Abstract
The sensory reception of vision, olfaction, hearing and balance are mediated by receptors that reside in specialized epithelial organs. Age-related degeneration of the photoreceptors in the retina and the hair cells in the cochlea, caused by macular degeneration and sensorineural hearing loss, respectively, affect a growing number of individuals. Although sensory receptor cells in the mammalian retina and inner ear show only limited or no regeneration, in many non-mammalian vertebrates, these sensory epithelia show remarkable regenerative potential. We summarize the current state of knowledge of regeneration in the specialized sense organs in both non-mammalian vertebrates and mammals, and discuss possible areas where new advances in regenerative medicine might provide approaches to successfully stimulate sensory receptor cell regeneration. The field of regenerative medicine is still in its infancy, but new approaches using stem cells and reprogramming suggest ways in which the potential for regeneration may be restored in individuals suffering from sensory loss.
Introduction
Our special senses, vision, olfaction, taste, hearing and balance are mediated by receptors that reside in specialized epithelial organs. To best capture the physical stimuli required for their function these receptors are “exposed” to the environment and subject to excesses in the very stimuli they are optimized to detect. Olfactory receptor cells have an average lifetime of a few months. Excessive noise leads to the degeneration of auditory hair cells; constant high levels of illumination can cause retinal photoreceptors loss. In addition, sensory receptor cells have many specialized proteins which are not present in other tissues; mutations in the genes coding for these proteins are often not lethal due to their very specific expression, but can cause sensory receptor degeneration, leading to devastating syndromes in humans. Individuals with Usher’s syndrome, for example, in which both the photoreceptors in the retina and the hair cells in the cochlea degenerate, ultimately become both blind and deaf. While thankfully these disorders are rare, more common degenerative disorders of the retina and cochlea, such as macular degeneration and most acquired sensorineural hearing loss, are age-related and affect a growing number of individuals as the aged human population increases. It is estimated that over 50% of the individuals over 60 have significant hearing loss (Zhan et al., 2010). The sense of smell also declines with age, and at least some part of this decline may be related to a reduction in receptor neurons; estimates of olfactory impairment range from 50% to 75% of people over the age of 65 (Doty et al., 1984). Although there are focused efforts in medical and gene therapy to treat these conditions and slow the degeneration of sensory receptor cells, there are many millions of individuals with varying degrees of impairment already. Moreover, many people do not seek treatment until a significant percentage of the sensory receptors have already degenerated. For these patients, prosthetic devices or regenerative medical approaches may be the only options.
What hope have we for stimulating the functional regeneration of sensory epithelial receptor cells in the human retina and inner ear? The field of regenerative medicine is still in its infancy, but it is rapidly developing. New approaches using stem cells and reprogramming have provided insights into the plasticity of cell identity, suggesting new ways in which the potential for regeneration may be restored. Moreover, although sensory receptor cells in the mammalian retina and inner ear show only limited or no regeneration, in many non-mammalian vertebrates, these sensory epithelia show remarkable regenerative potential. In newts, for example, most parts of the eye regenerate. In birds, the sensory receptors in the auditory and vestibular (balance) organs regenerate almost completely after various types of injury. In this review, we will summarize the current state of knowledge for regeneration in the specialized sense organs in both non-mammalian vertebrates and mammals, and discuss possible areas where new advances in regenerative medicine might provide approaches to successfully stimulate sensory receptor cell regeneration in patients.
Functional and structural features of sensory epithelia
The specialized sensory organs that have been most well studied for their regeneration are the olfactory epithelium, the auditory and vestibular epithelia of the inner ear, and the retina of the eye. The details of the structure and function of these organs is beyond the scope of this review, but a brief description of their common features and their differences will place the research on their regeneration in context.
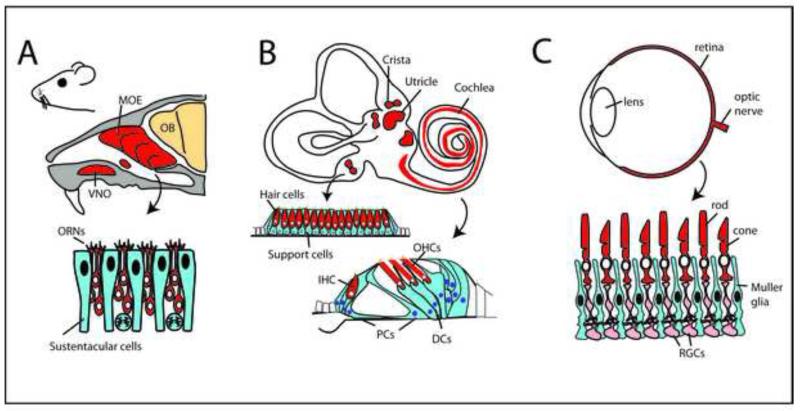
The olfactory epithelium is contained within the nasal cavity (Figure 1A). Most of the studies on regeneration have been done in the main olfactory epithelium, but many vertebrates also have additional sensory regions, like the vomeronasal organ. The olfactory receptor neurons have a single dendrite that extends to the apical surface of the epithelium and ends in a terminal knob, which has many small cilia extending into the mucosa. A single axon projects through the basal side of the epithelium through the lamina cribosa to terminate in the olfactory bulb. Each of the receptor neurons expresses one of a family of over 1000 olfactory receptor proteins, G-protein coupled receptor molecules, in their cilia (Kaupp, 2010) for recent review). The neurons are surrounded by glial-like cells, called sustentacular cells. Other cells in the epithelium contribute to the continual production of the new receptor neurons and will be described later in the review.
Figure 1.
Simplified schematic diagrams of the specialized sensory organs that have been most studied for their regeneration potential. (A) The main olfactory epithelium (MOE) and the vomeronasal organ (VNO) contain specialized sensory receptor neurons (ORNs) and supporting cells, called sustentacular cells. The axons of the ORNs project directly to the olfactory bulb (OB) in the brain. (B) The inner ear of vertebrates (top) contains two basic types of sensory receptor organs (red), the auditory sensory epithelia in the cochlea, known as the organ of Corti in mammals, and the vestibular epithelia-the three cristae, the utricle and the saccule. The vestibular epithelia (middle) are organized with alternating hair cells (red) and support cells (blue). In the organ of Corti (bottom), one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) alternate with various types of supporting cells, the pillar cells (PCs) and the Deiters’ cells (DCs). The hair cells are innervated by afferent fibers from associated ganglia (spiral ganglia) and from efferent fibers from the CNS. (C) The neurosensory retina (red) lines the back of the eye; it contains the sensory receptors, the rods and cones (red), supporting Müller glia (blue), and other neurons (light red) that process and relay the light responses of the photoreceptors to the brain via the optic nerve.
The vestibular and auditory epithelia in vertebrates have some structural similarities to the olfactory epithelia (Figure 1B). The mechanosensory receptor cells in these organs are called hair cells. There are five distinct regions of vestibular epithelia in the inner ear, the three cristae and the maculae of the utricle and saccule. Like the olfactory receptor neurons, the hair cells are surrounded on all sides by glial-like support cells, but are organized in a more regular mosaic than the olfactory receptor cells. In addition to the inner ear sensory epithelia, aquatic amphibians and fish have small mechanoreceptor organs distributed along the body, called the lateral line organs. All hair cells contain a mechanosensitive structure at their apical surface called the hair bundle, consisting of a group of approximately 100 actin-containing sterocilia and a microtubule containing kinocilium (for recent review, see,(Gillespie and Müller, 2009).
In the auditory sensory epithelium of non-mammalian vertebrates (the basilar papilla; BP), the hair cell and support cells have a similar organization to that in the vestibular organs, with alternating hair cells and support cells. However, in the mammalian auditory sense organ (the cochlea) the hair cells are organized in a striking pattern, with a single row of “inner” hair cells and three rows of “outer” hair cells, while the support cells assume a variety of specialized morphologies. The inner hair cells are the primary sensory receptors, while the outer hair cells act to amplify sound at least in part through regulation of cochlear stiffness. The inner hair cells are surrounded by specialized support cells, the inner phalangeal cells. Lning the space between the inner and outer hair cells, the tunnel of Corti, are the pillar cells, which provide rigidity and structure to the epithelium. Finally, the support cells associated with the outer hair cells are called Deiters’ cells, and each of these cells contain a process that reaches up around the outer hair cell and forms a contact with its apical surface. It is thought that the development of the tunnel of Corti and specializations of the cells may be an adaptation necessary for higher frequency hearing (Dallos and Harris, 1978; Hudspeth, 1985).
The sensory receptors for visual information, the rod and cone photoreceptor cells, are contained in a part of the CNS called the retina (Figure 1C). The retina is quite different in its embryology from the olfactory and inner ear sensory epithelia in that the former is derived from the neural plate with the rest of the CNS, while the latter two are derived from ectodermal placodes (Schlosser, 2010). There are several different types of cone photoreceptors, and the different types are most sensitive to a particular wavelength. In humans, cones with peak sensitivities to three different wavelengths (short, middle and long) provide us with trichromatic vision. Rods are specialized for high sensitivity at low light levels and are responsible for nighttime vision. All vertebrate retinas contain both rods and cones. The sensory receptors are concentrated at the apical surface of the retinal epithelium, organized in regular arrays and surrounded by glial cells, the Müller glia, that resemble the support cells and sustentacular cells of the inner ear and olfactory system, respectively. Phototransduction in the sensory receptors is mediated by G-protein coupled receptors, the opsins, which are concentrated in specialized cilia, the so-called outer segments. In addition to the sensory receptors and glia, the retina contains a group of projection neurons, called retinal ganglion cells, somewhat analogous to the spiral ganglion neurons in the auditory system as well as a diverse array of interneurons, more reminiscent of other CNS regions than the other sensory epithelia.
Ongoing Sensory Receptor Cell Production
Although most of the neurons in the nervous system of vertebrates are generated during a developmental period, some regions of the nervous system continue to add new neurons throughout life. For example, in mammals, neurons are generated in the hippocampus into adulthood (Hodge et al., 2008). In many vertebrates, new receptor cells are also added to the sensory organs. The cellular and molecular mechanisms that enable ongoing genesis of receptor cells in different specialized sensory epithelia in various species have some features in common that provide insights into what factors might be critical for regeneration (Figure 2).
Figure 2.
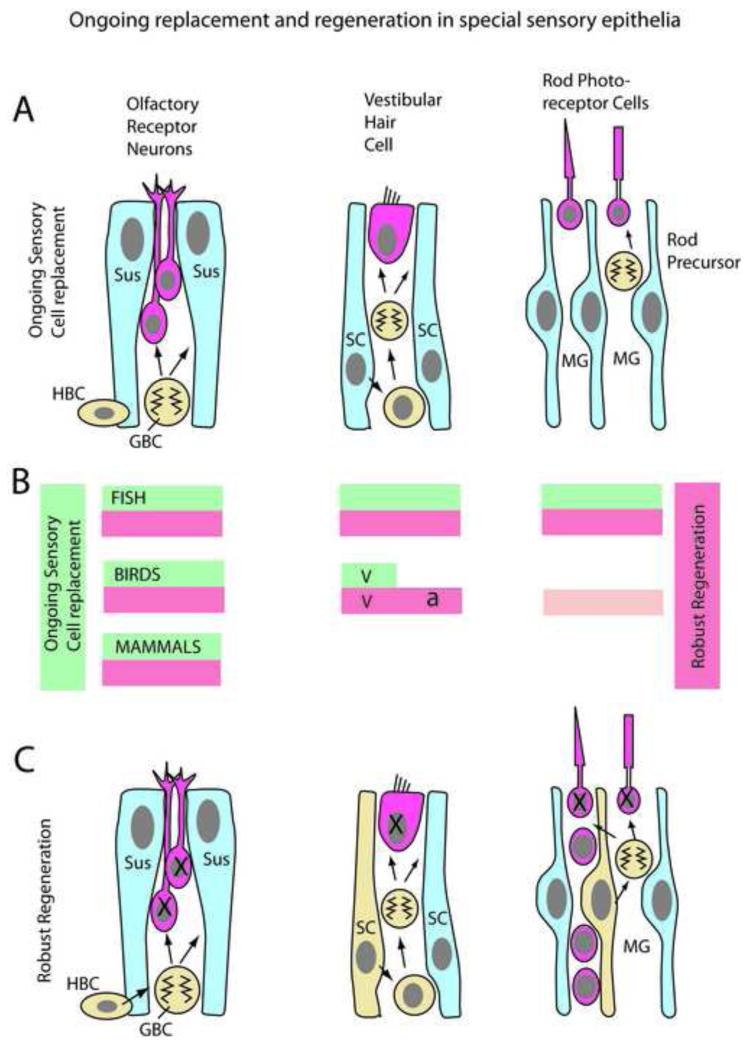
There is a very good correlation between the process of ongoing sensory receptor cell generation throughout life and the capacity for regeneration in the epithelium. (A) In the normal olfactory epithelium, vestibular system and retina in some vertebrates, there is a continual generation of new sensory receptor cells. The globose basal cells (GBCs; yellow) and Horizontal basal cells (HBCs; yellow) act as progenitors and stem cells for the other cells in the epithelium, the olfactory receptor neurons (red) and the sustentacular cells (blue). Similarly, in the vestibular epithelium of some vertebrates, the support cells (SC; blue) can enter the mitotic cell cycle (yellow) to generate additional support cells and hair cells (red). In the retina of fish, new rod photoreceptors (red) are continually added from mitotic divisions of the rod precursors (yellow), while the Müller glia (MG; blue) are quiescent. (B) The correlation between robust regeneration and ongoing sensory cell genesis is diagrammed as red (regeneration) and green (ongoing sensory cell production) bars. Exceptions to the general rule occur in the bird auditory epithelium (a) where there is no ongoing production of sensory receptor cells, but there is robust regeneration, whereas in the bird vestibular organs (v), both processes occur. The lighter red bar for the bird retina indicates that whereas there is robust proliferation of Müller glia after damage, much like that observed in fish, the number of new receptor cells generated is small. (C) The changes in these epithelia during regeneration are diagramed. In the olfactory epithelium, the GBCs increase in their proliferation after damage, and the HBCs are recruited when the damage is extensive. In the auditory system of birds, the support cells express Atoh1 and other developmental genes, and in some cases directly transdifferentiate into hair cells. If the retina is damaged in fish, the Müller glia re-enter the mitotic cell cycle and produce all types of retinal neurons, including both rods and cones.
The ongoing genesis of olfactory receptor cells is common to all vertebrates (see(Graziadei and Monti Graziadei, 1978),for review) and the rate of production is quite high. The production of new olfactory receptor cells is critical to the maintenance of this system, as the olfactory receptor cells only last a few months. The rate of production of new olfactory receptor cells is balanced by their loss so that a relatively stable population of these receptors is maintained. In the vestibular epithelium of fish (Corwin, 1981) amphibia (Corwin, 1985), and birds (Jorgensen and Mathiesen, 1988; Roberson et al., 1992), there is also ongoing production of the hair cells. However, in fish and amphibia, rather than the sensory receptor cell turnover that occurs in the olfactory epithelium, the ongoing production of new hair cells in vestibular epithelia results in an increase in the overall number of these cells as the animal grows (Corwin, 1985). The macula neglecta of skates for example, adds hair cells continuously through at least six years increasing more than 10 fold the number of hair cells with a 500 fold increase in sensitivity. The number of hair cells appears to scale with overall body size. In the toad sacculus, new hair cell addition occurs primarily at the peripheral edges; as a result, the epithelium is composed of concentric rings of progressively younger cells. The situation is somewhat different in the vestibular epithelia of birds. Although there is also good evidence for new hair cell production throughout life, the newly generated hair cells are frequently near apoptotic cells, and the number of hair cells does not increase over the life of the animal as it does in fish. Therefore, it is likely that the ongoing genesis of hair cells in birds may serve a maintenance role to replace dying hair cells, much like that in the olfactory epithelia (Jorgensen and Mathiesen, 1988; Roberson et al., 1992). In the retina of fish, there is also ongoing production of one type of sensory receptor, the rod photoreceptors (Johns and Easter, 1977; Raymond and Rivlin, 1987). Rod photoreceptor cells are not generated to replace dying cells, but rather they are generated as the retina grows, to keep the density of rod photoreceptors relatively constant with the growth of the animal, thereby maintaining light sensitivity (Fernald, 1990). In fish and amphibians, the retina also grows throughout life at its peripheral edge, adding new retinal cells of all types that seamlessly integrate with the existing retina (for review, Lamba et al, 2008). This process also occurs in birds to a limited extent (Fischer and Reh, 2000).
The continued production of sensory receptor cells in these epithelia requires a mitotic cell population that can act like the stem cells in non-neural epithelia. In the case of the olfactory epithelium, there are at least two types of mitotic cells, the globose basal cells (GBCs) and the horizontal basal cells (HBCs). The GBCs are mitotically active in the normal, undamaged epithelium and act as a multipotent progenitor to generate all of the other types of olfactory cells, including the sensory receptors (Caggiano et al., 1994; Chen et al., 2004; Huard et al., 1998). The more slowly cycling (or even quiescent) HBCs are more like “stem cells” serving both to replenish the more actively proliferating GBCs (Iwai et al, 2008) and as a reserve pool after more extensive damage to the receptors (Leung et al., 2007). The model of a slow-cycling stem cell (HBC) with a more rapidly-cycling, transit-amplifying progenitor cell (GBC) has similarities with non-neural epithelia, like the epidermis (Watt et al., 2006).The situation in the vestibular system of non-mammals is somewhat different, in that there does not appear to be a committed hair cell progenitor. Rather it appears that some or all of the support cells remain capable of mitotic division and divide at a low rate to produce both additional hair cells and support cells as the epithelium grows. In the retina, the source of the new rods in the fish is a group of cells called the rod precursors (Johns and Fernald, 1981) which typically generate only rod photoreceptors under normal conditions and are likely derived from the Müller glia (more on this later).
The different progenitors/precursors in these systems also share some common molecular expression patterns that are similar to those expressed during initial development (see Box). In the olfactory epithelium, for example, at least some of the GBCs express Ascl1, Neurog1, Sox2, and Pax6, genes critical during olfactory epithelial development (Guo et al., 2010; Manglapus et al., 2004). NeuroD1 is expressed at a slightly later stage, in the cells that will differentiate into the olfactory receptor neurons. In the inner ear,the support cells also express Sox2 (Oesterle et al., 2008), and many of the support cells that are in the S-phase or Mphase of the cell cycle, as well as the newly generated postmitotic daughters, express Atoh1 (Cafaro et al., 2007). In the retina, the rod precursor expresses NeuroD1 (Hitchcock and Kakuk-Atkins, 2004; Nelson and Reh, 2008), suggesting that these cells are at a slightly “later” stage in their development, consistent with their commitment to develop as rod photoreceptors and their expression of other photoreceptor specific transcription factors. The Müller glia, which act as the “stem” cell that gives rise to the rod precursors (Bernardos et al., 2007), express Sox2 and Pax6 (and Ascl1 after damage, see below), similar to the GBCs.
Box: Common pathways in the development of the sensory epithelia.
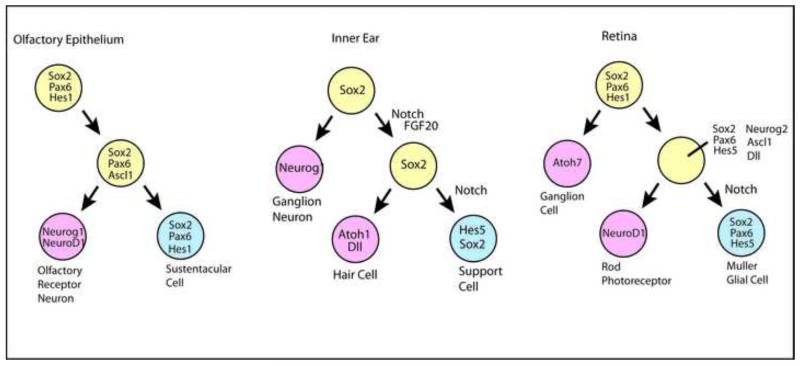
The development of the specialized sensory organs share many developmental mechanisms with one another and other regions of the nervous system (Figure). Paired-homeodomain (Pax), bHLH proneural/neural differentiation, SRY-related HMG-box (Sox), and homeodomain transcription factors, are all necessary for these sensory organs. Signaling factors and their receptors, including BMP, FGF, Shh, Wnt and Dll/Notch are also important in the development of these systems. In the following paragraphs, we will highlight a few key features of these molecules in the development of specialized sensory receptor cells that are particularly relevant to the studies on regeneration in these systems, but the reader is referred to several excellent reviews for a more comprehensive view (Chatterjee et al., 2010; DeMaria and Ngai, 2010; Driver and Kelley, 2009; Fuhrmann et al., 2000; Wallace, 2011; Swaroop et al., 2010).
In the development of all these sensory epithelia, Sox2 is one of the earliest required factors. Sox2 is required at a very early stage in the nasal placode for the initial formation of the olfactory sensory epithelium (Donner et al., 2007). In the inner ear, loss of Sox2 leads to the failure of production of hair cells and support cells in all inner ear sensory epithelia, including the auditory and vestibular sensory organs (Kiernan et al., 2005b). Sox2 is thus thought to specify the “sensory” identity in the otic vesicle, singling out those regions from the surrounding non-sensory epithelium. In the retina, a very similar phenotype occurs following conditional deletion of Sox2: no neurons of any type are produced and the proneural genes and neural differentiation genes are not expressed (Taranova et al., 2006). Another key regulator of sensory development is Pax6. Pax6, a member of the paired-homeodomain family of transcription factors, plays a critical role in eye development in animals as diverse as Drosophila to humans (Callaerts et al., 1997). In the retina, loss of Pax6 causes the progenitor cells to generate only retinal inter-neurons; photoreceptors are no longer produced (Marquardt and Gruss, 2002). Pax6 is thought to directly activate expression of the proneural genes Ascl1 and Neurog2 in the retina, thereby providing a link to the process of neurogenesis (Marquardt et al., 2001). Pax6 may play a similar role in the olfactory epithelium, since it is expressed throughout development and even in the mature epithelium, but there is an early requirement in olfactory placode that precludes the analysis of its functions in the later developmental stages. Pax2 is expressed in the inner ear sensory epithelia, and loss of Pax2 leads to defects in their development; however, few direct targets of Pax6 or Pax2 are known in the sensory epithelia, so it is difficult to know at this time whether they have similar functions in the eye and ear, respectively. In addition, it is important to note that Pax genes interact with many other transcription factors in combinatorial ways to regulate their targets. In the retina, for example, Pax6 is one of a group of “eye-field” transcription factors, which coordinately regulate one another in a concerted manner to specify the retinal fate (Zuber et al., 2003). An analogous set of organizing transcription factors may exist for the other sensory epithelia, though since both the olfactory and auditory/vestibular sensory cells emerge from placodes, instead of the neural tube, the specification mechanisms for these early stages may differ.
Once specific regions of the epithelia are specified as “sensory” by Sox2 and/or Pax genes, the process of neurogenesis begins in these domains, and several different bHLH genes become important in the production and differentiation of the sensory receptor cells in these regions. The proneural gene, Ascl1 is expressed in the developing retina and olfactory epithelium and is necessary for providing a neural competence in the progenitor cells (Cau et al., 2002; Cau et al., 1997; Jasoni et al., 1994; Nelson et al 2009). The proneural neurogenins are also expressed in the olfactory, retinal and inner ear epithelia, and play important roles in the production of specific types of neurons in each region. Loss of Neurogenins in the inner ear, for example, causes the failure of spiral ganglion neurons to develop (Ma et al., 2000). In addition to these proneural factors, other bHLH transcription factors are required for differentiation of the sensory receptor cells or their associated neurons. NeuroD1 is expressed in the photoreceptors in the retina, and targeted deletion of this gene in mice leads to a failure of normal cone photoreceptor differentiation and the degeneration of the rod photoreceptors (Liu et al., 2008). In the inner ear, NeuroD1 is required in the ganglion neurons that synapse with the hair cells (Jahan et al., 2010; Liu et al., 2000). One of the most important genes for hair cell development, Atoh1(Bermingham et al., 1999), is another member of the bHLH family of transcription factors. and is required for hair cell development, Targeted deletion of this gene results in the absence of hair cells in all the inner ear sensory epithelia, and over-expression of Atoh1 during development induces hair cells in nonsensory regions of the inner ear epithelium. Although not required for the sensory receptors in the retina, the related Atoh7/Math5 is necessary for the development of the retinal ganglion neurons (Brown et al., 1998). The similarity in the expression of the proneural and neural differentiation bHLH genes during development of the specialized sensory organs is quite striking and supports the idea that these systems have well conserved developmental mechanisms.
In addition to the transcription factors discussed above, the development of the specialized sensory structures is regulated by many different signaling factors. One of the most important is Notch signaling. Notch is required in all these systems and functions at several different stages of their development. For example, in the inner ear, Notch is initially required in the early specification of the Sox2 expressing presumptive sensory domain of the epithelium (Bermingham et al., 1999; Brooker et al., 2006; Daudet and Lewis, 2005; Kiernan et al., 2001; Kiernan et al., 2006). This requirement for Notch is called the prosensory function, and recent studies have shown that ectopic expression of active Notch is sufficient to produce sensory patches from the non-sensory epithelium (Hartman, 2010; Pan 2010). A later role for Notch signaling in the inner ear sensory epithelia is in its more traditional role: lateral inhibition. When hair cells begin to develop from the sensory epithelium, they signal via Dll1/Jag2/Notch1 interactions to suppress hair cell differentiation in the adjacent cells, and instead direct them to develop as support cells (Haddon et al., 1998). The Notch signal induces expression of Hes5, a downstream effector in the Notch pathway (Kageyama and Ohtsuka, 1999) and the Hes5 likely represses Atoh1, the bHLH class transcription factor necessary for hair cell development. In this way the alternating rows of hair cells and support cells are set up during development. Notch is also necessary for appropriate development of the retina. Maintained expression of Notch causes the progenitor cells to develop as Müller glia (Vetter and Moore, 2001), like the support cells in the ear, and loss Notch effectors, Hes5, Hes1 and Hesr2 leads to a reduction in Müller glial production (Hojo et al., 2000). Inhibition of the Notch pathway in the developing retina causes premature neural differentiation of the progenitor cells and the loss of Müller glia (Nelson et al., 2007).
Although Notch is perhaps the best studied signaling system in the sensory epithelia, several members of the FGF family of receptor tyrosine kinase ligands also are of critical importance. In the auditory epithelium of the inner ear, FGF20 and Fgfr1, are critical for the early stages of cochlear development, including the initiation of Atoh1 expression (Hayashi et al., 2008b; Pirvola et al., 2002). Later in cochlear development, FGF8 and Fgfr3 are necessary for the proper differentiation of one type of supporting cell, the pillar cells (Colvin et al., 1996; Dominguez-Frutos et al., 2009; Hayashi et al., 2007; Jacques et al., 2007; Puligilla et al., 2007). In the retina and olfactory system, FGF8 is also important for the early specification of the sensory domains, and several other FGFs and FGF receptors are expressed in these organs. Other signaling molecules, including members of the Wnt, BMP, EGF, and IGF families have been shown to be involved in the normal development in these systems, and although the details may be different, there are many conserved features.
BOX Figure.
Similarities in developmental mechanisms in the different specialized sensory epithelia relevant to studies on regeneration in these systems. The sensory receptor cells arise from specialized Sox2 expressing regions of the nasal and inner ear epithelia. The progenitor cells in these regions go on to express proneural bHLH transcription factors necessary to produce the receptor cells. A similar series of early events occurs in the retina, though it is derived from the neural tube. Notch signaling, and its downstream effectors Hes1 and Hes5, promote support cells and Müller glial fates in the inner ear and retina.
From this overview, several common features of ongoing sensory cell production emerge. First, the sensory receptor cells are derived from what might be called a “persistent progenitor” or “sensory receptor cell precursor”. In both the olfactory epithelium and the retina of fish, the immediate precursor to the receptor neurons/rods is a cell that seems to have a more limited capacity for cell division than a true “stem cell.” The rod precursor of fish is particularly committed to generating rod photoreceptors, and the GBC of the olfactory epithelium can generate most, though not all, of the cell types in the sensory epithelium. These cells have some similarity to the immediate neuronal precursors found in the cerebral cortex, or the progenitor/stem cells in the hippocampal and subventricular zone in that they are restricted to generate specific sub-types of neurons and their mitotic divisions do not occur at the ventricular surface (Hodge et al., 2008; Pontious et al., 2008). Second, many of the genes expressed in normal development in the lineages leading to the differentiated sensory receptor cells are also expressed in the progenitors responsible for the genesis of these cells in mature sensory epithelia. Third, the progenitors/precursors in the mature epithelia co-exist with differentiated, functioning sensory receptors, underscoring the fact that the maintenance of a “neurogenic” niche is not inconsistent with the environment of a mature neural tissue. Fourth, although the different systems have very different requirements for the maintenance of sensory cell addition throughout life, the addition of new sensory receptors seems to serve a very specific purpose in each system. Lastly, although the rate of new cell addition in the different systems varies considerably, where the olfactory epithelium generates new sensory receptors at a much higher rate than the other epithelia, the production of new cells appears to be under tight regulation, producing precisely the cell types necessary for maintenance and growth or regeneration of these structures.
Regeneration in olfactory epithelium
Of the specialized sensory epithelia, the olfactory epithelium shows the most robust regeneration in response to injury (Graziadei and Monti Graziadei, 1985). All cell types, including the sensory receptor neurons, can be regenerated in all species that have been examined. Severing the axons at the lamina cribosa in rats and mice causes extensive apoptosis in the olfactory receptor neurons within a few days (Cowan and Roskams, 2002). The epithelium at this point contains only the sustentacular cells and the globose and horizontal basal cells. The number of mitotic figures increases dramatically within the basal cell populations and within a few days new receptor neurons are evident. Labeling the epithelium with traceable thymidine analogs demonstrated that the proliferating cells generated new receptor neurons and sustentacular cells such that by 4 weeks after the injury, the epithelium was completely restored. The new receptor neurons extend their axons back to the olfactory bulb and they function normally. Other types of damage also trigger a regenerative response. Damage from the toxin methyl bromide (MeBr) causes an even more massive degeneration of the sensory epithelium, including the receptor neurons, the sustentacular supporting cells, and many of the GBCs; however, regeneration of the epithelium to the pre-lesion state occurs within 4 weeks of the insult.
Several in vitro and in vivo studies have attempted to identify the cells involved in the regeneration in this system (Beites et al., 2005; Calof et al., 2002; Carter et al., 2004; Huard et al., 1998; Kawauchi et al., 2004; Sicard et al., 1998). The two main candidates are the GBCs and the HBCs. The cells responsible for the regeneration of the epithelium under conditions of olfactory nerve transection, where the damage is largely confined to the olfactory receptor neurons, are likely the GBCs. Olfactory bulbectomy (essentially the same as olfactory nerve section) causes the GBCs to increase their rate of proliferation and quickly repopulate the missing cell types (Carr and Farbman, 1992). Under normal conditions, the HBCs are relatively quiescent, and even after bulbectomy, they are only occasionally found in the mitotic cycle. After the more extensive damage caused by MeBr, though, the HBCs also proliferate (Leung et al., 2007). Utilizing mice expressing Cre-recombinase under the keratin 5 (K5) promoter to label HBCs and track their progeny in vivo (Leung et al., 2007), these groups found that the lineage of the HBCs can include all the of different cell types of the epithelium, including the GBCs (even in normal mice, Iwai et al, 2008). However, after MeBr lesions, the proliferation of the HBCs is greatly increased, as is the production of GBCs (Leung et al, 2007).
Thus the current model is that the HBCs normally have a very low level of proliferation, sufficient to selfrenew and replenish the GBC population, while the GBCs act more like transit amplifying cells or immediate precursors to the cells of the sensory epithelium. A relatively small amount of damage activates the GBCs to produce receptor neurons at a higher rate, and these cells are certainly capable of generating the sustentacular cells as well. A large amount of damage to the epithelium recruits the HBCs to replace lost GBCs, which go on to generate receptor neurons and sustentacular cells. On a molecular level, many of the features of developmental neurogenesis are recapitulated. After MeBr lesion, the proneuronal factors reappear in a sequence that mimics their expression during the embryonic development of the olfactory epithelium (Cau et al., 2002; Manglapus et al., 2004): Mash1+ GBCs are destroyed by the MeBr, but they reappear in increased numbers two days after the MeBr. Ngn1/ NeuroD+ cells are also lost with MeBr damage, but are present three days after the damage (Guo et al., 2010) and precede production of new receptor neurons, which appear by four days post-lesion. Hes1 is expressed by the sustentacular cells in the normal epithelium, but after MeBr, GBCs also express Hes1, and some of these go on to differentiate into sustentacular cells.
Since the olfactory epithelium displays such robust regeneration it begs the question as to why we lose olfactory sensation as we age. The loss of sensory perception can result from changes in the sensory epithelia, or alternatively, from changes in the brain critical for processing the sensory information. There is evidence, however, that the number of receptor cells declines with age in humans. Moreover, in rats and mice, the density of proliferating (BrdU+) cells in the epithelium declines as the size of the epithelium grows (Weiler and Farbman, 1997); thus, while the overall number of proliferating cells does not decline by very much, the turnover of the receptor neurons, as indicated by the number of BrdU/OMP+ cells, declines with age (Kondo et al., 2010). This decline is also seen in the vomeronasal organ of mice, where Brann and Firestein reported that the number of proliferating cells declines with age (Brann and Firestein, 2010). Taken together, these studies suggest that the production of new receptor cells may not be able to keep pace with the increased loss of these cells that accompanies increasing age.
Regeneration in the inner ear
Some of the first evidence for regeneration of hair cells came from studies of the lateral line organs in fish and amphibia. The lateral line organs of fish and amphibia consist of mechanosensory neuromasts distributed along the body surface. In urodeles, after amputation of the tip of the tail, new neuromasts are generated in the lateral line organ at the stump and migrate to form new organs as the tail regenerates (Stone, 1937). Studies by Jones and Corwin demonstrated that a low level of ongoing hair cell production is dramatically up-regulated after hair cells in the lateral line are destroyed with a laser (Jones and Corwin, 1993, 1996). Direct time-lapse recordings demonstrated that the regenerated hair cells arose from support cells (Jones and Corwin, 1993). A similar increase in mitotic proliferation in the support cells occurs in zebrafish after various types of ototoxic damage (Hernandez et al., 2007; Ma et al., 2008; Williams and Holder, 2000), and the proliferating support cells go on to replace the hair cells within 48 hours of the insult.
Hair cell regeneration has also been extensively studied in both auditory and vestibular sensory organs. In the vestibular epithelia, regeneration of new hair cells has been reported in the cristae, and the saccule and macula (Warchol, 2010) of all non-mammalian vertebrates that have been studied such as fish (e.g., (Faucher et al., 2009), bullfrogs, newts and birds. In the Bullfrog saccule, many of the regenerated hair cells are newly generated and labeled with BrdU, but at least a fraction of the new hair cells arise from direct transdifferentiation of the support cells; ie. hair cells are regenerated even after inhibition of proliferation (Baird et al., 2000; Baird et al., 1993). In the newt, hair cell damage causes many support cells to enter the mitotic cell cycle, but in this system the proliferating BrdU+ cells do not contribute to the new hair cells (Taylor and Forge, 2005). Instead, all the new hair cells are thought to be due also to transdifferentiation.
Birds regenerate hair cells in both their vestibular epithelia and their auditory epithelia. Since the vestibular organs normally generate new hair cells throughout life in birds, like the olfactory epithelium, when the sensory receptor cells are destroyed, the proliferating cell population increases in the rate of new hair cell production and the normal number of sensory receptors is restored (Jorgensen and Mathiesen, 1988; Roberson et al., 1992; Weisleder and Rubel, 1993). The situation in the auditory epithelia (Basilar papilla) in birds is somewhat different. The BP in the bird shows robust regeneration after hair cells are destroyed with either oto-toxic drugs or from excessive noise (Cotanche et al., 1987; Cruz et al., 1987). In posthatch chicks, for example, experimental destruction of the hair cells causes the surrounding support cells to re-enter the cell cycle within 16 hours, and new hair cells appear within 2-3 days (Warchol and Corwin, 1996) (Corwin and Cotanche, 1988; Cotanche et al., 1994; Janas et al., 1995; Ryals and Rubel, 1988; Weisleder and Rubel, 1993) It is not clear if there are is a subset of support cells that can re-enter the cell cycle or if this is a property of all support cells in the BP, but it has been estimated that only 10-15% of the support cells enter the mitotic cell cycle after damage, and most of these are concentrated in the neural part of the damaged epithelium (Bermingham-McDonogh et al., 2001; Cafaro et al., 2007). In addition to the generation of new hair cells through support cell divisions, there is also evidence in birds that some of the regenerated hair cells come from direct transdifferentiation (Adler et al., 1997; Adler and Raphael, 1996; Roberson et al., 2004; Rubel et al., 1995, like that described above in the amphibian. The initial response occurs prior to even extrusion of the damaged hair cells and results in an upregulation of a key hair cell marker (Atoh1) in some cells with support cell morphology. Moreover, new hair cells appear to be produced even in the presence of mitotic inhibitors. The regeneration of hair cells after damage leads to functional recovery {Bermingham-McDonogh, 2003 #1889).
The story is quite different in the mammalian inner ear. In mammals, once the hair cells are lost there appears to be little if any spontaneous recovery. In the organ of Corti, the hair cells and surrounding support cells are mitotically quiescent, and hair cell damage does not induce their re-entry into the mitotic cell cycle. Further, in the adult mammalian cochlea, there appears to be no direct transdifferention of support cells into hair cells after damage. The same is true for the mammalian vestibular organs. Little proliferation is observed after hair cell damage or in undamaged organs, either in vivo or in vitro (Oesterle et al., 1993). Occasional H3-thymidine+ or BrdU+ support cells have been reported, but most investigators would agree that the level of proliferation in the mammalian inner ear epithelia is extremely low (Cotanche and Kaiser, 2010; Groves, 2010). So what are the differences in mammals that may account for this lack in proliferative potential? One striking difference in the auditory system is the structure of the organ itself. In birds the auditory epithelium resembles the vestibular epithelium, with relatively homogeneous support cells. By contrast, the support cells of the mammalian organ of Corti have highly specialized structures (Figure 1B) adapted for high frequency hearing. The morphological specializations in the mammalian organ of Corti may impose limits on proliferation of the support cells that preclude a regenerative response. However, this structural argument does not really apply to the mammalian vestibular organs, since they are quite similar to their counterparts in other vertebrates.
Investigations of regeneration of hair cells in the inner ear and lateral line have focused on two key questions: (1) what factors control cell proliferation in the support cells and (2) what factors control hair cell specification and support cell transdifferentiation. Cell proliferation accompanies most examples of hair cell regeneration in the inner ear sensory epithelia, and consequently many investigators in this field have focused on developing a better understanding of the mechanisms that control proliferation in normal and damaged epithelia. Attempts to stimulate proliferation of support cells in explant cultures of inner ear organs have shown some effects with mitogenic factors, including EGF, TGF-alpha, TNF-alpha and IGF (Doetzlhofer et al., 2004; Oesterle and Hume, 1999; Oesterle et al., 1997; Warchol, 1999; Yamashita and Oesterle, 1995; Zheng et al., 1997); however, FGF, which is mitogenic in many systems, appears to have the opposite effect in the inner ear epithelia (Oesterle et al., 2000), possibly related is the fact that Fgfr3 is downregulated in the chick basilar papilla after damage (Bermingham-McDonogh et al., 2001). Many mitogenic factors act via the upregulation of cyclin expression, and CyclinD is particularly important in regulating proliferation of hair cell precursors (Laine et al., 2010). The Cdkis are also important in the control of proliferation in the inner ear. During development of the sensory epithelium in the cochlea, the Cdki, p27kip is an early marker of the part of the presumptive sensory region that will generate the hair cells and support cells (Chen and Segil, 1999) and deletion of p27kip leads to an extension in the normal developmental limit in proliferation of cells in the cochlea (Kil et al., 2011; Lee et al., 2006; Lowenheim et al., 1999). Although these experiments demonstrated that p27kip is an important developmental regulator of support cell proliferation, recently it was shown that deletion of p27kip in adult animals also causes support cells to enter the mitotic cell cycle (Oesterle et al., 2011), albeit in small numbers, indicating that p27kip is one of the factors required in mature mice to maintain mitotic quiescence in the support cells. Taken together, the results suggest that methods to stimulate proliferation in the mammalian inner ear epithelia might be possible through manipulation of a combination of known pathways. However, even though some support cells proliferate in the postnatal cochlea in the p27kip knockout mice, very few, if any, generate mature new hair cells as they would during regeneration in non-mammalian vertebrates; rather, the proliferating cells appear to generate additional support cells, or else they undergo apoptosis. This leads to the second main difference between the non-mammalian vertebrates and mammals: the support cells of the auditory sensory epithelium of non-mammalian vertebrates have the capacity to transdifferentiate into hair cells, while mammalian cochlear support cells do not.
What factors enable the support cells of non-mammalian vertebrates to differentiate into hair cells after damage? Studies of the factors that control the fates of hair cells and support cells during regeneration have focused on the developmental regulators of hair cell determination/differentiation: the bHLH transcription factor, Atoh1, and the Notch pathway (Cafaro et al., 2007; Daudet et al., 2009; Stone and Rubel, 1999). Atoh1 is a critical transcription factor for the specification of the hair cells during development (Box), while Notch signaling has both a “prosensory” role and acts in a more conventional lateral inhibitory manner to regulate the ratios of hair and support cells. (Brooker et al., 2006; Kiernan et al., 2001; Kiernan et al., 2006) (Adam et al., 1998; Brooker et al., 2006; Haddon et al., 1998; Kiernan et al., 2005; Zine and de Ribaupierre, 2002). In the normal adult vestibular organs in birds, Atoh1 is expressed in scattered cells throughout the epithelium, suggesting a continued requirement for specification during the ongoing hair cell production in these organs (Cafaro et al., 2007). By contrast, in the normal avian adult BP, there is no Atoh1 expression; however, after hair cell damage a number of cells express Atoh1 and Notch pathway genes (Cafaro et al., 2007; Daudet et al., 2009), reflecting the hair cell production during regeneration. The expression of Atoh1 in support cells occurs rapidly after hair cell damage in the BP, near the time when these cells enter the mitotic cell cycle. Studies in zebrafish lateral line have shown that Notch pathway components are also upregulated very soon after hair cell damage in this system (Ma et al., 2008), and that blocking the Notch pathway using a gamma-secretase inhibitor leads to an excess of regenerated hair cells. Similar results were obtained in chick BP (Daudet et al., 2009). These studies indicate that the mechanisms of lateral inhibition function during regeneration much like they would during normal development; however, there are two additional interesting findings. First, in the chick, Atoh1 is up-regulated in support cells of the chick basilar papilla very soon after damage, possibly before the cells enter the cell cycle. Second, the experimental inhibition of Notch in the undamaged basilar papilla or lateral line does not activate Atoh1 expression in the support cells, and does not induce transdifferentiation of support cells to hair cells. These results indicate that it is only after damage, when the Notch pathway is up-regulated in the BP, that Notch-mediated lateral inhibition is important for defining the fates of the hair and support cells. Notch does not appear to be necessary for the maintenance of these fates in the undamaged epithelium. Since the loss of hair cells induces the rapid up-regulation of Atoh1 in the support cells, it also suggests that a different type of signal produced by the hair cells normally inhibits the expression of the proneural Atoh1 transcription factor in the neighboring support cells.
Although cell proliferation and transdifferentiation are virtually absent in the normal mature inner ear epithelia of mammals, these processes can occur in neonatal mammals. Dissociation of the support cells from the cochlea and vestibular epithelia from neonatal mice allows them to proliferate in vitro and turn on markers of hair cells, Myosin VIIa and Atoh1 (Diensthuber et al., 2009; Martinez-Monedero et al., 2007; Oshima et al., 2009; White et al., 2006). However, this proliferative ability is limited to the first two weeks of postnatal development in the cochlea of mice, though the vestibular organs may harbor these putative stem cells into adulthood. In addition to these examples of proliferation, the decline in potential for transdifferentiation with maturation has also been examined in the cochlea. The Notch pathway remains active for a brief period of postnatal development (Hartman et al., 2009); however, after postnatal day 3 in the mouse, inhibition of this pathway no longer leads to Deiters’ cell transdifferentiation (Hayashi et al., 2008; Yamamoto et al., 2006). Analysis of expression of Notch pathway components has shown that these decline in all of the epithelia of the inner ear during the first postnatal week, and even after damage of the hair cells in the cochlea, Notch signaling is not up-regulated in mice as it is in non-mammalian vertebrates (Hartman et al., 2009). A few studies have attempted to identify the genes responsible for this loss in the competence for hair cell transdifferentiation by cochlear support cells. One candidate is Sox2, since it is expressed in sensory epithelial precursors in the inner ear and is required for their formation. However, Sox2 is expressed in the mature Deiters’ cells, and therefore its presence does not correlate with the loss of hair cell competence in Deiters’ cells (Oesterle et al., 2008). Signaling molecules may also be critical for limiting the process of transdifferentiation in the organ of Corti: FGF signaling may also play a role in limiting the competence of pillar cells to transdifferentiate into hair cells, though Deiters’ cells may use a different mechanism (Doetzlhofer et al., 2009).
In sum, successful regeneration of hair cells in non-mammalian vertebrates requires a coordinated induction of Atoh1 and the Notch pathway in the support cells. Neonatal mammals still display some aspects of these phenomena in the cochlea, and they may extend into adulthood in the vestibular epithelia to a limited extent. In light of these results, several groups have asked whether expression of Atoh1 is sufficient to generate new hair cells from non-sensory cells in the inner ear (Gubbels et al., 2008; Zheng and Gao, 2000)(. Studies in the adult Guinea Pig have shown that over-expression of Atoh1 can promote new hair cell formation in the normal and damaged organ of Corti, by reprogramming of the remaining support cells (Izumikawa et al., 2005; Kawamoto et al., 2003), though most of the new hair cells appeared in non-sensory regions of the inner ear epithelium. The potential of support cells to generate hair cells using Atoh1 appears to be limited to a critical window, since infection 6 days after the damage no longer induces new hair cells (Izumikawa et al., 2008). Nevertheless, taken together with the chick and fish studies, it would appear that the expression of Atoh1 after damage might be sufficient for direct transdifferentiation of support/non-sensory cells to hair cells, and clearly represents a key step in the regeneration process.
Regeneration in the Retina
In amphibians, particularly urodeles (eg. salamanders), new retina can be generated from the nonneuronal cells of the retinal pigmented epithelial layer (RPE). The RPE cells respond to retinal damage by reentering the mitotic cell cycle, losing their pigmentation and acquiring gene expression patterns similar to the retinal progenitors found in embryonic development (Lamba et al., 2008; Moshiri et al., 2004) for review). These RPE-derived progenitors proliferate, recapitulate the sequence of normal histogenesis and ultimately generate a complete new retina, with new sensory receptors, and the other retinal neurons necessary to process and relay visual sensory information to central visual nuclei and restore visual function. The process by which the RPE cells acquire a retinal progenitor phenotype appears to be a critical one, since the process after this point resembles that of normal development. This phenomenon has been variously termed metaplasia, transdifferentiation (Okada, 1980), or de-differentiation (since the RPE cells are reverting to a developmentally earlier state). This phenomenon involves shifting the pattern of gene expression in a highly regulated way, and might be called “regulated reprogramming” to distinguish it from the “direct transdifferentiation” process that occurs in support cells in mechanosensory receptor regeneration in the inner ear or lateral line. Pigmented epithelial cells reprogram to a progenitor state in birds as well, however, this phenomenon is restricted to the earliest stages of eye development, a few days after the lineages of the retinal progenitors and the RPE progenitors have diverged (Coulombre and Coulombre, 1970).
Fish also have considerable ability to regenerate sensory receptor cells and other retinal neurons from sources within the retina. When the fish retina is damaged (via surgical, neurotoxic, or genetic lesions or excessive light), there is a burst of proliferation. As noted above, the fish retina contains a precursor cell that continues to generate new rod photoreceptors throughout the lifetime of the animal. For many years it was believed that the primary source of new retinal neurons was the rod precursor (Raymond et al., 1988). More recently, it became clear that the Müller glia were another source, if not the major source of proliferating cells after retinal damage in the fish (Fausett and Goldman, 2006; Wu et al., 2001), although rod precursors contribute as well, particularly when only rods are damaged. After retinal damage, the Müller glia in the fish retina undergo a de-differentiation process (Bernardos et al., 2007; Fausett and Goldman, 2006; Qin et al., 2009; Ramachandran et al., 2010; Raymond et al., 2006; Thummel et al., 2008), somewhat like that described for the RPE in the amphibian; they re-express many, if not all, of the genes normally expressed in retinal progenitors.
Thus, in both fish and amphibians, damage in the central retina causes non-neuronal cells to change their phenotype into retinal progenitors: in amphibians, the progenitors are derived from the RPE cells, while in fish these progenitors are derived from Müller glia. However, damage to cells in the peripheral retina can be repaired by a very different mechanism in these species. In both fish and amphibians, the retina contains a specialized zone of progenitor cells at the periphery, called the ciliary marginal zone (or CMZ), which adds new neurons of all types throughout the lifetime of the animal (see Lamba et al, 2008 for review). The CMZ progenitors and stem cells generate new neurons at a low rate, and the progeny are integrated with the existing retina cells extending the retina as the overall eye grows. This process matches the eye size with the overall size of the animal. Damage to cells in the peripheral retina causes an increase in the proliferation of the progenitor cells in the CMZ and replacement of the cells that were destroyed by the insult. However, the new cells regenerated by the CMZ do not migrate to central regions of retina, and only repair the peripheral damage. Nevertheless, the fact that the retina in fish and amphibians grows throughout their life may require developmental mechanisms are preserved and provide a partial explanation for their regenerative potential.
Because of its ability to regenerate and due to the excellent molecular tools developed in zebrafish, recent studies have begun to identify the molecular requirements for regeneration in this species. Neural progenitor genes are up-regulated in Müller glia after damage consistent with their shift to the phenotype of a retinal progenitor, while some Müller glial specific genes are down-regulated as the regenerative process proceeds. Although it is not yet known whether the Müller glia are fully reprogrammed to retinal progenitors in fish, several developmentally important genes have been shown to be necessary for successful regeneration; for example, knockdown of the proneural bHLH transcription factor Ascl1a blocks regeneration (Fausett et al., 2008), as does knockdown of proliferating cell nuclear antigen (PCNA) (Thummel et al., 2008). Signaling factors such as Midkine-a and –b, galectin and ciliary neurotrophic factor (CNTF) are up-regulated after injury, and potentially important in the proliferation of the Müller cells that underlies regeneration (Calinescu et al., 2009; Kassen et al., 2009).
Müller glia of posthatch chicks also respond to neurotoxin damage to the retina by re-entering the mitotic cell cycle (Fischer and Reh, 2001). Unlike the fish, however, the Müller glia in the posthatch chick progress through one or at most two cell cycles, but do not undergo multiple rounds of cell division. Attempts to stimulate the proliferation with injections of growth factors can prolong this process somewhat and possibly recruit additional Müller glia into the cell cycle. In addition to a tempered proliferative response by the Müller glia, posthatch chicks show a limited amount of neuronal regeneration. Damage to the retina causes some of the proliferating Müller cells to express most of the progenitor genes that are up-regulated in fish Müller glia after damage (Fischer et al., 2002; Fischer and Reh, 2001, 2003; Hayes et al., 2007). When the progeny of the proliferating Müller glia are tracked over the weeks after damage, BrdU+ cells are found that express markers of amacrine cells (calretinin+, HuC/D+), bipolar cells (Islet1) and occasional ganglion cells (Brn3; neurofilament). Although it is not known whether the expression of progenitor genes is required for successful regeneration in the chick, two studies have tested whether reactivation of Notch signaling is necessary. Blocking Notch signaling with the gamma-secretase inhibitor DAPT prior to the regenerative process blocks the Müller cells re-entry into the cell cycle (Ghai et al., 2010; Hayes et al., 2007). However, inhibition of Notch signaling after the proliferation has begun causes a higher percentage of cells to differentiate into amacrine cells than in the control retinas, a result much like that described in the previous section on hair cell regeneration in fish and chicks. Thus, Notch activation is critical early in regeneration, but dysregulated Notch activity might also limit the effectiveness of the process.
The above analysis indicates that retinal damage in both chick and fish causes a somewhat similar response in the Müller cells: they proliferate and upregulate expression of neural progenitor genes and Notch signaling. However, a key difference is that in the fish most of the progeny of the Müller glia differentiate into retinal neurons and sensory receptor cells, whereas in the bird only a small percentage of the progeny of the Müller glia differentiate as neurons, and few, if any, develop into rods or cone photoreceptor cells. Thus, for functional replacement of neurons after damage, the proliferative response of the Müller glia in birds is not very effective. Nevertheless, comparisons between the bird and fish are instructive as we discuss the regenerative response in mammals below.
Mammalian Müller glia show an even more limited regenerative response to injury than birds (Karl and Reh, 2010). In response to neuronal loss, the Müller glia in rodent retina become “reactive” like the astrocytic response to neuronal damage in other regions of the CNS, increasing their expression of GFAP; however, very few of them re-enter the mitotic cell cycle (Dyer and Cepko, 2000; Levine et al., 2000) (Ooto et al., 2004), Nevertheless, when the retinal damage is followed by treatment with specific mitogenic proteins (eg. EGF, FGF, IGF, Wnt3a), some Müller glial cells are stimulated to proliferate (Close et al., 2005; Close et al., 2006) (Karl et al., 2008). It is also possible to stimulate Müller glial proliferation in the absence of overt neuronal death with sub-toxic doses of alpha-aminoadipic acid (Takeda et al., 2008). In all of these studies, however, only a relatively small number of Müller glia enter the mitotic cell cycle after damage, when compared with the chick or the fish. Like the mammalian inner ear, one of the restrictions on the proliferation of Müller glia is the Cdki, p27kip, and in the retina, the expression of this inhibitor is known to be driven by TGF-beta (Close et al., 2005; Close et al., 2006; Levine et al., 2000).
Damage to the retina in fish and birds causes Müller glia to undergo a process of regulated reprogramming, allowing them to adopt a retinal progenitor pattern of gene expression which correlates with their ability to regenerate neurons after damage. Does this same type of reprogramming occur in the mammalian retina? Some aspects of the developmental program present in the progenitors are reinitiated in rodent Müller cells in the injured retina: Pax6, Dll1, Notch1 and Nestin are upregulated to some extent after NMDA damage (eg. Karl et al., 2008). However, many key progenitor genes do not appear to be reexpressed. Two key neurogenic transcription factors, Ascl1 and Neurogenin2, for example, are not upregulated in mammalian Müller glia after damage, even under conditions when these cells are induced to proliferate with growth factors (Karl et al., 2008; Karl and Reh, 2010). Thus, mammalian Müller glia appear to undergo only a partial reprogramming in contrast to the more complete reprogramming to the progenitor phenotype that is observed in fish and birds.
Although there is evidence for at least a partial reprogramming of Müller glia the evidence that neurons are generated from these cells is not nearly as clear. Following the BrdU+ cells for two to four weeks after NMDA damage, Ooto et al (2004) reported that some expressed markers of bipolar cells and photoreceptors. Karl et al (2008) reported that a combination of NMDA and mitogen treatments in adult mice led to regeneration of new amacrine cells from the Müller glia. Other studies have reported regeneration of photoreceptors in the mouse or rat retina after particular experimental manipulations. Wnt3a, MNU damage, sonic hedgehog (Shh), and alpha-AA all increase Müller glial proliferation, and after survival periods of several days to weeks, many of the BrdU+ cells expressed photoreceptor markers (Osakada et al., 2007; Takeda et al., 2008; Wan et al., 2008; Wan et al., 2007). However, in all these studies, the numbers of Müller glia that reenter the cell cycle is very low and the number that go on to differentiate into cells expressing neuronal markers of any type are lower still; overall leading to the conclusion that the regenerative response in the mammalian retina is very limited compared with what is observed in non-mammalian vertebrates.
General principles of specialized sensory regeneration
The specialized sensory epithelia show a range of regenerative capacities, from very good to not at all, depending on the species and the sense organ. Regeneration in the olfactory epithelium is very good in all species that have been studied. The auditory and vestibular hair cells regenerate in fish and amphibia and birds; in mammals, regeneration of new hair cells is very limited or non-existent. Retinas regenerate in fish, amphibians and to some extent in birds; the regenerative capacity in mammals is very limited. Why is there such variety in their potential for intrinsic repair? In the following paragraphs we will attempt to synthesize the common aspects of the response to injury in these three systems across species with the aim of developing general principles for sensory receptor cell regeneration.
One of the more clear conclusions that can be made concerning the regeneration in these organs is that those sensory epithelia with a continual ongoing replacement or addition of new sensory receptors have excellent regenerative potential. The olfactory epithelia of all vertebrates, the vestibular sensory structures of fish and birds, and the retinas of fish all maintain active processes for adding new sensory receptor cells throughout life, and coincidentally, they all regenerate very well after a variety of types of damage. Damage causes an active response of the proliferating cells and an increase in their overall output to produce the increased number of new cells required to restore the epithelium. An appropriate niche has been maintained to preserve a part of the embryonic environment in a functioning organ, and in some ways regeneration in these organs is similar to the phenomena known as “regulation” that occurs in embryonic development; ie. when parts of a developing organ are removed, the tissue “regulates” to replace the missing parts. The increases in cell proliferation and shifts in cell fate determination that occur during regeneration presumably reflect the complex developmental interactions among these cells that control their initial patterning and ratios.
Another conclusion that can be drawn concerning regeneration in sensory epithelia is that regeneration generally follows the normal pattern of development once the process has started. In the olfactory epithelium, for example, once the process of regeneration has begun, the progenitor cells go through the same sequence that was followed during development, first expressing Ascl1, then Neurog1 then NeuroD1, etc. This makes sense in the sensory epithelia that have an ongoing production of sensory receptors, like the olfactory epithelium. However, even in cases where a functioning, differentiated cell, like the RPE of the frog or the Müller glia in the fish retina undergo a process of reprogramming to generate a progenitor, the sequence of regeneration from the progenitors closely follows the embryonic developmental sequence. The Müller glialderived progenitors in the fish retina de-differentiate into progenitors which go on to generate neurons after surgical lesions, in spite of the fact that the regenerating progenitor cells are producing neurons and glia in a very different microenvironment than that which was present during embryonic development. Neurons in the adjacent, undamaged parts of the retina seem to have little impact on the progression of regeneration. For the inner ear, the same signaling regulators (eg. Notch) and transcription factors (eg. Atoh1) are employed during regeneration as were use to regulate the production of hair and support cells during embryonic development, and the process of lateral inhibition appears to function in much the same way as it did during development to generate the correct ratios of hair and support cells during regeneration.
A third general conclusion that can be made is that those specialized sensory epithelia that regenerate well, ultimately connect with the undamaged circuitry and restore function. The olfactory epithelium in all animals tested can regenerate functional sensory cells that grow axons through the lamina cribosa to reconnect with the olfactory bulb, providing there is not much scar tissue from the surgery. The lateral line of fish and amphibians and the inner ear of all non-mammalian vertebrates can regenerate new hair cells that will function nearly as well as those lost through injury. Birds that have recovered from noise damage have thresholds near normal (see (Bermingham-McDonogh and Rubel, 2003)for review). The same is true for the retina of the fish; visual acuity is nearly restored to normal, though there is some disruption in the details of cone patterning. The fact that function can be restored in these systems is a rather amazing feat, and so it gives us a bit of hope that stimulation of regeneration in the mammalian retina or inner ear sensory receptor cells might be sufficient to trigger the associated processes that must take place for effective restoration of function. Even in mammals, there is evidence from both the inner ear and the retina that considerable plasticity remains in the cells that synaptically connect with the sensory receptor cells; ectopic hair cells induced form Atoh1 over-expression can be innervated by the ganglion neurons, and rod photoreceptors transplanted into normal adult mice will reconnect to host bipolar cells and appear to function (MacLaren et al., 2006).
Lastly, we can conclude that those sensory epithelia that display little or no capacity for regeneration generally do not have ongoing proliferation or addition of new neural cells anywhere in the organ. What is even more striking about most of the systems where regeneration is absent is that the cells in these epithelia don’t respond to damage by increasing proliferation; in both the mammalian inner ear and retina there is very little proliferation of the support cells or Müller glia, respectively, after injury. In addition, the non-neuronal support/glial cells in these epithelia do not undergo extensive reprogramming after injury, but for the most part retain most morphological and gene expression characteristics as they have in the undamaged tissue (though they frequently become “reactive”). So in the specialized sensory epithelia that do not have ongoing new sensory cell addition, like the mammalian inner ear and retina, the tissue may no longer retain the “developmental niche” that is characteristic of the olfactory epithelium, or the retina and inner ear of nonmammalian vertebrates.
There are two interesting exceptions to some of the above conclusions: the retina and the cochlea of birds. The avian retina responds to damage with robust Müller glial proliferation, though the reprogramming of these cells to a progenitor pattern of gene expression is much more limited than in the fish, and very few of the BrdU+ Müller cells go on to differentiate into neurons. Results from studies of regenerating fish retina suggest that proliferation might be required for reprogramming in this system, since blocking the proliferation with antisense to PCNA blocks regeneration. However, most of the Müller glia in the chick retina enter the cell cycle after damage, so why do they not reprogram more effectively? One possible answer might be that chick Müller glial cells only go through a single round of cell division after damage, while fish Müller cells appear to undergo multiple rounds; it is possible that full reprogramming requires multiple rounds of division. In vitro studies of reprogramming also suggest that cell division is important for the more complete reprogramming required to generate iPS cells (eg. Takahashi and Yamanaka, 2006), though fibroblasts can be directly converted to neurons by misexpressing neurogenic transcription factors without multiple rounds of cell division (Vierbuchen et al., 2010). Examples from the other sensory systems also suggest that cell division is not absolutely required for reprogramming; the lateral line of the amphibian and the chick basilar papilla support cells can directly transdifferentiate to hair cells.
Another related puzzle concerns the chick inner ear. The avian vestibular system has ongoing proliferation, but the avian cochlea does not, but they both regenerate very well. How has the chick cochlea retained a “developmentally immature” state equivalent to that of the best regenerating epithelia, without apparently adding new cells? An analogous situation can be also seen in the regeneration of the newt retina from RPE cells, which are not actively dividing in the mature organism. Despite this lack of continual renewal, both the support cells of the chick basilar papilla and the RPE cells of the newt undergo robust proliferation and reprogramming after injury to replace the lost cells. An interesting feature of both systems, is that while they do not have ongoing cell replacement within the specific cells that provide the source for the regeneration, both of these organs have ongoing sensory cell replacement “nearby.” For the newt, the stem cells at the margin of the retina continue to produce new retinal neurons at its peripheral edge; in the chick inner ear, vestibular organs with ongoing hair cell genesis (ie.the lagena) are immediately adjacent to the basilar papilla in chick. It is possible that some type of long-distance non-autonomous property of the organs allows more plasticity in cell phenotype throughout the epithelia. Alternatively, the genetic program of development that allowed some part of the retina or inner ear to retain developmental character into adulthood might also enable regeneration more broadly across the sensory organ. For example, perhaps in the inner ear of the chick, the vestibular system and basilar papilla share a common developmental program that does not preclude new cell addition, and this is utilized continuously by the vestibular system and only after injury in the basilar papilla. Answers to these questions might provide insight into the reasons why mammalian regeneration in the retina and inner ear are so limited.
What have we learned from studies of regeneration in the systems capable of this process to inform our future progress in promoting regeneration in the mammalian retina and auditory/vestibular epithelia? Despite many years of study, it has proven to be very difficult to stimulate regeneration in an organ without any ongoing replacement or addition of sensory receptor cells, like the mammalian retina or inner ear. Nevertheless, we have really only scratched the surface in our understanding of the molecular mechanisms underlying successful regeneration, such as that in the olfactory epithelium. The studies of regeneration in both the retina and the inner ear have shown that cell proliferation is quite limited in the species that do not regenerate their sensory receptors in these organs. There are few, if any mitotic cells in the mouse retina or cochlea after photoreceptor or hair cell damage, respectively. At least some of the regulators of proliferation have been identified in these structures; proliferation of support cells and Müller glia in both the inner ear and the retina is regulated in part by the Cdki, p27kip1 and the tumor suppressor, Rb. Loss of p27kip1 leads to extra cell divisions in the Müller glia and inner ear support cells in mice, though the number of mitotic divisions is still very limited. Studies in other systems suggest that multiple pathways may need to be targeted to stimulate proliferation in otherwise quiescent tissues (Pajcini et al., 2010). More importantly, the new cells that are produced in the retina and inner ear of mammals, even when the proliferation is stimulated, for the most part do not generate sensory receptor cells. Simply getting the cells to divide again is not sufficient for regeneration; some reprogramming appears to be necessary for regeneration.
The reprogramming or transdifferentiation that occurs naturally during regeneration in the retinas of fish and newts involves the silencing of glial/RPE genes and the reactivation of a progenitor gene expression program. However, the molecular mechanisms that maintain cell identity are still not very well understood and further research into the epigenetic response of cells to injury and during regeneration is warranted. The degree of reprogramming that takes place in the retinas of these animals does not appear to be required in the inner ear, where the support cells seem poised to activate Atoh1 expression. Several rounds of cell division might be needed to effectively reprogram the RPE cells or the Müller glia, whereas no cell division at all is required in the inner ear of fish and chicks. In both the retina and the inner ear, Notch signaling also plays a role in regeneration. In the olfactory epithelium, the Notch pathway is upregulated after damage. In both the inner ear, and in the retina, Notch and its ligands are up-regulated after damage, and in the retina, the upregulation of Notch seems to be required at a very early stage in the process of reprogramming. By contrast, in the inner ear the support cells upregulate the Notch pathway, but inhibiting the pathway does not prevent support cell transdifferentiation to hair cells. These results suggest that the upregulation of Notch after damage may not be required in the inner ear, but nevertheless the presence (and upregulation) of Notch signaling is a reliable indicator of a regenerating system. This is an important conclusion in light of the fact that the downstream Notch effector Hes5 is not expressed in either the normal adult mouse cochlea, or after damage to the hair cells (Hartman et al., 2009). Unbiased screening for critical factors in the regeneration process, using small molecule libraries, microarray studies and genetics (Brignull et al., 2009) will certainly lead to a better understanding of the differences between the successful and non-successful regenerates.
Outlook: Regenerative medicine in the special senses
A process of ongoing sensory receptor cell replacement characterizes the sensory epithelia that show robust regeneration. This does not appear to be present in the retinas or cochleas of mammals. Therefore the main options for therapy will likely involve reinitiating the process of regulated reprogramming to a proliferative progenitor state in the glia and support cells. Although stimulation of regeneration in mammalian inner ear and retina to the level present non-mammalian vertebrates would be ideal, considerably less effective regeneration could still be useful for patients. For example, stimulation of proliferation in support cells in the cochlea may not be necessary for some recovery. In many individuals with age-related hearing loss, the inner hair cells are thought to survive, longer than the outer hair cells. The loss of outer hair cells causes dramatic loss in cochlear function (eg Chen et al., 2009). Therefore, the restoration of only 30-40% of the outer hair cells, by stimulation of transdifferentiation of the remaining Deiters’ cells, could result in a significant hearing improvement. The same may be true for the retina. The degeneration of foveal cones in late stages of macular degeneration leads to significant vision loss, though these cells make up less than 1% of the total retinal cell population. As the molecular pathways are further elucidated, one can imagine a scheme in which these pathways could be targeted by gene therapy to initiate a process of regulated reprogramming; in the mammalian inner ear, viral expression of Atoh1 has already been shown to restore some hair cells to damaged cochlea. Perhaps more promising are small molecule approaches to stimulate this process, since these have proven successful in the more drastic reprogramming that is required to generate iPS cells (Li et al., 2009). The types of phenomena that we currently lump together under the terms “transdifferentiation”, “de-differentiation” and “reprogramming” are complex and highly regulated processes that are key to regeneration in the specialized sensory epithelia. Our understanding of the mechanisms that underlie these phenomena are in their infancy, but there is a new sense of optimism that regenerative medicine approaches will someday provide treatments for sensory disorders.
Acknowledgements
The authors acknowledge the many discussions on regeneration in sensory epithelia that they have had over the years with the members of the current Reh/Bermingham-McDonogh labs, past members of our labs, especially Drs. M. O. Karl, B. Nelson, T. Hayashi and B. Hartman, and colleagues at the University of Washington, including Drs. E. Rubel, D. Raible, J. Stone, E. Oesterle and C. Hume. Research in the authors’ labs are supported by the following grants from the National Institutes of Health DC005953, DC009991, DC010862, EY021482, PO1 GM081619-01 and TA-CBT-0608-0464-UWA-WG from the Foundation Fighting Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Adler HJ, Komeda M, Raphael Y. Further evidence for supporting cell conversion in the damaged avian basilar papilla. International Journal of Developmental Neuroscience. 1997;15:375–385. doi: 10.1016/s0736-5748(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neuroscience Letters. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci U S A. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Torres MA, Schuff NR. Hair cell regeneration in the bullfrog vestibular otolith organs following aminoglycoside toxicity. Hearing Research. 1993;65:164–174. doi: 10.1016/0378-5955(93)90211-i. [DOI] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001;238:247–259. doi: 10.1006/dbio.2001.0412. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann JH, Firestein S. Regeneration of new neurons is preserved in aged vomeronasal epithelia. J Neurosci. 2010;30:15686–15694. doi: 10.1523/JNEUROSCI.4316-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Calinescu AA, Vihtelic TS, Hyde DR, Hitchcock PF. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. J Comp Neurol. 2009;514:1–10. doi: 10.1002/cne.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, Shou J, Wu HH. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58:176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- Carr VM, Farbman AI. Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium. Exp Neurol. 1992;115:55–59. doi: 10.1016/0014-4886(92)90221-b. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res. 2009;248:39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Müller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015–3026. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54:94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic production and aging in inner ear hair cells in sharks. J Comp Neurol. 1981;201:541–553. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Perpetual production of hair cells and maturational changes in hair cell ultrastructure accompany postembryonic growth in an amphibian ear. Proc Natl Acad Sci U S A. 1985;82:3911–3915. doi: 10.1073/pnas.82.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hear Res. 2010;266:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Lee KH, Stone JS, Picard DA. Hair cell regeneration in the bird cochlea following noise damage or ototoxic drug damage. Anat Embryol Berl. 1994;189:1–18. doi: 10.1007/BF00193125. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Saunders JC, Tilney LG. Hair cell damage produced by acoustic trauma in the chick cochlea. Hearing Research. 1987;25:267–286. doi: 10.1016/0378-5955(87)90098-0. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature. 1970;228:559–560. doi: 10.1038/228559a0. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Roskams AJ. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc Res Tech. 2002;58:204–215. doi: 10.1002/jemt.10150. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diensthuber M, Oshima K, Heller S. Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J Assoc Res Otolaryngol. 2009;10:173–190. doi: 10.1007/s10162-009-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev Biol. 2004;272:432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Faucher K, Aas-Hansen O, Damsgard B, Laukli E, Stenklev NC. Damage and functional recovery of the Atlantic cod (Gadus morhua) inner ear hair cells following local injection of gentamicin. Int J Audiol. 2009;48:456–464. doi: 10.1080/14992020902738029. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD. Teleost vision: seeing while growing. J Exp Zool Suppl. 1990;5:167–180. doi: 10.1002/jez.1402560521. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Müller glia. J Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Müller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei GA, Monti Graziadei AG. The Olfactory System: A Model for the Study of neurogenesis and Axon Regeneration in Mammals. In: Cotman CW, editor. Neuronal Plasticity. Raven Press; 1978. pp. 131–153. [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and plasticity of the olfactory sensory neurons. Ann N Y Acad Sci. 1985;457:127–142. doi: 10.1111/j.1749-6632.1985.tb20802.x. [DOI] [PubMed] [Google Scholar]
- Groves AK. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 2010;235:434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE. Expression of pax6 and sox2 in adult olfactory epithelium. J Comp Neurol. 2010;518:4395–4418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, Reh TA. Hes5 Expression in the Postnatal and Adult Mouse Inner Ear and the Drug-Damaged Cochlea. J Assoc Res Otolaryngol. 2009 doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker sox2 and proliferationdependent and-independent mechanisms of hair cell renewal. Dev Neurobiol. 2007;67:637–654. doi: 10.1002/dneu.20386. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Kakuk-Atkins L. The basic helix-loop-helix transcription factor neuroD is expressed in the rod lineage of the teleost retina. J Comp Neurol. 2004;477:108–117. doi: 10.1002/cne.20244. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Hudspeth AJ. The cellular basis of hearing: the biophysics of hair cells. Science. 1985;230:745–752. doi: 10.1126/science.2414845. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Janas JD, Cotanche DA, Rubel EW. Avian cochlear hair cell regeneration: stereological analyses of damage and recovery from a single high dose of gentamicin. Hear Res. 1995;92:17–29. doi: 10.1016/0378-5955(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Johns PR, Easter SS., Jr. Growth of the adult goldfish eye. II. Increase in retinal cell number. J Comp Neurol. 1977;176:331–341. doi: 10.1002/cne.901760303. [DOI] [PubMed] [Google Scholar]
- Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293:141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13:1022–1034. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. Journal of Neuroscience. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–19513. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Reh TA. Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med. 2010;16:193–202. doi: 10.1016/j.molmed.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Müller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil J, Gu R, Fero M, Lynch ED. The Cyclin Dependent Kinase Inhibitor p27Kip1 Maintains Terminal Differentiation in the Mouse Organ of Corti. The Open Otorhinolaryngology Journal. 2011;5:25–34. [Google Scholar]
- Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M, Yamaguchi T, Nibu K, Kaga K, Yamasoba T. Age-related changes in cell dynamics of the postnatal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol. 2010;518:1962–1975. doi: 10.1002/cne.22316. [DOI] [PubMed] [Google Scholar]
- Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev Biol. 2010;337:134–146. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Levine EM, Close J, Fero M, Ostrovsky A, Reh TA. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev Biol. 2000;219:299–314. doi: 10.1006/dbio.2000.9622. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Oshima K, Heller S, Edge AS. The potential role of endogenous stem cells in regeneration of the inner ear. Hear Res. 2007;227:48–52. doi: 10.1016/j.heares.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Close J, Reh TA. Retinal stem cells and regeneration. Int J Dev Biol. 2004;48:1003–1014. doi: 10.1387/ijdb.041870am. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Reh TA. Relationship between Delta-like and proneural bHLH genes during chick retinal development. Dev Dyn. 2008;237:1565–1580. doi: 10.1002/dvdy.21550. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J Comp Neurol. 2000;424:307–326. doi: 10.1002/1096-9861(20000821)424:2<307::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27 (Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10 doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Hume CR. Growth factor regulation of the cell cycle in developing and mature inner ear sensory epithelia. J Neurocytol. 1999;28:877–887. doi: 10.1023/a:1007074222659. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Reh TA, Rubel EW. Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hearing Research. 1993;70:85–108. doi: 10.1016/0378-5955(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor-I and insulin. Journal of Comparative Neurology. 1997;380:262–274. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Okada TS. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr Top Dev Biol. 1980;16:349–380. [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–162. doi: 10.1007/978-1-59745-523-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajcini KV, Corbel SY, Sage J, Pomerantz JH, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell. 2010;7:198–213. doi: 10.1016/j.stem.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106:9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19:431–463. doi: 10.1002/neu.480190504. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Rivlin PK. Germinal cells in the goldfish retina that produce rod photoreceptors. Dev Biol. 1987;122:120–138. doi: 10.1016/0012-1606(87)90338-1. [DOI] [PubMed] [Google Scholar]
- Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res. 1992;57:166–174. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Dew LA, Roberson DW. Mammalian vestibular hair cell regeneration. Science. 1995;267:701–707. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int Rev Cell Mol Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Sicard G, Feron F, Andrieu JL, Holley A, Mackay-Sim A. Generation of neurons from a nonneuronal precursor in adult olfactory epithelium in vitro. Ann N Y Acad Sci. 1998;855:223–225. doi: 10.1111/j.1749-6632.1998.tb10570.x. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–973. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone L. Further experimental studies of the development of lateral-line sense organs in amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115. [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takamiya A, Jiao JW, Cho KS, Trevino SG, Matsuda T, Chen DF. alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–1150. doi: 10.1167/iovs.07-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–120. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Müller glia after retinal degeneration in adult rat. Vision Res. 2008;48:223–234. doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J Neurocytol. 1999;28:889–900. doi: 10.1023/a:1007026306730. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Sensory regeneration in the vertebrate inner ear: Differences at the levels of cells and species. Hear Res. 2010 doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17:3610–3622. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. Journal of Comparative Neurology. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest Ophthalmol Vis Sci. 2001;42:2115–2124. [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Oesterle EC. Induction of cell proliferation in mammalian inner-ear sensory epithelia by transforming growth factor alpha and epidermal growth factor. Proc Natl Acad Sci U S A. 1995;92:3152–3155. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Cruickshanks KJ, Klein BE, Klein R, Huang GH, Pankow JS, Gangnon RE, Tweed TS. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Helbig C, Gao WQ. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cell cultures. J Neurosci. 1997;17:216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, de Ribaupierre F. Notch/Notch ligands and Math1 expression patterns in the organ of Corti of wild-type and Hes1 and Hes5 mutant mice. Hear Res. 2002;170:22–31. doi: 10.1016/s0378-5955(02)00449-5. [DOI] [PubMed] [Google Scholar]