Abstract
The use of stem cells (SCs) as carriers for therapeutic agents has by now progressed to early clinical trials. These clinical trials exploring SC-mediated delivery of oncolytic adenoviruses will commence in the near future, hopefully yielding meritorious results that could provoke further scientific inquiry. Preclinical animal studies have demonstrated that SCs can be successfully loaded with conditionally-replicative adenoviruses and, then, delivered to the tumor, upon which they may evoke pronounced therapeutic efficacy in the animal (Ahmed et al., 2011; Ahmed et al., 2012; Thaci et al., 2012; Tobias et al., 2013). Here in this protocol, we describe the maintenance of SCs, provide an analysis of optimal adenoviral titers for SC loading, and evaluate the optimized viral loading on SCs.
INTRODUCTION
As stem cell-mediated delivery of oncolytic adenoviruses has shown promising therapeutic efficacy, it is very necessary to standardize the protocol to enhance the efficacy and consistency of this approach. Only when healthy and minimally passaged SCs are loaded with an optimized number of virus particles/cell will these SCs migrate to its destined place(s) around the tumor area during the period of viral progeny production (48-72hr) and then release the virus progenies (~104 particles/cell). We describe here how to properly culture and prepare the SCs for oncolytic virus loading. We further describe how to optimize a virus titer for loading on SCs to maximize the oncolytic virus progenies delivered to the target site.
BASIC PROTOCOL 1. Culture of neural stem cells
Through this protocol, we describe the means of culture of different type of stem cells for the loading of adenoviral vector.
Materials
Stem cells: Neural stem cells (HB1.F3-CD) or Mesenchymal stem cells (either bone marrow derived or adipose derived)
100X stock of Pen/Strep: Penicillin sodium (100 units/mL) and streptomycin (100 µg/mL)
100X stock of Glutamine (200 mM, light sensitive): It is only needed when you purchase DMEM without Glutamine.
Fetal Bovine Serum (FBS)
1X PBS
Dulbecco's Modified Eagle's medium (DMEM, 500 mL)
Dimethyl sulfoxide (DMSO)
0.25% Trypsin-EDTA solution
T75 cell culture flask
15ml and 50ml Conical tubes
Microscope
37°C, 5% CO2 humidified incubator
Note: All cell culture practices must be conducted under sterile conditions, including the reagents and workspace involved.
Before putting any materials in the sterile hood, 70% Et-OH has to be sprayed and let it completely dry up in the sterile hood. Do not wipe out. Sanitization can be only done with dehydration of Et-OH.
Initial culture from a frozen stock of SCs
This protocol is tailored to a thawing of a cryotube containing 2×106cells in 1 mL.
1. Add 15 mL of cell growth media (10% FBS and 1X Pen/Step in DMEM) to a T75 flask.
2. Spread the cell growth media throughout the flask.
3. Thaw the frozen stock of SCs in 37°C water bath.
4. Transfer thawed cells in the cryotube into a sterile hood.
5. Slowly add the SCs to the growth media in the T75 flask.
Be cautious not to cause osmotic shock to the cells by slowly adding the SCs to the culture media.
6. Gently mix the cells in the media to evenly extend their presence on the surface of the T75 flask
7. Incubate the cells with growth media in a 37°C, 5% CO2 humidified incubator.
8. On the following day, aspirate the media from the T75 flask and immediately add 15 mL of the fresh cell growth media.
In this step, the DMSO from the freezing media and any dead cells may be aspirated in removal.
Please be advised that it will take approximately 3-4 days for SCs to fully recover and propagate efficiently.
Subculture of SCs
SCs can grow rapidly until the passage limit reaches 25-30 (Aboody et al., 2008; Ahmed et al., 2012). During these passages, carefully check their growth every day. As the media becomes acidic (denoted by a yellow change of color), change the media.
9 Aspirate the existing cell growth media from the T75 flask.
10. Add 5ml of 1X PBS and gently spread to wash.
11. Add, and thoroughly spread, 1.5 mL of 0.25% Trypsin-EDTA solution to the T75 flask.
To accelerate cell detachment, the flask can be placed and incubated in a 37°C, 5% CO2 humidified incubator.
12. When obvious cell detachment is observed, add 5 mL of the fresh cell growth media to the T75 flask.
13. Wash and homogenize the cells in the cell growth media.
Be especially gentle throughout this repeated pipetting. Strong agitation causes the cells to die.
One of confluent T75 flask of SCs can generate 3 of T75 flasks to be cultured.
14. Transfer the evenly divided volume of the cell-containing media (~2.3ml) to a new T75 flask.
15. Add 12mL of fresh cell growth media to the flask.
Preparation of Frozen Stocks of SCs
A minimum of 2-3 × 106 cells per 1 mL of freezing media in cryotube is required to restore the cells in a T75 flask. At high confluency, approximately 1.0 × 107 cells can grow in a T75 flask, therefore, 3 cryotubes of frozen stock can be made.
16. Follow the subculture directives from steps 9-13.
17. Transfer the cell-containing media (~6.5 mL) to a 50 mL tube.
18. Centrifuge the tube at 1,200rpm for 5 minutes.
19. While centrifuging, make an appropriate amount of the cell freezing media (10% DMEM in FBS: 300 µL of DMSO + 2.7 mL of FBS).
20. Aspirate the supernatant media and add the cell freezing media.
21. Homogenize the cells well and transfer to 3 different cryotubes.
22. Store the cryotubes at −80 °C overnight and transfer to −180°C the following day.
Basic Protocol 2. Optimizing the Loading Capability of Stem Cells
As the primary receptor, Coxsackievirus and adenovirus receptor (CAR) of the wild type human adenovirus serotype 5 (HAd5) is not expressed on the SCs, the Ad5 fiber domain which dictates the receptor binding, has been genetically modified to achieve the efficient, enhanced and/or directed specific infection in most of presently used adenoviral vectors. However, when this structural protein (fiber) is modified, it could in some cases lead to a lower number of virus progeny production (Kim et al., 2013; Tyler et al., 2009; Zheng et al., 2007).
Therefore, depending on the modification(s), the infectivity and/or production of viral progenies can be different in SCs. As such, Basic Protocol 2 is necessary to investigate in vitro the optimal virus titer that can keep the SCs alive until in vivo delivery and yet produce the maximum number of virus progenies. In the following protocol, we did not include the generation or production of adenoviral vectors since the defined protocol can be found in UNIT 12.4.
Materials
24-well plates
1.5 mL tubes
Hemocytometer
Trypan blue
Adeno-X™ Rapid Titer Kit (Clontech)
Determine the viability of SCs post virus loading
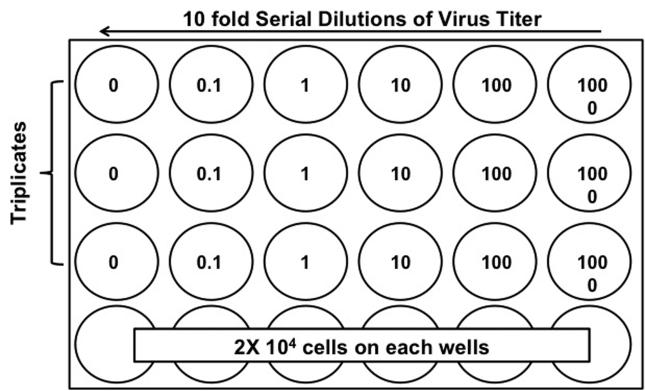
1. Prepare one 24-well plate by adding 2 × 104 cells per well (Figure 1) and incubate overnight in a 37°C, 5% CO2 humidified incubator.
Figure 1.
NSC plating and virus dilution for cell viability and viral progeny production assay. To assess the optimal titer of adenovirus for NSC infection, NSCs can be seeded in the 24 well plate with 2×104 cells in each well followed by different virus titers (from 0.1 to 1,000). Each virus titer should be evaluated in triplicates to minimize experimental errors.
2. On the following day, prepare a 100-fold dilution of virus stock by mixing 10 µL of original virus stock with 990 µL of 1X PBS). Keep this dilution readily available on ice.
For the most part (with some exception), adenovirus stocks are in 10~50% glycerol with a high number of virus particles (Unit 12.4).
The dissociation factor for adenovirus is very low (Shabram et al., 2002); therefore, it is highly recommended to make a 100-fold diluted virus stock first and use it for further virus infection to have better homogeneous mixture in the virus infection media.
3. By following Table 1, prepare a virus dilution: multiple of infection (MOI) of 0.1-1,000 vp/cell or pfu/cell with virus infection media (2% FBS and 1X Pen/Step in DMEM).
Table 1.
Formula for viral infection
| Name | Vp/ml or pfu/ml |
Cell/wells | MOI | Wells | Amount (mL) | Virus titer | 100 dil. titer | Add amount(μL) in E_ ml |
|---|---|---|---|---|---|---|---|---|
| virus unit | The number of cells in one well |
Multiple of infection : how many viruses per cells |
Number of total wells to use |
Amount of the total media needed |
Virus titer to be used |
100 fold diluted virus titer |
Required Amount of
100 fold diluted virus |
|
| A | B | C | D | E |
F
= B × C × D |
G
= A/100 |
H
=F / G × 1000 |
|
| Ex. You want to use 1.012 virus to infect 3 well of 2X104 cells with 1000 MOI | ||||||||
| JK | 1.0×1012 | 2.0×104 | 1000 | 4wells (3 wells + 1 extra) |
2mL (500μL X4well) |
8.0×107 | 1.0×1010 | 8μL |
| → You can add 8μL of 100 fold diluted stock into 2ml of infection media. Then add 500μL to each well to achieve 1000 MOI virus infection. | ||||||||
4. Aspirate the media and add a virus-containing media with proper titers.
5. After incubation for 2 hours in a 37°C, 5% CO2 humidified incubator, aspirate the virus-containing media and wash with 0.5 mL of 1 × PBS.
6. After washing, add 0.5 mL of cell growth media and incubate for 72 hours in a 37°C, 5% CO2 humidified incubator.
7. After 72 hours, transfer the media to 1.5 mL tubes.
8. Add 150 µL of 0.25% Trypsin-EDTA and incubate until the cells detach.
9. Add 300 µL of 1X PBS and transfer to the corresponding tubes from step 7.
In the time between 48 and 72 hours, the initially added viruses will propagate and start to induce the detachment of the cells. To analyze the cell viability, both adhered and detached cells have to be collected.
10. Homogenize the cells in the tube.
11. Mix 20 µL of the cell suspension and 20 µL of trypan blue.
12. Quantify the live and dead cells with hemocytometer.
Only dead cells will uptake trypan blue which is visibly dark coloured microscopically. The ideal percentage of viable cells should be at least 80-90%. If the percentage of live cells is less than this, the virus titer should be reduced.
Analysis of viral progeny production in SC
13. Follow the steps from 1 – 9 from the above SC Cell viability post virus loading protocol.
14. Centrifuge at 1,200 rpm for 5 minutes.
15. Aspirate the supernatant media and resuspend in 50 µL of PBS.
16. Freeze the cell-containing tubes at −80°C, thaw the cell containing tubes at 37°C, and vortex well.
17. Repeat step 16, three times.
Adenovirus particles are relatively stable during the ‘freezing and thawing’ steps. These steps will, however, rupture the cell membrane and release the virus progenies into the supernatant.
18. Check the virus titer with Adeno-X™ Rapid Titer Kit (Clontech).
Around 104 virus particles can be produced from one cell. Therefore with an optimized viral titer (between 10-100 infectious units) loaded 1.0 × 106 SCs, it is expected to produce and release approximately 1.0 × 108 to 1010 of virus particles with 72hr.
Basic Protocol 3. Loading of adenovirus into Stem Cells
Through the above analysis, optimal adenovirus titers that can achieve maximum neural stem cell viability and viral progeny production should be determined and these parameters used in the final loading of adenovirus (Ahmed et al., 2013; Bolontrade et al., 2012; Yong et al., 2009).
1. Follow steps 9-13 from the subculture of SC (Basic protocol 1).
2. Centrifuge at 1,200 rpm for 5 minutes.
3. Aspirate the supernatant media and add 5 mL of 1X PBS.
4. Quantify the cell number using a hemocytometer.
5. Transfer approximately 1.0 × 106 cells into a new 15 mL tube and add 5 mL of 1X PBS for the second wash.
6. Centrifuge at 1,200 rpm for 5 minutes.
7. While centrifuging, prepare optimal virus titers, as determined in Basic Protocol 2, in a solution of 100 µL of 1X PBS.
8. Aspirate the cell supernatant and add 100 µL of optimal virus titer containing solution.
9. After gently mixing, incubate for 2 hours at room temperature.
10. After incubation, add 0.5 mL of 1X PBS and centrifuge at 1,200 rpm for 5 minutes.
11. Remove the supernatant and add 0.5 mL of 1X PBS.
12. Repeat steps 10-11 three times.
13. After washing, resuspend the cells in a desired amount of 1XPBS (Ahmed et al., 2013; Bolontrade et al., 2012; Yong et al., 2009).
The amount of 1X PBS needed will vary depending on the application. For intracranial injection , 5ul/injection would be the maximum volume so it should be less than 5ul but in other cases, it could be 100ul.
14. Keep the virus-loaded SCs cool on ice until their use.
It is recommended to use the virus-loaded SCs as soon as possible but they can be stored on ice for up to an hour.
Commentary
Background Information
Initially isolated from human adenoids as infectious particles in the mid-20th century, adenoviruses may be now engineered for use as delivery vehicles for gene therapy and for their oncolytic activity against cancer (Nandi and Lesniak, 2009). More specifically, adenovirus-mediated cancer therapy has steeped particular interest in treating glioma (Nandi and Lesniak, 2009). This is due to the fact that these viruses merit particularly well as delivery vehicles, offering specific tropism to the engineered site so desired (Kim et al., 2013; Tyler et al., 2009; Zheng et al., 2007). However, despite many advantages of adenoviral vectors, there have been major challenges for their use such as pre-existing anti-adenoviral immunity within the population, and minimal penetration and spreading of viruses in the tumor mass (Hendrickx et al., 2014). The use of the stem cell carrier can overcome these challenges and enhance the oncolytic activity of adenovirus.
Since the discovery of stem cells (SCs), their biological classification, function, and potential for therapeutic use has been investigated in a litany of preclinical and translational studies. The self-renewal and regenerative capabilities of SCs make them attractive agents of treatment for neurodegenerative disorders. However, it is their tropism to brain tumors that motivated the study of their potential as a therapeutic delivery vehicles (Aboody et al., 2008). This remarkably intrinsic tumor tropism of neural stem cells allows them to specifically deliver a therapeutic agent, such as an oncolytic adenovirus, to the tumor, drastically reducing the off-target side effects all too commonly experienced. Using SCs to deliver oncolytic viruses can enhance the viral distribution throughout the tumor bed while simultaneously shielding the viruses from the immune system (Ahmed et al., 2010). Furthermore, SCs can deliver oncolytic viruses across the blood brain barrier to the tumor, making multiple, less invasive intravenous doses of therapy possible (Aboody et al., 2000).
The groundbreaking use of SCs as a carrier for therapeutic agents has already progressed to early clinical trials, and hopefully clinical trials exploring stem cell-mediated delivery of oncolytic adenoviruses will commence in the near future. Preclinical studies have demonstrated that SCs can successfully be loaded with conditionally replicative viruses, deliver them to tumor, and provide a therapeutic benefit in animal models (Ahmed et al., 2011; Ahmed et al., 2012; Thaci et al., 2012; Tobias et al., 2013). The innate limitations and obstacles facing oncolytic virotherapy can be overcome by delivering them with stem cell carriers like NSCs and MSCs, and the future is bright for this promising combination of molecular agents against currently devastating malignancies.
Critical Parameters and Troubleshooting
Several steps are critical for the efficient loading of adenoviruses on SCs. The initial recovery of cells from the freezing stock takes about 3 days. At this point, SCs grow slowly. However, SCs grow exponentially after 2-3 passages. Therefore during this initial passage step, extra careful observation is absolutely required.
SCs can be cultured for 25-30 passages. After this time point, SCs start to grow extremely slow; therefore, it is highly recommended to make frozen stocks at the earliest time point. It is not recommended to culture and use the cells for any experiments after 30 passages.
In addition, SCs are highly sensitive to pH change. When the SCs are confluent and left to themselves for too long, the pH of the media become acidified which causes the death of SCs. Observe the SCs on a daily basis and change the media if any media acidification is observed. Optimization of virus titer to maximize virus progeny production and minimize cytopathic effects on SCs is the most important step for a stem cell-mediated adenovirus delivery approach. Depending on how well it is optimized, it will completely change the therapeutic effect from the SC-mediated adenovirus delivery approach. As mentioned above, within 72hrs the viral progeny production should reach the maximum without killing the SCs. At least 80-90 % of virus loaded SCs should be healthy but detached from the bottom of the well which will generate approximately 104 virus particles per cell.
For optimized virus titer, it is recommended to dilute an original virus stock to 100-fold in 1XPBS and use it for infection. Keep in mind that the dissociation factor of adenovirus particles is low and the sample must be thoroughly mixed to achieve homogeneity of virus particles in the solution, minimizing any variation on each experiment.
Anticipated Results
It takes approximately 48 to 72 hours for adenovirus to produce the maximum number of virus progenies (104 virus particles) in a host cell and to be released from the host cell. When healthy and minimally passaged, SCs are loaded with optimized virus particles/cell and these SCs will migrate to its destined place(s) around the tumor area during this progeny production period and then release the virus progenies. When adenoviruses with an optimized titer (between 10-100 infectious units) loaded 1.0 × 106 SCs are used, it is expected to produce and release approximately 1.0 × 108 to 1010 of virus particles in their destination.
Time Considerations
Initial cell recovery time from a frozen stock can vary depending on the condition of the cell stock and personal skills however, sub-culturing of SCs should be done on a basis of less than 72 hours to keep them healthy. When the SCs do not grow well at the initial recovery or during the subculture period, aspirate and discard the old media and replace with a fresh cell growth media. All of the procedures, including the preparation of SCs and adenovirus infection (loading) until in vivo delivery, should be done in 3-4 hours to maximize the therapeutic efficacy of SC-mediated adenovirus delivery.
Acknowledgement
This work was supported by NIH/NINDS grant U01NS069997 to MSL.
Literature Cited
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- Ahmed AU, Alexiades NG, Lesniak MS. The use of neural stem cells in cancer gene therapy: predicting the path to the clinic. Curr Opin Mol Ther. 2010;12:546–552. [PMC free article] [PubMed] [Google Scholar]
- Ahmed AU, Thaci B, Alexiades NG, Han Y, Qian S, Liu F, Balyasnikova IV, Ulasov IY, Aboody KS, Lesniak MS. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AU, Thaci B, Tobias AL, Auffinger B, Zhang L, Cheng Y, Kim CK, Yunis C, Han Y, Alexiades NG, Fan X, Aboody KS, Lesniak MS. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J Natl Cancer Inst. 2013;105:968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AU, Ulasov IV, Mercer RW, Lesniak MS. Maintaining and loading neural stem cells for delivery of oncolytic adenovirus to brain tumors. Methods Mol Biol. 2012;797:97–109. doi: 10.1007/978-1-61779-340-0_8. [DOI] [PubMed] [Google Scholar]
- Bolontrade MF, Sganga L, Piaggio E, Viale DL, Sorrentino MA, Robinson A, Sevlever G, Garcia MG, Mazzolini G, Podhajcer OL. A specific subpopulation of mesenchymal stromal cell carriers overrides melanoma resistance to an oncolytic adenovirus. Stem Cells Dev. 2012;21:2689–2702. doi: 10.1089/scd.2011.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Human gene therapy. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Glasgow JN, Nakayama M, Ak F, Ugai H, Curiel DT. An adenovirus vector incorporating carbohydrate binding domains utilizes glycans for gene transfer. PLoS One. 2013;8:e55533. doi: 10.1371/journal.pone.0055533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Lesniak MS. Adenoviral virotherapy for malignant brain tumors. Expert Opin Biol Ther. 2009;9:737–747. doi: 10.1517/14712590902988451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabram P, Vellekamp G, Scandella C. Purification of Adenovirus Elsevier. 2002 [Google Scholar]
- Thaci B, Ahmed AU, Ulasov IV, Tobias AL, Han Y, Aboody KS, Lesniak MS. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19:431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias AL, Thaci B, Auffinger B, Rincon E, Balyasnikova IV, Kim CK, Han Y, Zhang L, Aboody KS, Ahmed AU, Lesniak MS. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Transl Med. 2013;2:655–666. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler MA, Ulasov IV, Sonabend AM, Nandi S, Han Y, Marler S, Roth J, Lesniak MS. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]