Abstract
Background
The authors conducted a prospective cohort study to determine whether poor glycemic control is a contraindication to implant therapy in patients with type 2 diabetes.
Methods
The study sample consisted of 117 edentulous patients, each of whom received two mandibular implants, for a total of 234 implants. Implant-retained mandibular overdentures were loaded after a four-month healing period and followed up for an additional one year. The authors assessed implant survival and stability (by means of resonance frequency analysis) relative to glycated hemoglobin A1c (HbA1c) levels, with baseline levels up to 11.1 percent and levels as high as 13.3 percent over one year.
Results
Implant survival rates for 110 of 117 patients who were followed up for one year after loading were 99.0 percent, 98.9 percent and 100 percent, respectively, for patients who did not have diabetes (n = 47), those with well-controlled diabetes (n = 44) and those with poorly controlled diabetes (n = 19). The authors considered the seven patients lost to follow-up as having had failed implants; consequently, their conservative estimates of survival rates in the three groups were 93.0 percent, 92.6 percent and 95.0 percent (P = .6510) . Two implants failed at four weeks, one in the nondiabetes group and the other in the well-con trolled diabetes group. Delays in implant stabilization were related directly to poor glycemic control.
Conclusions
The results of this study indicate that elevated HbA1c levels in patients with type 2 diabetes were not associated with altered implant survival one year after loading. However, alterations in early bone healing and implant stability were associated with hyperglycemia.
Practical Implications
Within the clinical parameters of this study, the findings indicate likely implant success among patients with type 2 diabetes who lacked good glycemic control. Further investigation, including longer-term evaluation, is needed.
Keywords: Implantology, diabetes, bone biology, resonance frequency analysis, implant stability quotient
Diabetes mellitus has long been considered a contraindication to dental implant therapy, depending on the patient's glycemic control.1-4 Patients with well-controlled diabetes are considered candidates for implant therapy, whereas patients with poor glycemic control may be denied this treatment option. However, the potential benefits of implant therapy may be important for diabetic patients dependent on dietary management to aid in controlling their condition.
More than 90 percent of patients with diabetes in the United States have type 2 diabetes, and it affects more than 25 million people in the country. More-over, it has been estimated that as many as 30 to 50 percent of patients with diabetes lack good glycemic controt, having hemoglobin A1c (HbA1c) levels greater than 9.0 percent.5 This HbA1c level is consistent with an estimated mean glucose level of 212 milligrams/deciliter.5,6 Although the results of numerous animal and human studies suggest that poor glycemic control is a contraindication to implant therapy, limitations in these studies leave in question our understanding of the role of glycemic status in patients with diabetes.7,12
Potential compromises in bone metabolism shown with animal models of hyperglycemia suggest alterations in implant integration.8,12 The clinical impact on the integration process may be understood better through a longitudinal assessment of implant stability by means of resonance frequency analysis (RFA).13-19 Researchers in previous studies have assessed implant stability over the first four to six months after placement in patients with type 2 diabetes who had HbA1c levels as high as 12 percent.13,20,21 They reported high levels of implant survival regardless of patients’ glycemic status, as well as few clinical complications. It is important to point out that in the first few weeks after implant placement, implant stability decreased more in patients with diabetes who had an HbA1c level of greater than 8 percent, and they demonstrated delayed integration compared with that in patients without diabetes and in patients with well-controlled diabetes. However, these studies were of a short duration and designed primarily to assess implant-related outcomes before restoration of the implants. The high levels of implant survival identified in these studies support a longer-term assessment after restoration.
The goal of this observational study was to examine the effects of elevated glycemic levels on implant survival and stability over 16 months, including one year after implant restoration.
METHODS
This single-center, prospective cohort study was designed to evaluate the effects of glycemic levels on implant-related outcomes among edentulous patients receiving mandibular implant-supported overdentures. We recruited patients seeking treatment at the School of Dentistry, University of Texas Health Science Center at San Antonio (UTHSCSA). We enrolled participants in the study from September 2007 through June 2012. This study was approved and conducted in compliance with the institutional review board at UTHSCSA.
Inclusion criteria
We included in the study edentulous patients 25 years or older who required treatment with two dental implants in the mandibular anterior region to support an implant-retained complete overdenture. We verified a diagnosis of type 2 diabetes at enrollment on the basis of the patient’s medical record. We included participants who did not have diabetes and had a baseline HbA1c level that was less than 5.9 percent or a fasting blood glucose level that was 100 mg/dL or lower. We used a single Clinical Laboratory Improvement Amendments–certified commercial laboratory to measure HbA1c values. Medical management of diabetes by means of diet, oral hypoglycemic agents, insulin or combination therapy was allowed.
We limited required dental implant therapy to the anterior mandible (canine-premolar region) that had sufficient bone volume to allow for the placement of 4.1-millimeter–diameter implants with a length of 8 to 12 mm. We also limited participation to implants in sites that required at least four months of healing after tooth extraction before implant placement. We required participants to have had clinically acceptable complete mandibular and maxillary dentures for at least six months.
Exclusion criteria
Criteria for exclusion from the study included an HbA1c level of greater than 12 percent at screening and systemic conditions other than type 2 diabetes mellitus considered to be a contraindication to implant surgery. We also excluded patients receiving antiresorptive drug therapy, pregnant women, patients with a self-reported smoking habit, patients with untreated oral infections, patients with viral or autoimmune disease, implant sites that underwent bone-grafting procedures involving use of autogenic or allogeneic materials within one year of the study or implant sites that had undergone alloplastic grafting procedures.
Implant therapy
Patients received two transmucosal implants (4.1-mm diameter and 8-, 10- or 12-mm length, SLActive, Straumann, Basel, Switzerland) in the anterior mandible to support a mandibular overdenture. The surgeons determined implant length on the basis of existing bone dimensions and, in appropriate cases, they placed implants of the same length in both sites. Implant surgery, consistent with standard surgical protocols, was performed by multiple clinicians, including residents and faculty members (P.G., G.H.-B.), under supervision of the principal investigator (PI) (T.W.O.). The surgeon and PI made clinical assessments jointly regarding bone type at implant placement, according to a four-tiered scale: high density (type I), moderate density (type II), low density (type III) and very low density (type IV).22 After implant placement, the surgeon placed transmucosal healing caps and made denture base adjustments to eliminate any contact between the implant and denture. Patients received prescriptions for a postoperative antibiotic regimen consisting of amoxicillin (500 mg) or clindamycin (150 mg) three times per day for seven days and chlorhexidine gluconate (0.12 percent) mouthrinse twice a day for two weeks. After a four-month healing period, two of us (P.A., A.V.G.) restored the implants by using Locator abutments and attachments (Zest Anchors, Escondido, Calif.).
Study procedures
Two examiners (P.A., A.V.G.) who underwent calibration and were masked to the diabetic and glycemic status of participants evaluated all implant-related outcomes. They assessed implants over a four-month healing period after placement and then at three, six and 12 months after implant restoration. We defined implant survival as an implant lacking any signs of clinical mobility, peri-implant radiolucency, clinical findings consistent with failure of the implant to integrate, pain or other reasons preventing restoration of the implant at four months or requiring removal of the implant.23 The examiners obtained panoramic radiographs at the three-month postloading visit for evaluation of any radiographic signs of implant complications or evidence of implant failure.
The examiners assessed implant stability at each study visit by means of two readings from each parallel and perpendicular direction relative to the alveolar ridge using RFA to determine the implant stability quotient (ISQ) (on a 0-100 scale, with higher numbers indicating greater stability) (Osstell ISQ Meter, Osstell, Gothenburg, Sweden) in accordance with the manufacturer’s protocol.
The two examiners obtained venous blood samples at screening for measurement of HbA1c levels to determine eligibility for participation. They also obtained venous blood samples at the time of implant placement (baseline), two months after surgery, four months after surgery (implant loading) and at three, six and 12 months after loading.
The examiners assessed implant-related biological complications at each study visit. Biological complications included any clinical sign of infection such as pain, swelling or suppuration that did not require removal of the implant but may have been sufficient to warrant antibiotic or other therapeutic intervention or an alteration in clinical management procedures.
Statistical analysis
For each patient, four ISQ measurements were made for each of the two implants at each of the visits, as described earlier. We calculated the mean of these four readings for each implant at each time point, as well as the median, minimum and maximum values. Correlation coefficients of the mean ISQ measurements with the median, minimum and maximum measurements were ≥ 0.96. Because the high correlation coefficients indicated that the same information was obtained regardless of the summary measure, we used the mean of the four ISQ measures in the statistical analyses.
We categorized HbA1c levels as follows: less than or equal to 5.9 percent, between 6.0 and 8.0 percent, and 8.1 percent or higher. There is no set lower limit for HbA1c for patients with type 2 diabetes, but recommended therapeutic targets range between 6.5 and 7.0 percent.24,25 Complications from hyperglycemic conditions have been shown to be directly proportional to glycemic levels, as measured with glycated hemoglobin, with the greatest risks of clinical complications occurring at HbA1c levels greater than 8.0 percent.26,27 Therefore, we classified participants who had HbA1c levels of 8.1 percent or greater as having poorly controlled diabetes at study enrollment.
We calculated implant stability measurements by using analysis of variance (ANOVA) for repeated measurements. We also determined the time required for an implant to attain an ISQ greater than or equal to the ISQ at surgery (baseline). In addition, we determined the time needed to attain 90 percent and 95 percent of the ISQ at surgery. We analyzed these times by using ANOVA for repeated measurements. We used Kaplan-Meier survival curves to analyze the times needed for the poorer-performing implants to achieve ISQs greater than or equal to the ISQs at surgery. We defined the poorer-performing implant per patient as the one requiring more time to return to baseline ISQ levels. In addition, we tested differences between groups by using the log-rank test.
We used the proportional hazards model28 to analyze the associations of time required for each participant’s poorer-performing implant to achieve an ISQ greater than or equal to the ISQ at surgery with HbA1c category, sex, age group (≤ 65 years [median age] and > 65 years) and implant length. We investigated use of the HbA1c level at surgery as a predictor, as well as a time-varying covariate, constructed as the mean HbA1c level over the period from surgery until the visit at which the implant achieved an ISQ greater than or equal to the ISQ at surgery; we assumed that implant performance depended on the patient’s history of glucose control and not just on the most recent glycemic level.
We applied the following definitions, which resulted in our findings being conservative in estimating complete healing. For patients lost to follow-up, we assumed that the ISQs for implants that had not returned to baseline ISQs never returned. Similarly, we included the two implants that were replaced and the implant for which the baseline ISQ was missing in the group in which the ISQ never returned to the baseline ISQ.
RESULTS
Patients
We included a total of 117 edentulous patients with 234 implants in this study. This group consisted of 52 men and 65 women ranging in age from 38 to 83 years, with a mean age of 64 years. The patient population included four Asian Americans, three African Americans, 54 Hispanic whites and 56 non-Hispanic whites. Baseline (surgical) HbA1c values ranged from 5.1 percent to 11.1 percent. The highest HbA1c value at two months after surgery was 13.3 percent; at implant loading, 12.1 percent; at three months after loading, 11.9 percent; at six months after loading, 12.0 percent; and at 12 months after loading, 12.6 percent. At the time of implant placement, 50 patients (42.7 percent of the 117 participants) did not have diabetes (HbA1c value ≤ 5.9 percent), 47 (40.2 percent) were in the well-controlled diabetes group (HbA1c value 6.0-8.0 percent) and 20 (17.1 percent) were in the poorly controlled diabetes group (HbA1c value ≥ 8.1 percent).
We found no significant differences in overall HbA1c levels from implant placement (baseline) through the one-year postloading evaluation. Intraclass correlation coefficients, as a measure of agreement between the HbA1c level at baseline and those at other visits, were ≥ 0.80. The study findings showed that the lowest intraclass correlation coefficient was for the period between baseline and one year after loading—that is, the visits separated by the most time. These results indicate that the HbA1c levels were relatively stable in patients over this period and support our using the baseline HbA1c level as a predictor variable in assessing the association between HbA1c and outcome.
The mean (standard deviation) baseline HbA1c levels were 5.65 percent (0.20) for the group without diabetes, 6.54 percent (0.58) for the group with well-controlled diabetes and 9.20 percent (0.85) for the group with poorly controlled diabetes. Baseline HbA1c categories were unchanged for 61 to 90 percent of participants when we compared values with the three-, six- and 12-month postloading averages. The HbA1c levels in patients changed by no more than one category from baseline (surgery) to preloading or postloading times.
Postsurgical complications
Early healing outcomes were consistent across the three HbA1c groups, with expected minor postoperative complications; the exception was two failed implants that were removed during the study period, as described below. There were no reports of signs of infection (purulence, unanticipated pain or unusual swelling) or compromised healing that required interventions such as antibiotic extension, incision and drainage, or additional treatment for any implant.
Implants excluded from RFA analysis
Two of the 117 patients (1.7 percent) experienced failure of one of their two implants (two of 234 [0.85 percent]) during the study period, requiring removal. Both of the implants were removed four weeks after surgical placement as a result of failure to osseointegrate. One of these patients had a baseline HbA1c level of 5.8 percent, and the other patient had a baseline HbA1c level of 7.4 percent. Both failed implants were replaced successfully and followed up by means of the protocol described earlier. By 12 months after loading, the mean ISQs for the patient without diabetes (baseline HbA1c 5.8 percent) were 79.5 for the replacement implant and 79.75 for the contralateral implant. In the patient with well-controlled diabetes, the ISQ for the replacement implant was 72.75, and for the contralateral implant, the ISQ was 70.75. Thus, complications that required implant removal did not compromise the eventual success of the replacement implant. These limited data pertaining to implant removal provide little information about any association with HbA1c. We did not include data pertaining to these two replaced implants in the statistical analyses for ISQ comparisons.
Overall, 110 patients were evaluated for implant survival over the 12-month postloading period, representing a retention of 94 percent, with no differences between HbA1c categories (Table 1). One year after loading, implant survival rates on the basis of implants were 99.0, 98.9 and 100 percent, and at the patient level were 97.9, 97.7 and 100 percent for the nondiabetes (n = 47), well-controlled diabetes (n = 44) and poorly controlled diabetes (n = 19) groups, respectively. Seven patients (three who did not have diabetes, three with an HbA1c level of 6.0-8.0 percent and one with an HbA1c level ≥ 8.1 percent) were lost to follow-up. One of these patients was not seen after implant loading, whereas the other patients had at least one visit after implant loading. None of the seven patients lost to follow-up experienced any implant-related complications before they dropped out of the study. If we consider these seven patients lost to follow-up as each representing two implant failures, then the conservative estimate of implant failures is seven (7.0 percent) in patients without diabetes, seven (7.4 percent) in patients with well-controlled diabetes and two (5 percent) in those with poorly controlled diabetes (P = .6510).
TABLE 1.
Number of patients at follow-up times, according to HbA1c* level.
| FOLLOW-UP TIME (NO. OF PATIENTS) |
NUMBER OF PATIENTS, ACCORDING TO HbA1c LEVEL |
IMPLANT FAILURES AND PATIENTS LOST TO FOLLOW-UP |
||
|---|---|---|---|---|
| ≤ 5.9 Percent | 6.0-8.0 Percent | ≥ 8.1 Percent | ||
|
Implant Placement
(n = 117) |
50 | 47 | 20 | Baseline |
|
Implant Loading
(n = 117) |
50 | 47 | 20 | Two implants lost in two patients |
|
Three Months After Loading
(n = 115) |
49 | 47 | 19 | Two patients lost to follow-up |
|
Six Months After Loading
(n = 112) |
48 | 45 | 19 | Three patients lost to follow-up |
|
12 Months After Loading
(n = 110)† |
47 | 44 | 19 | Two patients lost to follow-up |
HbA1C: Hemoglobin A1c.
Ninety-three percent of implants survived in patients who did not have diabetes (HbA1c ≤ 5.9 percent), 92.6 percent survived in those with well-controlled diabetes (HbA1c 6.0-8.0 percent) and 95.0 percent survived in patients with poorly controlled diabetes (HbA1c ≥ 8.1 percent). Implants in patients lost to follow-up were considered to have failed. The overall implant retention rate was 94 percent.
Implant stability
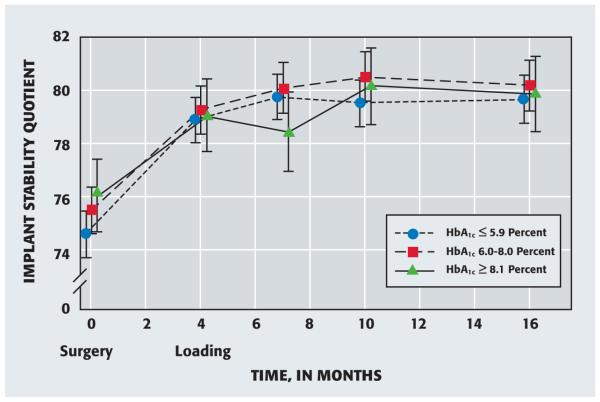
Implant stability increased significantly from baseline through all follow-up times for each HbA1c group (P ≤ .0045) (Figure 1). The mean baseline ISQs were not significantly different between the HbA1c groups (P = .1585). In the group with an HbA1c of 5.9 percent or lower, the mean ISQs at three, six and 12 months after loading were higher than the ISQ at implant loading (P = .0604); in addition, the results showed no differences in mean ISQ values at three, six and 12 months after loading (P = .8909). Similarly, for participants with an HbA1c level of between 6.0 and 8.0 percent, the mean ISQs at three, six and 12 months after loading were higher than the ISQ at implant loading (P = .0192), with no differences between the mean ISQ values at three, six and 12 months after loading (P = .7115).
Figure 1.
Mean implant stability quotient—as determined by means of resonance frequency analysis—according to hemoglobin A1c (HbA1c) level at implant placement (surgery) and follow-up times. Error bars represent 95 percent confidence intervals.
Implant integration was delayed in the group with poorly controlled diabetes compared with that in the other two groups. In the former group, the mean ISQs at six and 12 months after loading were higher than the mean ISQs at implant loading and three months after loading (P = .0337). We found no difference between the mean ISQ values at implant loading and three months after loading (P = .4515) or between the mean ISQ values at six and 12 months after loading (P = .7296).
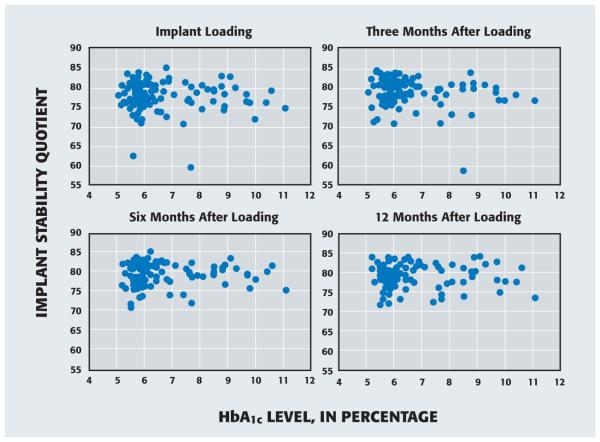
To evaluate the effects of HbA1c as a continuous variable at each time point, we identified the implant in each patient for which the ISQ was lowest (that is, the more poorly performing implant). In Figure 2, we present scatter diagrams of ISQs for the more poorly performing implant relative to the patient’s HbA1c level at baseline. No association between ISQs and HbA1c levels is evident over this one-year postloading period.
Figure 2.
Scatter diagrams of implant stability quotients—as determined by means of resonance frequency analysis—for the poorer-performing implant in each patient and hemoglobin A1c (HbA1c) levels.
Our evaluation of ISQ levels over time allowed an assessment of the integration—or healing—rate for HbA1c groups. The study results showed no statistically significant association of ISQ levels with bone type or HbA1c level at placement. We used baseline ISQ levels per patient to define the level of implant integration consistent with a healed implant, as evaluated at the time of loading and one year after loading. We explored healing broadly by using three different thresholds of baseline stability and observing the percentage of implants with an ISQ value equal to or greater than the patient’s baseline ISQ, 95 percent of the patient’s baseline ISQ or 90 percent of the patient’s baseline ISQ (Table 2). Regarding implants and patients lost to follow-up, we considered those for which or whom the ISQ had not returned to threshold percentages (100, 95, 90 percent) before loss to follow-up as never having achieved the threshold. Similarly, we considered the one implant for which the baseline ISQ was missing as never having achieved a return to threshold ISQ. Table 2 presents conservative estimates for the percentages of implants meeting the ISQ thresholds.
TABLE 2.
Percentage of implants for which ISQ* measurement at implant loading or 12 months after loading was equal to or greater than ISQ at implant placement.
| HbA1c† GROUP | NUMBER OF IMPLANTS |
PERCENTAGE OF IMPLANTS‡ | |||||
|---|---|---|---|---|---|---|---|
|
Implant Loading
(Four Months After Placement) |
12 Months After Loading | ||||||
|
Threshold (percentage of ISQ
at placement) |
Threshold (percentage of ISQ
at placement) |
||||||
| 100 | 95 | 90 | 100 | 95 | 90 | ||
| ≤ 5.9 Percent | 100 | 90.0 | 97.0 | 98.0 | 93.0 | 98.0 | 98.0 |
| 6.0-8.0 Percent | 94 | 86.2 | 96.8 | 98.9 | 92.6 | 97.9 | 98.9 |
| ≥ 8.1 Percent | 40 | 67.5 | 95.0 | 100 | 82.5 | 97.5 | 100 |
|
Statistical
Significance |
Not applicable |
P = .0033§
P = .0010¶ P = .4096* |
P = .7937§
P = .6276¶ P = .9386* |
P = .8216§
P = .6448¶ P ≈ 1.0000# |
P = .1162§
P = .0617¶ P = .9043* |
P ≈ 1.0000§
P ≈ 1.0000¶ p = .9504* |
P = .8216§
P = .6448¶ P ≈ 1.0000# |
| TOTAL | 234 | 84.6 | 96.6 | 98.7 | 91.0 | 97.9 | 98.7 |
ISQ: Implant stability quotient.
HbA1c: Hemoglobin A1c.
Unless otherwise specified.
Test of differences among the three HbA1c groups.
Test of difference between an HbA1c level of 8.1 percent or greater and a combined HbA1c level of 5.9 percent or lower and an HbA1c level between 6.0 and 8.0 percent.
Test of difference between an HbA1c level of 5.9 percent or lower and an HbA1c level between 6.0 and 8.0 percent.
By the time of implant loading, all implants except the two replaced implants and the implant for which the baseline ISQ was missing achieved ISQs greater than or equal to 90 percent of the baseline ISQ. Using the 95 percent threshold for healing, we found no significant differences between HbA,c groups at loading or at one year after loading. We should point out that using the 100 percent ISQ threshold, we found fewer implants in the poorly controlled diabetes group (HbA1c ≥ 8.1 percent) that attained the threshold than we did in the combined nondiabetes (≤ 5.9 percent) and well-controlled diabetes (6.0-8.0 percent) groups. There was no difference between the nondiabetes and well-controlled diabetes groups.
Regarding the time required to achieve the 100 percent level of healing (that is, the mean time required for ISQ values to return to baseline stability), implants in the poorly controlled diabetes group (that is, HbA1c ≤ 8.1 percent) required about twice the time to return to baseline levels compared with implants in the other two groups (Table 3). The study findings showed no difference between the nondiabetes and well-controlled diabetes groups. Evaluation of the time to healing using the 95 or 90 percent thresholds yielded no significant differences between groups (data not shown). However, these times may be underestimates because we used 16 months as the time for implants that had not yet attained an ISQ equal to or greater than the ISQ at implant placement.
TABLE 3.
| HbA1c‡ AT PLACEMENT (BASELINE) |
NUMBER OF PATIENTS |
MEAN TIME (95% CONFIDENCE INTERVAL), IN MONTHS, TO ISQ ≥ BASELINE ISQ§ |
|
|---|---|---|---|
| Poorer- Performing Implant |
Better- Performing Implant |
||
| ≤ 5.9 Percent | 50 | 3.8 (2.3-5.4) | 2.4 (1.0-3.9) |
| 6.0-8.0 Percent | 47 | 4.0 (2.4-5.6) | 2.8 (1.3-4.3) |
| ≥ 8.1 Percent | 20 | 7.3 (4.9-9.8) | 5.4 (3.1-7.7) |
|
Statistical
Significance |
Not applicable |
P = .0482¶
P = .0144# P = .8446** |
P = .0879¶
P = .0309* P= .6801** |
| TOTAL | 117 | 5.1 (3.9-6.2) | 3.6 (2.5-4.6) |
ISQ: Implant stability quotient.
For patients lost to follow-up before the implant attained an ISQ greater than or equal to the baseline ISQ, the authors assumed that the implant did not achieve such an ISQ during the study.
HbA1c: Hemoglobin A1c.
Unless otherwise specified.
Test of differences among the three HbA1c groups.
Test of difference between an HbA1c level of 8.1 percent or greater and a combined HbA1c level of 5.9 percent or lower and an HbA1c level between 6.0 and 8.0 percent.
Test of difference between an HbA1c level of 5.9 percent or lower and an HbA1c level between 6.0 and 8.0 percent.
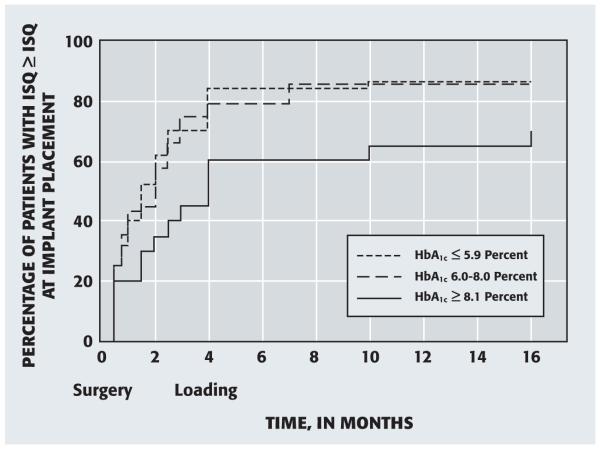
Figure 3 shows the percentage of patients for whom the more poorly performing implant had attained an ISQ greater than or equal to the ISQ at placement (surgery) at various follow-up times. There was no difference between the nondiabetes and well-controlled diabetes groups (P = .9473). The curve for the poorly controlled diabetes group (HbA1c ≥ 8.1 percent) was significantly different from those for the nondiabetes and well-controlled diabetes groups combined (P = .0355).
Figure 3.
Percentage of patients in whom the more poorly performing implant attained an implant stability quotient (ISQ)—as determined by means of resonance frequency analysis—greater than or equal to the ISQ at implant placement (surgery), according to the hemoglobin A1c (HbA1c) level at placement.
Using the proportional hazards model, we found that the group of patients with poorly controlled diabetes required longer times for the more poorly performing implant to achieve an ISQ greater than or equal to the ISQ at surgery than the combined group of patients without diabetes (HbA1c ≤ 5.9 percent) and the group with well-controlled diabetes (HbA1c 6.0-8.0 percent) (P = .0588). The hazard ratios at surgery were 1.00 for HbA1c less than or equal to 5.9 percent (reference category), 0.99 (95 percent confidence interval [CI], 0.65-1.52) for HbA1c between 6.0 and 8.0 percent and 0.58 (95 percent CI, 0.31-1.06) for HbA1c greater than or equal to 8.1 percent. Using the mean HbA1c levels averaged from surgery to the time the more poorly performing implant achieved an ISQ greater than or equal to the ISQ at surgery, we found that the hazard ratios at surgery were 1.00 for HbA1c less than or equal to 5.9 percent, 0.97 (95 percent CI, 0.63-1.49) for HbA1c between 6.0 and 8.0 percent and 0.55 (95 percent CI, 0.29-1.01) for HbA1c 8.1 percent or higher (HbA1c ≤ 5.9 percent and HbA1c 6.0-8.0 percent combined versus HbA1c ≥ 8.1 percent; P = .0481). Thus, we found little difference between HbA1c levels at surgery and the longer-term mean HbA1c levels as a predictor of the time required for a patient to achieve implant stability.
The study findings revealed no significant effects of sex, age category (≤ median age of 65 years and > 65 years) or implant length—after accounting for the effects of HbA1c at implant placement—on the time for the more poorly performing implant to achieve an ISQ greater than or equal to the ISQ at surgery. The hazard ratio for men was 1.00 and for women, 1.36 (95 percent CI, 0.90-2.06; P = .1402); for 65 years or younger, 1.00 and for older than 65 years, 1.04 (95 percent CI, 0.70-1.55; P = .8461). For implant length of 8 mm, the hazard ratio was 1.00, for implant length of 10 mm, 1.23 (95 percent CI, 0.65-2.36) and for implant length of 12 mm, 1.23 (95 percent CI, 0.53-2.82; P = .8157).
DISCUSSION
Our study findings revealed no differences in implant survival during the 16-month observational period relative to diabetes diagnosis or glycemic levels, even when we considered implants in patients lost to follow-up to be failures. We consider this to be a conservative approach, because we believe that a patient who experiences a problem with an implant is more likely, not less likely, to return for a follow-up visit.
Poor glycemic control has long been considered a contraindication to implant therapy.1-3 Strict glycemic control remains a critical goal in minimizing diabetes-related comorbidities for patients with type 2 diabetes.26 Thus, patients with poorly controlled diabetes are not considered to be appropriate candidates for implant therapy.1-3 However, a closer look at the literature reveals that the effects of hyperglycemia on implant therapy remain uncertain.7 Furthermore, patients with compromised glycemic control may gain important benefits from implant therapy with respect to dietary management of their diabetic condition. The potential benefits of and questionable risks involved with implant therapy make it more difficult for the practitioner to determine the best treatment option for the patient with type 2 diabetes who lacks good glycemic control.
We should point out that this study was conducted by using a delayed healing period (four months before restoration) to allow for adequate implant integration, and the restorative treatment was limited to the use of mandibular implant-supported overdentures for edentulous patients. Other than the failure of two implants, there were no clinical complications in this patient population during the study that would suggest important compromises on the basis of glycemic levels. The clinical results of this study are consistent with those of previous studies in which investigators reported similar rates of implant survival for patients with type 2 diabetes who had documented HbA1c levels approaching 12 percent.20,21,29,30
Our assessments of implant integration on the basis of changes in implant stability over time revealed subtle delays for patients with poor glycemic control. However, the degree of delay appears relatively minor, as it is evident when using 100 percent of baseline stability as a threshold for healing, but is not apparent when using 90 or 95 percent of baseline stability as thresholds. The minor role of this extended healing process also is supported by our survival data, which seem unaffected by these compromises in healing. The suggestion of compromises in bone metabolism during the integration process is consistent with results seen in hyperglycemic animal models. Investigators in these animal studies identified compromises in implant integration, bone metabolism, bone formation, bone strength and fracture healing.31-38
Although our study findings demonstrated positive clinical outcomes up to one year after loading that were independent of glycemic status, and we evaluated a larger patient population than those in previous studies, any extension of these findings to clinical care must be made with caution. We used specific surgical and prosthetic protocols that may have been important factors in the positive outcomes attained. At the time of implant placement, patients received systemic antibiotic treatment for seven days and used chlorhexidine rinse for two weeks to minimize postoperative complications. Also, prosthetic loading was delayed for twice the time (that is, four months rather than two months) used in standard protocols. Although our study design does not identify the value of any component of our clinical protocol as being critical to our outcomes, the stability findings support the use of a delayed prosthetic loading protocol. In addition, one may consider the use of panoramic radiographs rather than periapical radiographs a limitation in the radiographic assessment of implants owing to the potential for distortion in the mandibular midline area. However, this approach was necessary because many patients in this study had minimal alveolar ridge height, making it unfeasible to obtain periapical radiographs because of difficulty in film positioning and angulation.
The results of this study suggest that differences in bone metabolism and healing do not last much beyond six months after loading. Even with these differences in healing rates, implants seem to perform similarly for patients with poor glycemic control up to 12 months after loading. Certainly, clinicians should take into account the underlying differences in bone metabolism when considering implant therapy for patients with type 2 diabetes who have poor glycemic control. This finding provides further evidence of the need for careful evaluation of the effects of systemic conditions in these patients in dental practices. Investigators in future research need to examine the value of the restorative and anti-infective protocols used in our study, assess the clinical implications of hyperglycemia over longer periods with regard to peri-implant inflammation and bone loss, and clarify the benefits and risks of implant therapy for patients with type 2 diabetes.
CONCLUSIONS
The results of this study failed to show that elevated glycemic levels as high as 13.3 percent had a significant effect on implant survival over a one-year postloading period in patients with type 2 diabetes. However, early bone healing and implant stability may be affected by hyperglycemia, indicating the need for further investigation of the effects of elevated HbA1c levels and appropriate clinical management.
Acknowledgments
This study was supported by grants DE017882 and DE023518 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md.
ABBREVIATION KEY
- HbA1c
Hemoglobin A1c
- ISQ
Implant stability quotient
- PI
Principal investigator
- RFA
Resonance frequency analysis
- UTHSCSA
University of Texas Health Science Center at San Antonio
Footnotes
Disclosure. None of the authors reported any disclosures.
Materials support was provided by Straumann USA, Andover, Mass., and Osstell, Gothenburg, Sweden.
Contributor Information
Thomas W. Oates, Jr., Department of Periodontics, School of Dentistry, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, MCS 7888, San Antonio, Texas 78229-3900.
Patrick Galloway, Department of Periodontics, School of Dentistry, University of Texas Health Science Center at San Antonio.
Peggy Alexander, Department of Periodontics, School of Dentistry, University of Texas Health Science Center at San Antonio.
Adriana Vargas Green, Department of Comprehensive Dentistry, School of Dentistry, University of Texas Health Science Center at San Antonio.
Guy Huynh-Ba, Department of Periodontics, School of Dentistry, University of Texas Health Science Center at San Antonio.
Jocelyn Feine, Oral Health and Society Research Unit, Faculty of Dentistry, McGill University, Montreal, Quebec, Canada.
C. Alex McMahan, Department of Pathology, University of Texas Health Science Center at San Antonio.
References
- 1.National Institutes of Health Consensus Development Conference statement on dental implants June 13-15, 1988 J Dent Educ. 1988;52(12):824–827. [PubMed] [Google Scholar]
- 2.American Academy of Periodontology Proceedings of the 1996 World Workshop in Periodontics: consensus report—implant therapy II. Ann Periodontol. 1996;1(1):816–820. [Google Scholar]
- 3.Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol. 2009;80(11):1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 4.Marchand F, Raskin A, Dionnes-Hornes A, et al. Dental implants and diabetes: conditions for success. Diabetes Metab. 2012;38(1):14–19. doi: 10.1016/j.diabet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.National Committee for Quality Assurance . The State of Health Care Quality 2008. National Committee for Quality Assurance; Washington: www.ncqa.org/Portals/0/Newsroom/SOHC/SOHC_08.pdf. Accessed Sept. 11, 2014. [Google Scholar]
- 6.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the hemoglobin A1c assay into estimated average glucose values (published correction appears in Diabetes Care 2009;32[1]:207) Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Implants Res. 2013;24(2):117–127. doi: 10.1111/j.1600-0501.2011.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998;13(5):620–629. [PubMed] [Google Scholar]
- 9.Gerritsen M, Lutterman JA, Jansen JA. Wound healing around bone-anchored percutaneous devices in experimental diabetes mellitus. J Biomed Mater Res. 2000;53(6):702–709. doi: 10.1002/1097-4636(2000)53:6<702::aid-jbm13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Casap N, Nimri S, Ziv E, Sela J, Samuni Y. Type 2 diabetes has minimal effect on osseointegration of titanium implants in Psammomys obesus. Clin Oral Implants Res. 2008;19(5):458–464. doi: 10.1111/j.1600-0501.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants. 2008;23(2):237–246. [PubMed] [Google Scholar]
- 12.Schlegel KA, Prechtl C, Möst T, Seidl C, Lutz R, von Wilmowsky C. Osseointegration of SLActive implants in diabetic pigs. Clin Oral Implants Res. 2013;24(2):128–134. doi: 10.1111/j.1600-0501.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- 13.Oates TW, Dowell S, Robinson M, McMahan CA. Glycemic control and implant stabilization in type 2 diabetes mellitus. J Dent Res. 2009;88(4):367–371. doi: 10.1177/0022034509334203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HM, Pan LC, Lee SY, Chiu CL, Fan KH, Ho KN. Assessing the implant/bone interface by using natural frequency analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):285–291. doi: 10.1067/moe.2000.108918. [DOI] [PubMed] [Google Scholar]
- 15.Barewal RM, Oates TW, Meredith N, Cochran DL. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18(5):641–651. [PubMed] [Google Scholar]
- 16.Ersanli S, Karabuda C, Beck F, Leblebicioglu B. Resonance frequency analysis of one-stage dental implant stability during the osseointegration period. J Periodontol. 2005;76:1066–1071. doi: 10.1902/jop.2005.76.7.1066. [DOI] [PubMed] [Google Scholar]
- 17.Valderrama P, Oates TW, Jones AA, Simpson J, Schoolfield JD, Cochran DL. Evaluation of two different resonance frequency devices to detect implant stability: a clinical trial. J Periodontol. 2007;78(2):262–272. doi: 10.1902/jop.2007.060143. [DOI] [PubMed] [Google Scholar]
- 18.West JD, Oates TW. Identification of stability changes for immediately placed dental implants. Int J Oral Maxillofac Implants. 2007;22(4):623–630. [PubMed] [Google Scholar]
- 19.Makary C, Rebaudi A, Sammartino G, Naaman N. Implant primary stability determined by resonance frequency analysis: correlation with insertion torque, histologic bone volume, and torsional stability at 6 weeks. Implant Dent. 2012;21(6):474–480. doi: 10.1097/ID.0b013e31826918f1. [DOI] [PubMed] [Google Scholar]
- 20.Dowell S, Oates TW, Robinson M. Implant success in people with type 2 diabetes mellitus with varying glycemic control: a pilot study. JADA. 2007;138(3):355–361. doi: 10.14219/jada.archive.2007.0168. [DOI] [PubMed] [Google Scholar]
- 21.Khandelwal N, Oates TW, Vargas A, Alexander PP, Schoolfield JD, McMahan CA. Conventional SLA and chemically modified SLA implants in patients with poorly controlled type 2 diabetes mellitus: a randomized controlled trial. Clin Oral Implants Res. 2013;24(1):13–19. doi: 10.1111/j.1600-0501.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 22.Lekholm U, Zarb G. Patient selection and preparation. In: Branemark P-I, Zarb GA, Albrektsson T, editors. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. Quintessence; Hanover Park, Ill.: 1985. pp. 199–210. [Google Scholar]
- 23.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11–25. [PubMed] [Google Scholar]
- 24.American Diabetes Association Standards of medical care in diabetes: 2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–1298. [PubMed] [Google Scholar]
- 27.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33): UK Prospective Diabetes Study (UK-PDS) Group (published correction appears in Lancet 1999;354[9178]:602) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Wiley; New York City: 1999. [Google Scholar]
- 29.Tawil G, Younan R, Azar P, Sleilati G. Conventional and advanced implant treatment in the type II diabetic patient: surgical protocol and long-term clinical results. Int J Oral Maxillofac Implants. 2008;23(4):744–752. [PubMed] [Google Scholar]
- 30.Turkyilmaz I. One-year clinical outcome of dental implants placed in patients with type 2 diabetes mellitus: a case series. Implant Dent. 2010;19(4):323–329. doi: 10.1097/ID.0b013e3181e40366. [DOI] [PubMed] [Google Scholar]
- 31.Ajami E, Mahno E, Mendes VC, Bell S, Moineddin R, Davies JE. Bone healing and the effect of implant surface topography on osteoconduction in hyperglycemia. Acta Biomater. 2014;10(1):394–405. doi: 10.1016/j.actbio.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Funk JR, Hale JE, Carmines D, Gooch HL, Hurwitz SR. Biomechanical evaluation of early fracture healing in normal and diabetic rats. J Orthop Res. 2000;18(1):126–132. doi: 10.1002/jor.1100180118. [DOI] [PubMed] [Google Scholar]
- 33.Amir G, Rosenmann E, Sherman Y, Greenfeld Z, Ne’eman Z, Cohen AM. Osteoporosis in the Cohen diabetic rat: correlation between histomorphometric changes in bone and microangiopathy. Lab Invest. 2002;82(10):1399–1405. doi: 10.1097/01.lab.0000032378.19165.e2. [DOI] [PubMed] [Google Scholar]
- 34.Beam HA, Parsons JR, Lin SS. The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J Orthop Res. 2002;20(6):1210–1216. doi: 10.1016/S0736-0266(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 35.Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144(1):346–352. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- 36.Kayal RA, Tsatsas D, Bauer MA, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560–568. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41(2):81–85. doi: 10.3109/03008200009067660. [DOI] [PubMed] [Google Scholar]
- 38.He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT. Diabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology. 2004;145(1):447–452. doi: 10.1210/en.2003-1239. [DOI] [PubMed] [Google Scholar]