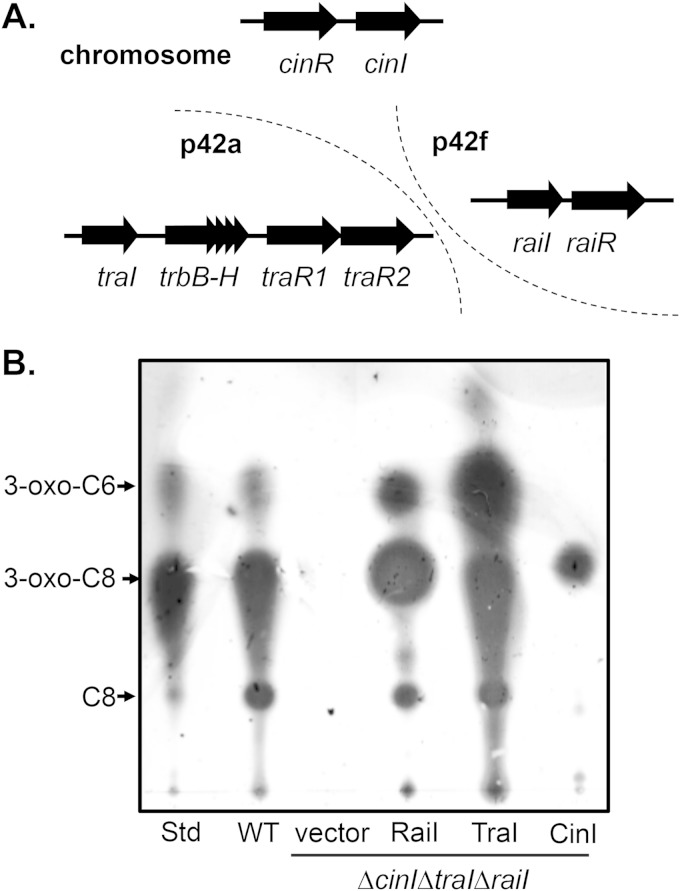
FIG 1.
AHL production in R. etli CFN42. (A) Genomic location and organization of R. etli QS systems. p42f and p42a are two plasmids in R. etli CFN42. (B) TLC analysis. Cell-free supernatants of R. etli wild-type and ΔcinI ΔraiI ΔtraI mutant strains harboring either the vector control, Ptac-raiI (RaiI), Ptac-traI (TraI), or Ptac-cinI (CinI) plasmid were subjected to TLC analysis. Each equivalent of 1 ml of culture extract was loaded on a C18 reverse-phase TLC plate, followed by an overlay of agar medium seeded with AHL bioassay strains (16). An extract from A. tumefaciens R10(pCF218) was used as the standard, and the migration positions of 3-oxo-C8, 3-oxo-C6, and C8-HSL, as indicated, were based on a previously published TLC analysis (16, 38). WT, wild type.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Rhizobium etli CFN42 streptomycin-resistant CE3 derivative strains were cultured at 28°C in peptone-yeast extract (PY) medium (13), Escherichia coli was cultured at 37°C in Luria-Bertani (LB) medium, and Agrobacterium tumefaciens was grown at 28°C in AT medium (1). The following antibiotics were added as appropriate to maintain selection for plasmids (final concentration): streptomycin (100 μg/ml), gentamicin (20 μg/ml), spectinomycin (100 μg/ml), and tetracycline (2 μg/ml). When required, medium was supplemented with 1.5% agar. To construct the in-frame deletion mutants, overlap extension PCR was first used to create the deletions. In-frame deletion plasmids in the cinI, cinR, raiI, raiR, traI, traR, traR2, and traR1-traR2 genes were constructed by overlapping PCR of flanking regions of the target genes and cloning the resulting product in the pEX18Gm suicide vector containing an sacB counterselectable marker (14). The resulting plasmids were introduced into R. etli by conjugation, and deletion mutants were selected for double homologous recombination events. Double-crossover events were selected on sucrose plates (10%) after the first “cross-in” homologous recombination. The deletions in each mutant strain were confirmed by PCR examination and sequence analysis. Each plasmid that constitutively expressed cinI, cinR, raiI, raiR, traI, and traR1-traR2 was constructed by cloning these genes into the pYC12 vector (15), and constructs were introduced into R. etli and E. coli strains by electroporation. Primers used in this study are listed in Table S1 in the supplemental material.
AHL bioassays and identification.
Spent medium (2%, vol/vol) from R. etli strains grown in PY medium were collected at various time points as indicated in Fig. 2 and added into AT medium (1) (optical density at 600 nm of 0.2 to 0.8), and approximately 107 cells per ml of AHL bioassay strain were added. We used A. tumefaciens KYC55(pJZ372)(pJZ384)(pJZ410) as the AHL bioassay strain (16). This strain cannot make its own AHLs, and the AHL receptor TraR of A. tumefaciens is overexpressed such that it sensitively detects exogenous AHLs with diverse structures and activates the traI-lacZ reporter. These cultures were incubated with aeration for 12 h and assayed for β-galactosidase-specific activity (17).
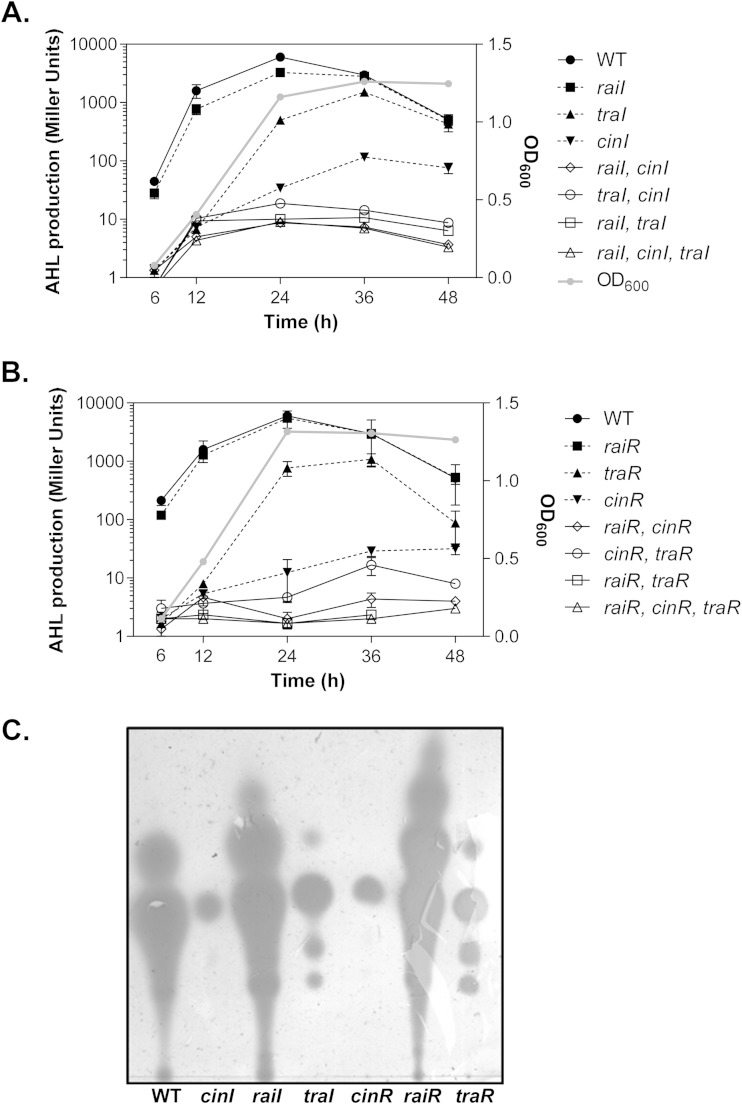
FIG 2.
AHL production in R. etli QS mutants. Graphs represent the time course of accumulation of AHLs in the spent medium from LuxI-type QS mutants (A) and LuxR-type QS mutants (B). Bacterial cultures were withdrawn at the time points indicated to measure cell density (OD600; gray lines) and AHL activity (black lines). traR represents the traR1-traR2 double mutant. The data shown represent the means of three independent experiments, the standard deviations for which are indicated by the error bars. (C) TLC analysis of AHL contents. The wild-type and QS mutant cultures were extracted and loaded on a C18 reverse-phase TLC plate, followed by an overlay of the agar medium seeded with AHL bioassay strains. Each lane was loaded with 1 ml of culture extract. traR represents the traR1-traR2 double mutant.
Thin-layer chromatography (TLC) analysis of AHLs released by R. etli strains was performed as previously described (18). R. etli was grown for 24 h in 50 ml of medium. After centrifugation, the supernatant was extracted twice with an equal volume of dichloromethane. The extract was evaporated to dryness by vacuum rotation at 35°C and redissolved in 50 μl of dichloromethane. One microliter of this suspension was spotted on a C18 reverse-phase thin-layer chromatography (TLC) plate (RP-18F254S; Merck), which was then developed with 60% methanol. The plate was removed from the chromatography tank and dried in air for 4 h. The air-dried chromatography plate was overlaid with a thin film of AT medium soft agar (1% [wt/vol]) containing 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal) and seeded with A. tumefaciens KYC55(pJZ372)(pJZ384)(pJZ410) indicator cells (16). The plate was incubated at 28°C for 12 to 16 h and examined for X-Gal hydrolysis.
Transcriptional analysis.
LacZ fusion reporters of cinR, raiR, traR, cinI, raiI, and traI were constructed by cloning promoter sequences of the genes of interest (∼0.5-kb sequences upstream of the start codon and ∼30 bp into the reading frame) into pRA302 (19), which contains a promoterless lacZ reporter. The coding sequence of the gene of study was fused in frame with the lacZ reading frame. The resulting plasmids were introduced into R. etli wild type and various QS mutants by electroporation. Bacteria containing lacZ translational reporters were grown in PY medium with appropriate antibiotics in the absence and in the presence of the AHL indicated. We tested the samples at the time points indicated in the figure legends, and β-galactosidase activity was measured as described previously (17).
Quantitative reverse transcription-PCR (RT-qPCR) was also used to examine raiR expression to include possible read-through transcription from the upstream raiI gene. The primers for raiR transcripts (raiRRT-1 and raiRRT-2) (see Table S1 in the supplemental material) were within the coding sequences of the raiR deletion construct. Wild-type and raiR mutants were grown to the late exponential phase (optical density at 600 nm [OD600] of 0.8, and RNA was purified using an RNA extraction kit (TaKaRa). The cDNA was then synthesized using 5× All-In-One RT MasterMix (Applied Biological Materials), and real-time PCR was performed with SYBR Premix Ex Taq (Applied Biological Materials, Inc.) using a StepOne real-time PCR system (Eppendorf Realplex2). The quantity of cDNA measured by using a real-time PCR system was normalized to the abundance of 16S cDNA.
CinR purification and EMSA.
The full-length CinR open reading frame (ORF) was amplified and cloned into pET28a(+). Recombinant CinR-His6 was purified by Ni2+-loaded nitrilotriacetic acid (NTA) resin (GE Healthcare) from cultures of E. coli BL21(DE3) carrying the resulting plasmid, according to the manufacturer's protocol. A biotin-labeled fragment containing the predicted cinI promoter region was PCR amplified with 5′ end biotin-labeled primers. CinI-producing AHLs were extracted from the cell-free supernatant of an E. coli strain expressing CinI or an R. etli triple AHL synthase mutant containing Plac-cinI. The supernatants were extracted with dichloromethane, evaporated to dryness, and redissolved in a 1/1,000 volume of distilled H2O (dH2O). When indicated, 1 μl of prepared AHL solution was included in the electrophoretic mobility shift assay (EMSA) reaction mix. Binding reaction mixtures contained 1 nM DNA and the amounts of CinR-His6 indicated in Fig. 4 with or without AHLs in a buffer containing 10 mM Tris-HCl (pH 7.9), 1 mM EDTA, 1 mM dithiothreitol (DTT), 60 mM potassium chloride, and 10% glycerol. After 30 min of incubation at 25°C, samples were size fractionated with the use of 5% polyacrylamide gels in 0.5× Tris-borate-EDTA (TBE) buffer. The band shifts were detected and analyzed by using a Chemiluminescent Nucleic Acid Detection Module kit (Thermo) according to the manufacturer's instructions. The images were obtained using an ImageQuant LAS 4000 Mini camera system (GE, Piscataway, NJ).
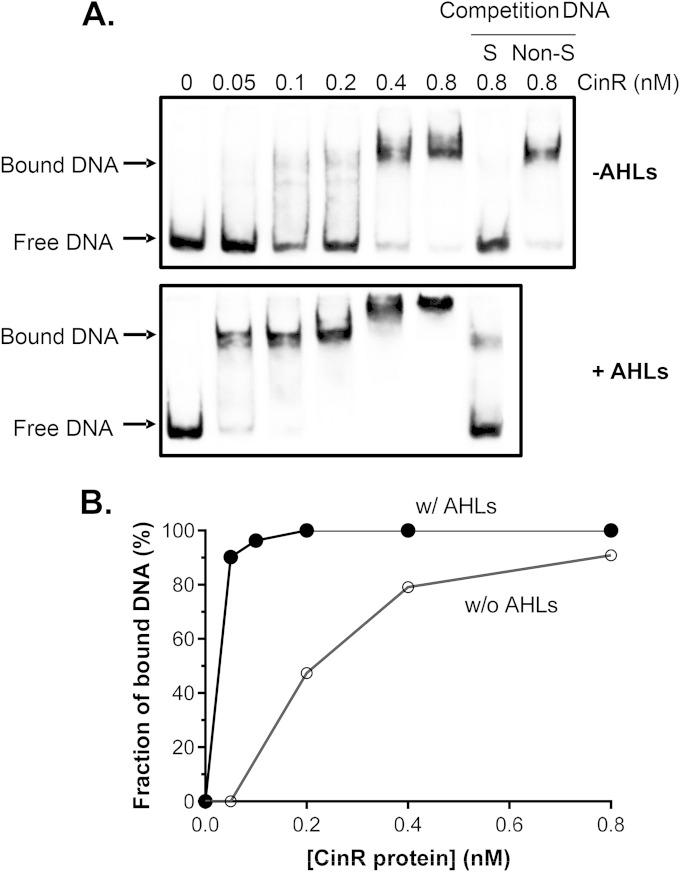
FIG 4.
CinR protein binds to the cinI promoter DNA. (A) EMSA of purified recombinant CinR on cinI promoter DNA. Each lane contained 1 nM biotin-labeled cinI promoter DNA and the indicated concentration of CinR-His6 protein in the absence (top panel) or presence (bottom panel) of AHLs produced by CinI expressed in E. coli. A 30-fold excess of either unlabeled cinI promoter DNA (S, specific) or random DNA fragments (Non-S, nonspecific) was used as competition DNA. (B) DNA binding affinity of CinR-His6 in the absence (w/o) and the presence (w/) of AHLs. Results obtained from the experiment described in panel A were quantified by plotting the fraction of shifted specific DNA versus CinR-His6 concentration.
Plant assays.
Common bean seeds were treated with 75% ethanol for 1 min and subsequently with 1.6% NaClO for 1 min. The treated seeds were then placed in sterilized culture dishes, germinated in the dark at 28°C for 2 days, and then planted in sterilized pots (five seeds per dish) filled with autoclaved vermiculite and a plant nutrient solution of Fahraeus medium (20) supplemented with 28 mg N/kg of vermiculite. Simultaneously, the autoclaved vermiculite was inoculated with approximately 108 R. etli cells (either wild type or mutant) cultured in PY medium. Plants were grown in a plant growth room at 28°C with 60% relative humidity and a 16-h–8-h day-night cycle. At the time indicated in Table 1, the plants were pulled out to count the number of nodules or to measure nitrogen fixing activity. There were at least 16 replicates per strain per experiment. In vivo nitrogenase activity was measured using an acetylene reduction assay according to previously described protocols (21, 22). In the absence of N2, the enzyme catalyzes the conversion of acetylene (C2H2) to ethylene (C2H4) gas. Briefly, all nodules were collected from each plant, placed in 20-ml empty bottles with 2 ml of acetylene (10% vol/vol), and incubated upside down at 28°C for 2 h. The ethylene production was detected by using an HP 7280A series gas chromatograph system. Gas chromatography was conducted to measure peak height of ethylene and acetylene with 100 μl of gas. The approximate nitrogenase activity was expressed as μmol of C2H4 of acetylene production per gram of nodule dry weight.
TABLE 1.
Symbiosis phenotypes of wild-type and QS mutant strainsa
| Strainb | First appearance of nodules (day) | Dry wt (mg/nodule) | Total no. of nodules | Pink nodule formation (%)c | Acetylene reduction (μmol/g nodules) |
|---|---|---|---|---|---|
| Wild type | 7.3 (1.0) | 59.6 (38.2) | 93.3 (34.0) | 89.5 | 20.4 (12.1) |
| cinR strain | 7.4 (0.4) | 48.3(33.1) | 60.0 (20.7)** | 100* | 8.1 (4.3) ** |
| cinI strain | 8.0 (1.5) | 56.3 (24.9) | 103.9 (35.4) | 84.8 | 15.0 (10.1) |
| raiR strain | 7.1 (0.8) | 45.4 (14.8) | 73.9 (25.7) | 82.5 | 11.4 (6.1) * |
| raiI strain | 8.5 (0.3) | 52.6 (17.4) | 106.1 (49.8) | 74.7* | 17.1 (6.1) |
| traR strain | 7.4 (0.6) | 47.6 (18.1) | 72.9 (31.3) | 83.7 | 10.5 (9.9) |
| traI strain | 6.8 (0.7) | 51.7 (17.0) | 80.4 (19.3) | 87.2 | 7.8 (4.6) ** |
Values are means (standard deviations) based on the examination of at least 16 replicates per strain. *, P < 0.05; **, P < 0.005 (Student t test).
Blank inoculum did not form any nodules.
Percentage of the total number of nodules.
RESULTS AND DISCUSSION
R. etli CFN42 possesses at least three LuxRI regulatory systems with different structures of AHLs.
The R. etli CFN42 genome contains three different LuxI-type AHL synthases (CinI, RaiI, and TraI) and four cognate LuxR-type regulators (CinR, RaiR, TraR1, and TraR2) (10) separately located on the chromosome and plasmids p42f and p42a (Fig. 1A). In addition to the previously reported TraI-TraR1-TraR2 system (12), sequence analysis demonstrated that putative CinI and CinR in R. etli also show a high degree of similarity at the amino acid level (95% and 97%, respectively) to CinI (GenBank accession number YP_768958.1) and CinR (YP_768957.1) from R. leguminosarum biovar viciae (AAF89900). Putative RaiI from R. etli shares 37% identity to RaiI (GenBank accession number CAK08866.1) from R. leguminosarum biovar viciae. The raiR gene, located 162 bp downstream of raiI, encodes a 212-amino-acid protein similar to BisR (GenBank accession number YP_768957.1) (31% identity) of R. leguminosarum biovar viciae.
To determine whether these LuxI family proteins are responsible for AHL synthesis in the R. etli CFN42 streptomycin-resistant derivative CE3, we first visualized AHL production in cell-free culture supernatant collected from the early stationary phase of the wild type and the triple LuxI deletion mutant ΔcinI ΔraiI ΔtraI strain by applying a thin-layer chromatography (TLC) assay (16). As shown in Fig. 1B, high AHL activity was produced in wild-type cells, but no AHL was detected in the ΔcinI ΔraiI ΔtraI mutants, indicating that in R. etli, CinI, RaiI, and TraI are responsible for all AHLs synthesized, at least under the growth condition tested. To determine the contents of AHL production of annotated AHL synthases, we expressed the raiI, traI, and cinI genes from a Ptac promoter carried on the low-copy-number plasmid pYC12 (23) in ΔcinI ΔraiI ΔtraI mutants. TLC analysis shows that these three AHL synthases produced distinct AHLs in R. etli (Fig. 1B). Of note, expression of raiI and traI in the triple AHL synthase mutant produced additional spots in our TLC assays (Fig. 1B), possibly due to the overexpression of these AHL synthase genes. The structures of these AHLs produced in R. etli are under investigation.
R. etli QS deletion mutants alter AHL production.
To further investigate the functions of the R. etli QS regulatory genes, we first examined AHL production and AHL content changes in a series of in-frame mutations of three pairs of LuxRI homologues. Wild-type R. etli produced AHL molecules in a typical cell density-dependent manner, with low AHL activity at early time points and maximal levels in early-stationary-phase cultures (Fig. 2A and B, filled circles). Mutations in raiI and raiR did not significantly alter AHL production (Fig. 2A and B, filled squares). To ensure that this phenotype was not due to excessive input of supernatant into the bioassay strain, a series of dilutions for cell-free culture supernatants were assayed, and no significant difference in AHL production levels of these strains was observed (data not shown). Intriguingly, both raiI and raiR mutants produced more AHLs than the wild type in the TLC analysis (Fig. 2C) but not in a more quantitative liquid assay (Fig. 2A and B). The reason for this is not clear. Deletion of the TraI-TraR circuit, on the other hand, altered AHL production in R. etli, particularly during the early log phase (Fig. 2A and B, filled triangles). Complementation of traI or traR on a plasmid restored AHL production of the traI or traR mutant to the wild-type level (see Fig. S1A and B in the supplemental material), suggesting that the TraI-TraR system may be functional as early as early log phase. Mutation of cinI or cinR resulted in a dramatic decrease in AHL accumulation (Fig. 2A and B) as well as AHL content (Fig. 2C). Complementation of cinI or cinR on a plasmid in a cinI or cinR mutant restored AHL production (see Fig. S1A and B). In addition, AHL production was significantly reduced in all QS double and triple mutants tested (Fig. 2A and B, open symbols). These data imply that CinRI may be situated at the top of a cascade of other QS regulators.
CinR is required for cinI expression, and CinI-produced AHLs enhance CinR activity.
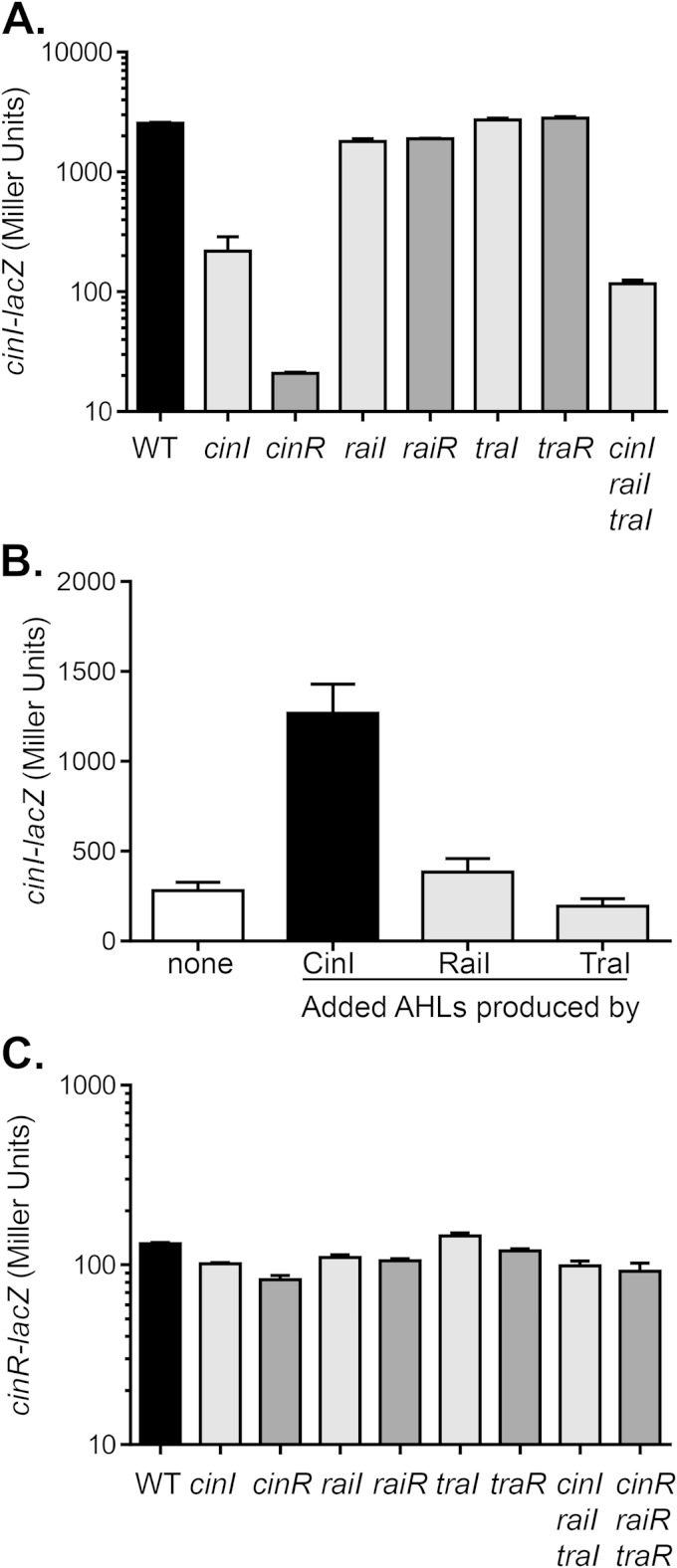
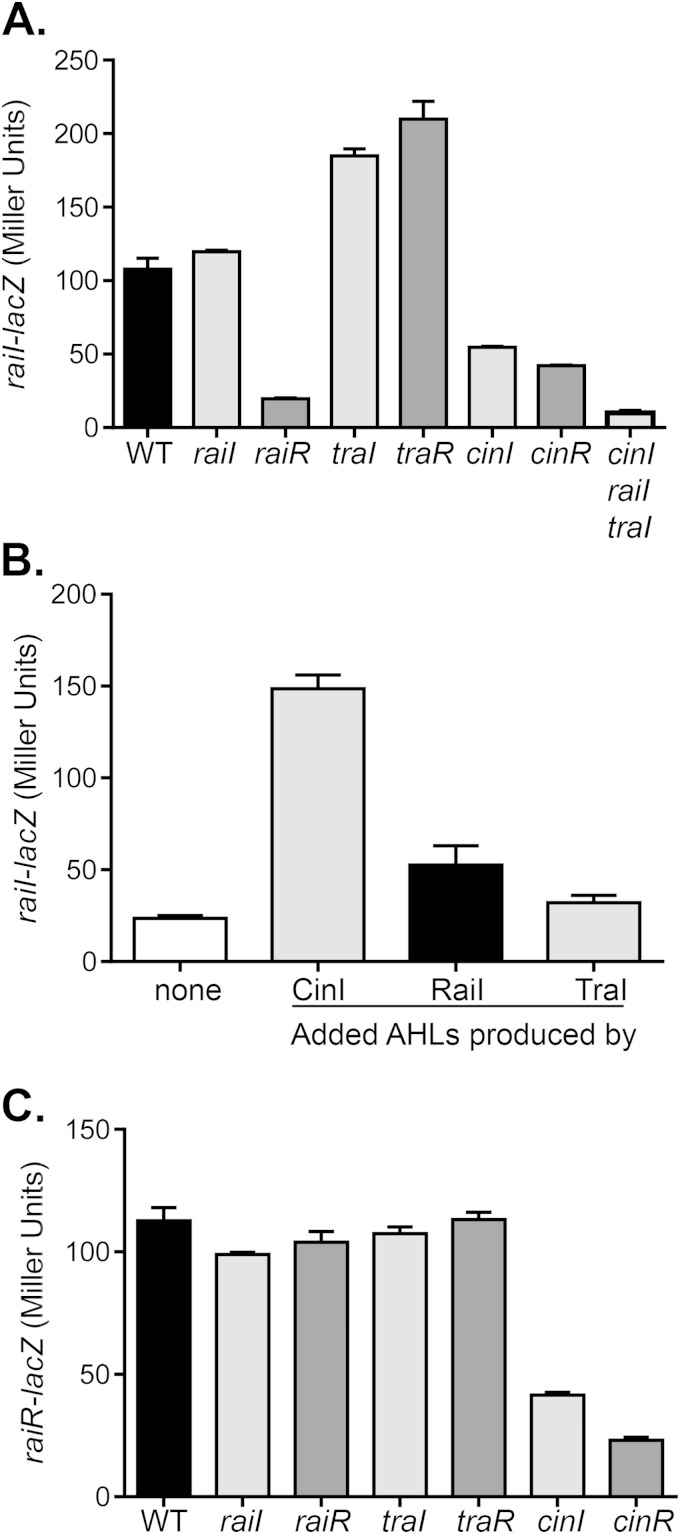
To understand the possible interactions between different QS systems, we constructed QS regulatory gene promoter-lacZ fusion plasmids and examined β-galactosidase activity in the wild type and QS mutants. Compared to the wild type, cinI expression was abrogated in cinR mutants (Fig. 3A), indicating that CinR is required for cinI activation. Interestingly, cinI expression was reduced in cinI mutants as well as in triple AHL synthase mutants but not as severely as in cinR mutants (Fig. 3A), suggesting that CinR may be functionally independent of cognate AHLs. RaiRI and TraRI did not regulate cinI as cinI expression was unaltered in these mutants (Fig. 3A). To examine cinI expression in response to exogenous AHLs, we added AHLs produced by CinI, RaiI, or TraI to cinI mutants. We found that the expression of cinI was enhanced only in the presence of its cognate AHLs (Fig. 3B). We also examined the expression of cinR. We found that cinR expression was not regulated by other QS components, including CinR itself (Fig. 3C). These data suggest that CinR is required for cinI expression and that CinR may be active in the absence of AHLs.
FIG 3.
Roles of QS genes in cinRI expression. (A) The wild type and QS mutants containing cinI-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. (B) cinI-lacZ expression in cinI mutants grown in PY medium supplemented with 5% cell-free supernatants from ΔcinI ΔraiI ΔtraI mutants containing Ptac-cinI, Ptac-raiI, or Ptac-traI for 24 h. β-Galactosidase levels were then measured and reported in Miller units. (C) The wild type and QS mutants containing cinR-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. The amount of β-galactosidase of a wild type containing the pRA302 vector control under the same growth condition was <10 units. The data shown represent the means of three independent experiments, the standard deviations for which are indicated by the error bars.
To further investigate how CinR acts on the cinI promoter, we constructed and purified His-tagged CinR fusion recombinant proteins. We found that, unlike many other LuxR family proteins (24), CinR was soluble in the absence of its cognate AHLs (data not shown). This allowed us to evaluate DNA-binding affinity in the absence and presence of AHLs by electrophoretic mobility shift assays (EMSAs) using a biotin-labeled 300-bp DNA fragment containing the cinI promoter. The CinR protein displayed a specific affinity for the cinI promoter fragment in the absence of AHLs (Fig. 4A, top panel). Addition of unlabeled specific DNA abolished the shift, whereas adding unlabeled nonspecific competitive DNA did not alter the shift (Fig. 4A, top panel, last two lanes), indicating that the binding of CinR to the promoter is specific. Addition of cognate AHL (purified from cultures of E. coli that overexpressed cinI) in the binding reaction mixture caused CinR to form DNA-protein complexes at lower protein concentrations (Fig. 4A, lower panel). We estimate that the DNA-binding affinity of CinR to the cinI promoter in the presence of AHLs was approximately 20-fold higher than that of CinR in the absence of the signals (Fig. 4B). Additionally, we used AHLs purified from an R. etli triple AHL synthase mutant containing Plac-cinI and found that these AHLs could also enhance CinR binding of the cinI promoter, similar to those purified from E. coli (see Fig. S2 in the supplemental material). These results suggest that apo-CinR is able to bind its target promoter and that in the presence of its cognate ligands, CinR-AHL complex may bind to the target with higher affinity. The EMSA data are consistent with the cinI-lacZ expression profiles (Fig. 3A).
CinRI activates both RaiRI and TraRI circuits.
To test whether the CinRI QS system affects the expression of the other two QS circuits, we examined the expression of raiRI and traRI in the wild-type and QS mutant strains. As shown in Fig. 5A, mutation of raiR completely abolished induction of raiI, but raiI itself did not significantly affect raiI expression. Addition of exogenous AHLs made by RaiI did not significantly enhance raiI expression (Fig. 5B). Mutation of raiR or raiI had almost no detectable effect on raiR expression (Fig. 5C). We also examined chromosomal raiR expression using RT-qPCR to verify if there are additional promoters upstream of raiR (e.g., from raiI). We found that raiR expression was not altered in the wild type or in the raiR mutants (see Fig. S2 in the supplemental material). Taken together, these results suggest that raiI expression is independent of the cognate AHLs but dependent on RaiR, the expression of which is not autoregulated. Moreover, although the TraRI system had little, if any, effect on raiRI expression, CinRI was apparently required for the full induction of raiRI (Fig. 5A and C). Additional AHLs made by CinI also stimulated raiI expression (Fig. 5B), which is again suggestive of the induction of raiI by CinRI.
FIG 5.
Roles of QS genes in raiRI expression. (A) The wild type and QS mutants containing raiI-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. (B) raiI-lacZ expression in raiI mutants grown in PY medium supplemented with 5% cell-free supernatants from ΔcinI ΔraiI ΔtraI mutants containing Ptac-cinI, Ptac-raiI, or Ptac-traI for 24 h. β-Galactosidase levels were then measured and are reported in Miller units. (C) The wild type and QS mutants containing raiR-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. The amount of β-galactosidase of a wild type containing the vector pRA302 control under the same growth condition was <10 units. The data shown represent the means of three independent experiments, the standard deviations for which are indicated by the error bars.
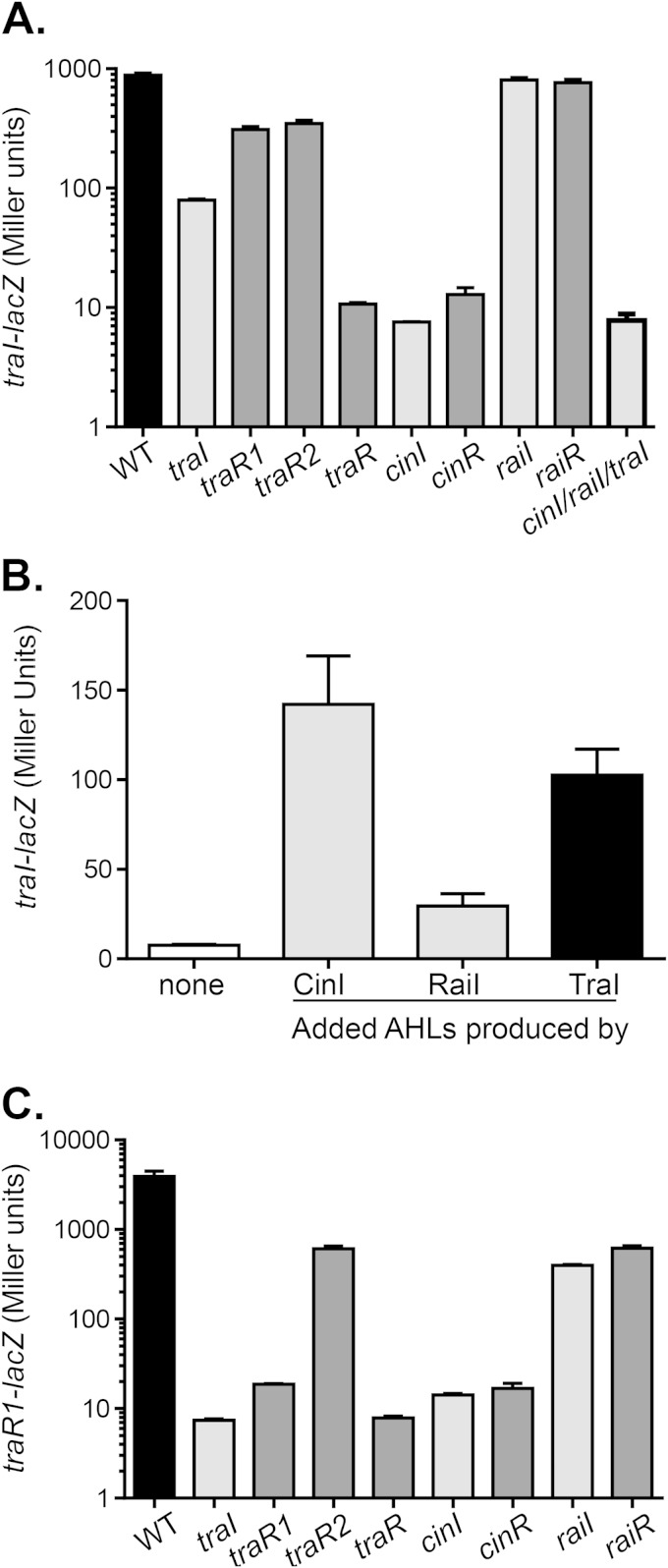
We then examined traRI expression. We found that the level of traI expression was reduced by approximately 10-fold compared to that in the wild type. In the alignment of the tra locus, there are two adjacent LuxR-type QS regulatory genes, traR1 and traR2, both of which had been reported to be required for inducing traI expression (12). As shown in Fig. 6A, each strain carrying a mutation in either of the two traR homologues resulted in about a 2.5-fold decrease in traI expression. Similarly, addition of exogenous AHLs produced by TraI to traI mutants restored traI expression (Fig. 6B). Moreover, traI expression was completely abolished in the traR1-traR2 double mutants, suggesting that both TraR1 and TraR2 are involved in traI expression and that they function in parallel, similar to the regulation of the tra system in R. leguminosarum biovar viciae (25). Since traR1 and traR2 are likely to form an operon (www.microbesonline.org) (26), we studied only traR1 expression. As shown in Fig. 6C, we found that the traR1 mutation by itself, but not mutation of traR2, greatly reduced the expression of traR1, and the combination of mutations in traR1 and traR2 further abolished its expression. Furthermore, the traI mutation abrogated traR1 expression. These results suggest that traR1 expression is autoregulated in a cognate AHL-dependent fashion. In terms of cross talk regulation, RaiRI did not affect traI expression but had a slight impact on traR1, whereas CinRI was critical for the expression of both traI and traR1 (Fig. 6A and C), and addition of AHLs produced by CinI stimulated traI expression (Fig. 6B), suggesting that CinRI is at the top of the QS regulatory network.
FIG 6.
Roles of QS genes in traRI expression. (A) The wild type and QS mutants containing traI-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. (B) traI-lacZ expression in traI mutants grown in PY medium supplemented with 5% cell-free supernatants from ΔcinI ΔraiI ΔtraI mutants containing Ptac-cinI, Ptac-raiI, or Ptac-traI for 24 h. β-Galactosidase levels were then measured and are reported in Miller units. (C) The wild type and QS mutants containing traR1-lacZ plasmids were grown in PY medium for 24 h. Gene expression was analyzed by measuring β-galactosidase levels and is reported in Miller units. traR represents the traR1-traR2 double mutant. The amount of β-galactosidase of a wild type containing the pRA302 vector control under the same growth condition was <10 units. The data shown represent the means of three independent experiments, the standard deviations for which are indicated by the error bars.
QS regulatory systems in R. etli CFN42 are important for symbiosis.
Previous works have shown that QS is involved in symbiotic processes in the R. etli CNPAF512 strain (8, 9). However, it is not known how R. etli CFN42 QS contributes to symbiosis. We thus examined wild-type R. etli CFN42 and its QS mutants for their ability to nodulate and fix nitrogen on the common bean (Phaseolus vulgaris Linn). Although in all mutants nodules started forming at the same time as in the wild type and had an average size similar to that of the wild type, cinR mutants had reduced numbers of nodules formed (Table 1). Moreover, the N2 fixation efficiency of nodules formed by cinR, raiR, and traI mutants was significantly lower than that of the wild type. Intriguingly, unlike traI mutants, traR mutants did not display a N2 fixation deficiency. It is possible that an additional regulator is involved in TraI-produced AHL signals in nodules. Taken together, these data suggest that all three QS regulatory systems are involved in nodule formation and nitrogen fixing in R. etli CFN42. How QS regulates these symbiosis processes is under investigation.
QS systems in rhizobia have been shown to be complex and play important roles in symbiosis processes (2). In this study, we have identified three pairs of QS regulatory systems in R. etli CFN42: CinRI, RailRI, and TraRI. We found that CinRI regulates the other two circuits, which are also autoregulated (the working model is shown in Fig. 7). This QS regulatory pathway bears similarity to that in R. leguminosarum biovar viciae, which contains four different LuxRI-type QS systems, identified as rai, tra, rhi, and cin loci. As with R. etli, the cin locus is situated at the top of the hierarchical regulatory network and induces the other three QS systems (25, 27). CinR induces cinI expression in response to its signal, 3-OH-C14:1-HSL. Mutation of cinI or cinR causes decreased production of all other short-chain AHLs synthesized by RaiI (28), TraI (25), and RhiI (29). RaiR induces raiI in response to RaiI-made AHLs and other noncognate AHLs, but mutations in raiI and raiR have no observable phenotypic effects on symbiosis (28). Both TraI-made and CinI-made AHLs induce the expression of traR, the gene product of which is necessary for symbiotic plasmid transfer (25). RhiR strongly induces rhiI in response to RhiI-made AHLs, and it is hypothesized that the rhi operon may play a role in the early stages of the symbiotic process (30). Although CinR-CinI is at the top of the regulatory cascade, the mechanisms of regulation of tra, rai, and rhi loci are different. The cin genes activate the tra QS regulon via yet another LuxR-type regulator, BisR, the gene of which is located directly upstream of traR (25). CinR regulation of rai and rhi is coordinated by the cinS gene, which encodes a small regulatory protein cotranscribed with cinI (31). Additionally, the LuxR-type regulator ExpR is also required for full induction of the raiR gene (31).
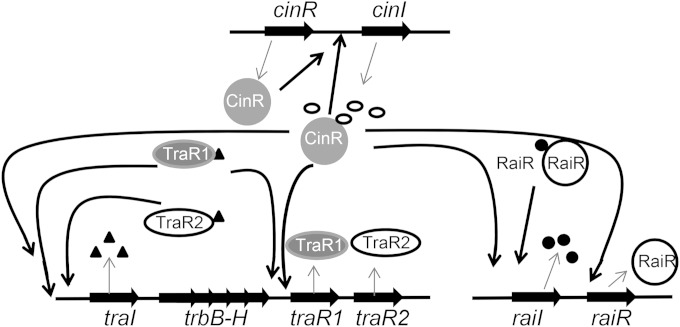
FIG 7.
Model of the QS regulation network in R. etli. R. etli harbors three QS systems. The CinRI system activates CinR-mediated regulation of cinI to form a positive-feedback loop. CinRI also positively controls the rai and tra systems. raiI is activated by RaiR. In the tra system, TraR1 and TraR2 positively influence traI expression, which activates traI itself.
Interestingly, CinR proteins in R. etli are soluble in the absence of their cognate ligands and can bind to cinI promoter DNA independent of AHLs (Fig. 4). Many other LuxR-type proteins, such as TraR (24), LasR (32), and LuxR (33), form inclusion bodies when overexpressed in E. coli in the absence of their cognate AHLs, whereas some LuxR-type proteins fold independent of their ligand, including MrtR in Mesorhizobium tianshanense (34), EsaR in Pantoea stewartii (35), ExpR in Erwinia chrysanthemi (36), and CarR39006 in Serratia (37). However, these LuxR family proteins either require cognate AHLs (MrtR), are antagonized by their cognate AHLs (EsaR and ExoR) or are unaffected by AHLs (ligand-independent CarR39006). In contrast, the CinR protein in R. etli is already active in the absence of AHLs and further activated by the cognate AHLs, a pattern never observed before and thereby representing a novel LuxR family protein. It is not clear whether this regulation has any physiological significance for R. etli biology and symbiosis. Further investigation is needed to fully understand how these three QS regulatory systems regulate R. etli symbiosis in natural environments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susana Brom for providing the R. etli CE3 strain and Xu Zhang for technical support and helpful discussions.
This study was supported by 973 projects (2010CB126502 and 2015CB15060002) and NSFC grants (31470202 and 31170077).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00003-15.
REFERENCES
- 1.Fuqua WC, Winans SC. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol 176:2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez JE, Marketon MM. 2003. Quorum sensing in nitrogen-fixing rhizobia. Microbiol Mol Biol Rev 67:574–592. doi: 10.1128/MMBR.67.4.574-592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Contreras M, Bauer WD, Gao M, Robinson JB, Allan Downie J. 2007. Quorum-sensing regulation in rhizobia and its role in symbiotic interactions with legumes. Philos Trans R Soc Lond B Biol Sci 362:1149–1163. doi: 10.1098/rstb.2007.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao M, Chen H, Eberhard A, Gronquist MR, Robinson JB, Rolfe BG, Bauer WD. 2005. sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J Bacteriol 187:7931–7944. doi: 10.1128/JB.187.23.7931-7944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marketon MM, Gronquist MR, Eberhard A, Gonzalez JE. 2002. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J Bacteriol 184:5686–5695. doi: 10.1128/JB.184.20.5686-5695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marketon MM, Gonzalez JE. 2002. Identification of two quorum-sensing systems in Sinorhizobium meliloti. J Bacteriol 184:3466–3475. doi: 10.1128/JB.184.13.3466-3475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sourjik V, Muschler P, Scharf B, Schmitt R. 2000. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J Bacteriol 182:782–788. doi: 10.1128/JB.182.3.782-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels R, De Vos DE, Desair J, Raedschelders G, Luyten E, Rosemeyer V, Verreth C, Schoeters E, Vanderleyden J, Michiels J. 2002. The cin quorum sensing locus of Rhizobium etli Cnpaf512 affects growth and symbiotic nitrogen fixation. J Biol Chem 277:462–468. doi: 10.1074/jbc.M106655200. [DOI] [PubMed] [Google Scholar]
- 9.Rosemeyer V, Michiels J, Verreth C, Vanderleyden J. 1998. luxI- and luxR-homologous genes of Rhizobium etli Cnpaf512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriol 180:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Case RJ, Labbate M, Kjelleberg S. 2008. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J 2:345–349. doi: 10.1038/ismej.2008.13. [DOI] [PubMed] [Google Scholar]
- 11.Crossman LC, Castillo-Ramirez S, McAnnula C, Lozano L, Vernikos GS, Acosta JL, Ghazoui ZF, Hernandez-Gonzalez I, Meakin G, Walker AW, Hynes MF, Young JP, Downie JA, Romero D, Johnston AW, Davila G, Parkhill J, Gonzalez V. 2008. A common genomic framework for a diverse assembly of plasmids in the symbiotic nitrogen fixing bacteria. PLoS One 3:e2567. doi: 10.1371/journal.pone.0002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tun-Garrido C, Bustos P, Gonzalez V, Brom S. 2003. Conjugative transfer of P42a from Rhizobium etli Cfn42, which is required for mobilization of the symbiotic plasmid, is regulated by quorum sensing. J Bacteriol 185:1681–1692. doi: 10.1128/JB.185.5.1681-1692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noel KD, Sanchez A, Fernandez L, Leemans J, Cevallos MA. 1984. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol 158:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 15.Cai T, Cai W, Zhang J, Zheng H, Tsou AM, Xiao L, Zhong Z, Zhu J. 2009. Host legume-exuded antimetabolites optimize the symbiotic rhizosphere. Mol Microbiol 73:507–517. doi: 10.1111/j.1365-2958.2009.06790.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Chai Y, Zhong Z, Li S, Winans SC. 2003. Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl Environ Microbiol 69:6949–6953. doi: 10.1128/AEM.69.11.6949-6953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 18.Shaw PD, Ping G, Daly SL, Cha C, Cronan JE, Rinehart KL, Farrand SK. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci U S A 94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akakura R, Winans SC. 2002. Mutations in the occQ operator that decrease OccR-induced DNA bending do not cause constitutive promoter activity. J Biol Chem 277:15773–15780. doi: 10.1074/jbc.M200109200. [DOI] [PubMed] [Google Scholar]
- 20.Fåhraeus G. 1957. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol 16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- 21.Hunt S, Layzell DB. 1993. Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Biol 44:483–511. doi: 10.1146/annurev.pp.44.060193.002411. [DOI] [Google Scholar]
- 22.Silvester WB, Sollins P, Verhoeven T, Cline SP. 1982. Nitrogen fixation and acetylene reduction in decaying conifer boles: effects of incubation time, aeration, and moisture content. Can J Forest Res 12:646–652. doi: 10.1139/x82-098. [DOI] [Google Scholar]
- 23.Wang H, Zhong Z, Cai T, Li S, Zhu J. 2004. Heterologous overexpression of quorum-sensing regulators to study cell-density-dependent phenotypes in a symbiotic plant bacterium Mesorhizobium huakuii. Arch Microbiol 182:520–525. doi: 10.1007/s00203-004-0735-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Winans SC. 1999. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci U S A 96:4832–4837. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danino VE, Wilkinson A, Edwards A, Downie JA. 2003. Recipient-induced transfer of the symbiotic plasmid Prl1ji in Rhizobium leguminosarum bv. viciae is regulated by a quorum-sensing relay. Mol Microbiol 50:511–525. doi: 10.1046/j.1365-2958.2003.03699.x. [DOI] [PubMed] [Google Scholar]
- 26.Price MN, Huang KH, Alm EJ, Arkin AP. 2005. A novel method for accurate operon predictions in all sequenced prokaryotes. Nucleic Acids Res 33:880–892. doi: 10.1093/nar/gki232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lithgow JK, Wilkinson A, Hardman A, Rodelas B, Wisniewski-Dye F, Williams P, Downie JA. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol Microbiol 37:81–97. doi: 10.1046/j.1365-2958.2000.01960.x. [DOI] [PubMed] [Google Scholar]
- 28.Wisniewski-Dye F, Jones J, Chhabra SR, Downie JA. 2002. raiIR genes are part of a quorum-sensing network controlled by cinI and cinR in Rhizobium leguminosarum. J Bacteriol 184:1597–1606. doi: 10.1128/JB.184.6.1597-1606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodelas B, Lithgow JK, Wisniewski-Dye F, Hardman A, Wilkinson A, Economou A, Williams P, Downie JA. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J Bacteriol 181:3816–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cubo M, Economou A, Murphy G, Johnston A, Downie J. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J Bacteriol 174:4026–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards A, Frederix M, Wisniewski-Dyé F, Jones J, Zorreguieta A, Downie JA. 2009. The cin and rai quorum-sensing regulatory systems in Rhizobium leguminosarum are coordinated by ExpR and CinS, a small regulatory protein coexpressed with CinI. J Bacteriol 191:3059–3067. doi: 10.1128/JB.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster M, Urbanowski ML, Greenberg EP. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci U S A 101:15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbanowski ML, Lostroh CP, Greenberg EP. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J Bacteriol 186:631–637. doi: 10.1128/JB.186.3.631-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Giel JL, Cai T, Zhong Z, Zhu J. 2009. The Luxr family quorum-sensing activator MrtR requires its cognate autoinducer for dimerization and activation but not for protein folding. J Bacteriol 191:434–438. doi: 10.1128/JB.01247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minogue TD, Carlier AL, Koutsoudis MD, von Bodman SB. 2005. The cell density-dependent expression of stewartan exopolysaccharide in Pantoea stewartii ssp. stewartii is a function of EsaR-mediated repression of the rcsA gene. Mol Microbiol 56:189–203. doi: 10.1111/j.1365-2958.2004.04529.x. [DOI] [PubMed] [Google Scholar]
- 36.Castang S, Reverchon S, Gouet P, Nasser W. 2006. Direct evidence for the modulation of the activity of the Erwinia chrysanthemi quorum-sensing regulator ExpR by acylhomoserine lactone pheromone. J Biol Chem 281:29972–29987. doi: 10.1074/jbc.M601666200. [DOI] [PubMed] [Google Scholar]
- 37.Poulter S, Carlton TM, Spring DR, Salmond GP. 2011. The Serratia LuxR family regulator CarR 39006 activates transcription independently of cognate quorum sensing signals. Mol Microbiol 80:1120–1131. doi: 10.1111/j.1365-2958.2011.07634.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Beaber JW, More MI, Fuqua C, Eberhard A, Winans SC. 1998. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol 180:5398–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.