Abstract
Unwarranted breast cancer adjuvant chemotherapy dose reductions have been documented in black women, women of lower socioeconomic status, and those who are obese. No information on the quality of chemotherapy is available in Hispanic women. The purpose of this study was to characterize factors associated with first cycle chemotherapy dose selection in a multi-ethnic sample of low-income women receiving chemotherapy through the Breast and Cervical Cancer Prevention Treatment Program (BCCPT) and to investigate the impact of Hispanic ethnicity and patient self-efficacy on adjuvant chemotherapy dose selection. Survey and chemotherapy information were obtained from consenting participants enrolled in the California BCCPT. Analyses identified clinical and non-clinical factors associated with first cycle chemotherapy doses less than 90 % of expected doses. Of 552 patients who received chemotherapy, 397 (72 %) were eligible for inclusion. First cycle dose reductions were given to 14 % of the sample. In multivariate analyses, increasing body mass index and non-academic treatment site were associated with doses below 90 % of the expected doses. No other clinical or non-clinical factors, including ethnicity, were associated with first cycle doses selection. In this universally low-income sample, we identified no association between Hispanic ethnicity and other non-clinical patient factors, including patient self-efficacy, in chemotherapy dose selection. As seen in other studies, obesity was associated with systematic dose limits. The guidelines on chemotherapy dose selection in the obese may help address such dose reductions. A greater understanding of the association between type of treatment site and dose selection is warranted. Overall, access to adequate health care allows the vast majority of low-income women with breast cancer to receive high-quality breast cancer chemotherapy.
Keywords: Breast cancer, Chemotherapy, adjuvant, Health disparities, Hispanic, Quality of care
Introduction
Racial and ethnic disparities in breast cancer outcome in the United States have been attributed to younger age at presentation [1, 2] and more advanced disease among these minority groups [1, 3], a high proportion of estrogen receptor (ER)-negative [1] and “triple-negative” tumors [4, 5], higher rates of some comorbid conditions among blacks and Hispanics, and lower socioeconomic status (SES) [6], which may pose barriers to guideline-concordant care [7] when compared to non-Hispanic whites and Asians.
In an attempt to improve access to breast and cervical cancer screening and treatment, Congress passed the Breast and Cervical Cancer Prevention Treatment Program (BCCTP) Act in 2000 that allowed Medicaid funds to pay for treatment for uninsured women whose income was ≤200 % of the federal poverty level. The California BCCTP program is federal and state funded for un- and under-insured women with income levels of 200 % of the federal poverty level or less [8].
An in-depth review of the care delivered to women with breast cancer in the BCCTP demonstrated that the care was of extremely high quality. Using 29 quality measures developed by the National Initiative for Cancer Care Quality (NICCQ), the investigators demonstrated that patients received 93 % of recommended care [9]. No information was collected at that time, however, on chemotherapy dosing patterns. Unwarranted chemotherapy dose reductions may compromise the efficacy of therapy and increase the risk of cancer recurrence and death [10, 11].
Previous work has demonstrated that black women, women of lower SES, and obese women are more likely to be underdosed with chemotherapy beginning with the first cycle of chemotherapy [12–16]. Adjuvant chemotherapy dosing patterns in Hispanics have not been described in the literature. The purpose of this study was to investigate patient demographic, social, and psychosocial factors associated with receipt of optimal adjuvant breast cancer chemotherapy doses in women enrolled in the BCCTP. We hypothesized that Hispanic women would receive lower chemotherapy doses than non-Hispanic white women. This hypothesis was based on the following—Hispanics have a particularly difficult time accessing high-quality care, and immigrants with lower levels of proficiency in English have the most difficulty of all [17, 18]. Moreover, primary care physicians have described challenges in providing high-quality care to Hispanics [19], and it is possible that oncologists experience similar challenges. Our second hypothesis is that higher levels of self-efficacy when communicating with physicians, regardless of race or ethnicity, would increase the prescriber's confidence that patients could ask for help if and when a chemotherapy side effect arises. Higher self-efficacy might thus in turn protect against chemotherapy dose reductions.
We selected the first cycle of chemotherapy as the dependent variable because it is the first cycle of therapy that is most highly influenced by the physician. That is, first cycle doses are not selected based on a patient's previous experience or side effects with chemotherapy. In addition, the dose given with the first cycle of chemotherapy is highly predictive of the overall dose for the entire regimen [13].
Patients and methods
Study sample and data collection
The study sample and recruitment procedures have been published previously but are described here [9]. Potential participants were identified with the assistance of the California Department of Health Services [20]. Consecutive patients over the age of 18 who were diagnosed with Stage I, II, or III breast cancer between February 2003 and September 2005 were invited to participate in this study. All participants signed written informed consent in English or in Spanish according to their language preference. Participation included completion of a telephone survey and review of medical records among consenting patients. Data were collected by telephone surveys, conducted approximately 6 months after enrollment in the BCCTP, and exhaustive medical record abstraction, including medical oncology records, in consenting patients.
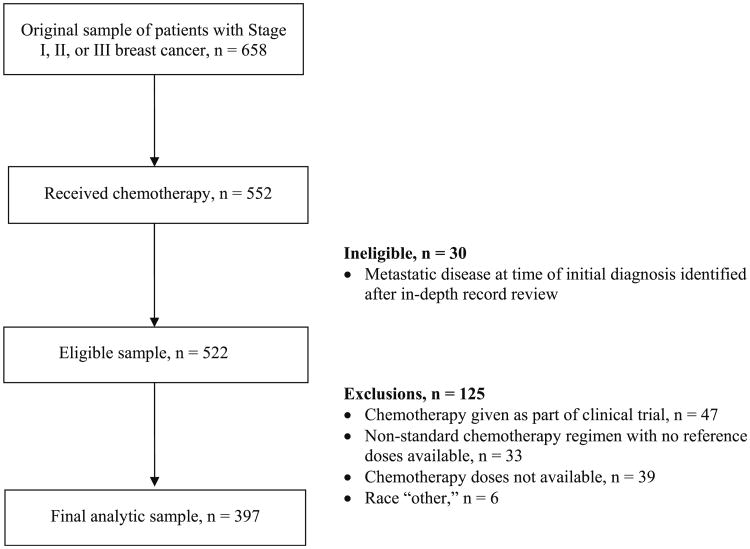
The original sample has been described in detail elsewhere [20]. The original sample for this study was 658. Further exclusions applied for this analysis were enrollment in a chemotherapy clinical trial, patients who received a non-standard adjuvant chemotherapy regimen based on the published literature, and patients in whom chemotherapy doses could not be obtained (see Figure). Patients found, via detailed chart review, to have metastatic disease early in the course of their adjuvant therapy were also excluded.
Measures
The dependent variable for the analyses was the chemotherapy dose ratio for the first cycle of therapy. We created a dichotomous variable of actual:expected doses using a cutoff of under 0.9 (that is, under 90 % of the expected chemotherapy doses) as in our previous work [13]. For each patient, the chemotherapy regimen was classified as either standard or non-standard based on regimens that were included in the guidelines of the National Comprehensive Cancer Network during the years the patients were treated [21]. Patients who received non-standard regimens (n = 33) were excluded from the analyses as shown in the Figure.
For those patients who received a standard regimen, the body surface area (BSA) was calculated using height and actual weight and the Mosteller formula [22]. The expected dose for each chemotherapeutic agent was calculated using standard published chemotherapy regimens as in our previous work [13, 16]. The actual documented chemotherapy dose given for the first cycle of chemotherapy was then divided by the expected chemotherapy dose to obtain a dose ratio for each drug. This was repeated for each drug in the first cycle; all the drugs were then averaged to obtain a regimen ratio as in our previous work and that of others [13, 14, 16, 23].
The independent variables included age at the time of diagnoses (obtained from the patient and the medical record), the presence or absence of comorbid conditions using the Katz adaptation of the Charlson comorbidity index obtained via the survey and confirmed via medical record review [24], race and ethnicity obtained by the survey, years of education obtained from the survey, and social support measured using the question, “How often did someone go to your doctor appointments with you?” for which the response options were, “sometimes,” “rarely,” “always,” or “usually.” The responses to this question were dichotomized into “sometimes/rarely” versus “always/usually.” Patient self-efficacy was measured using the perceived efficacy in patient–physician interactions (PEPPI), a validated self-administered scale that measures a patient's efficacy in getting her information and other needs met in interactions with physicians [25–27]. The PEPPI, composed of five items, has a scale ranging from 0 to 50, higher scores indicating higher perceived self-efficacy in patient–physician interactions. Higher patient self-efficacy measured with the PEPPI has been shown to correspond to greater success in managing post-treatment side effects after multimodality breast cancer treatment [25].
The medical record was used to collect information on comorbid conditions (confirming patient survey responses) and tumor characteristics (tumor size, lymph node status, and hormone receptor status). The medical record was also used to obtain data on height and weight and detailed information on chemotherapy regimen and doses as described above. Obesity status was measured using body mass index (BMI), which in turn was calculated using the Quatelet index (kg/cm2) and the criteria of the World Health Organization [28]. Data abstraction was conducted by a trained abstractor with continuous data quality checks.
The type of treatment site (academic vs. non-academic) was obtained from the State of California Office of Statewide Health Planning and Development, Hospital 2004 Annual Utilization Data [29].
Statistical analyses
Descriptive statistics were generated for each of the key variables. Bivariate analyses were performed for each of the clinical and pathologic variables. We then performed multivariable logistic regression including all independent variables in the model. We tested for interactions between treatment center and age, ethnicity/race, comorbidity, BMI, and education. All statistical tests were two-sided, and a p value less than 0.05 was considered statistically significant. All analyses were performed using SAS (Cary, NC).
All study procedures were approved by the University of California, Los Angeles Human Subjects Protection Committee, and the University of Michigan Institutional Review Board.
Results
The Figure shows the original sample size and the final sample after exclusions were applied. Of the original sample of 658 patients, 552 (85 %) received chemotherapy. Planned exclusions were applied and are shown in Fig. 1 as described in the “Patients and methods” section.
Fig. 1. Study sample flow diagram.
After examining the data, we imposed an additional exclusion criteria—patients who self-identified their race as “other” (n = 6) due to the small number. The final sample thus included eligible 397 patients. This includes 46 patients who received more than the standard number of cycles of a standard regimen (e.g., doxorubicin and cyclophosphamide for six rather than the standard four cycles). The final sample represented 72 % of those who received adjuvant chemotherapy.
Sample characteristics
The final sample included 122 non-Hispanic white women, 21 black women, 226 Hispanic women, and 28 Asian/Pacific Islander women. There were no Hispanic blacks. Sample characteristics are shown in Table 1. In this uniformly low-income sample, age at diagnosis, BMI, proportion of those who had completed high school, and patient self-efficacy in physician encounters were significantly different according to race and ethnicity as follows. The mean age at diagnosis among Hispanics was 47.8 years (SD 9.2), among blacks 53.8 years (SD 8.8), among non-Hispanic whites 51.4 years (SD 8.8), and among Asians/Pacific Islanders 50.5 years (SD 7.4) (p ≤ 0.001). The proportion or tumors that were hormone receptor-positive was 71.3 % among non-Hispanic whites, 62.4 % among Hispanics, 38.1 % among blacks, and 85.7 % among Asians (p = 0.002). The mean BMI among Hispanics was 30.1 (SD 5.8), among blacks 34.8 (SD 7.2), among non-Hispanic whites 30.4 (SD 7.8), and among Asian/Pacific Islanders 28.0 (SD 7.7) (p = 0.005). Mean patient self-efficacy scores in interactions with physicians as measured by the PEPPI (described above in “Measures” section) were 35.2 (SD 13.2) among Hispanics, 42.7 (SD 8.8) among blacks, 40.9 (SD 8.9) among non-Hispanic whites, and 35.8 (SD 12.1) among Asian/Pacific Islanders (p < 0.0001).
Table 1. Sample characteristics, N = 397.
| N | % | |
|---|---|---|
| Age group (years) | ||
| <40 | 59 | 14.9 |
| 40–49 | 138 | 34.8 |
| 50–59 | 146 | 36.8 |
| ≥60 | 54 | 13.6 |
| Number of comorbid conditions | ||
| None | 293 | 73.8 |
| 1 | 67 | 16.9 |
| 2 or more | 37 | 9.3 |
| Stage | ||
| I | 77 | 19.4 |
| II | 223 | 56.2 |
| III | 97 | 24.4 |
| Body mass index | ||
| Healthy weight (≥18.5 to <25) | 80 | 20.2 |
| Overweight (≥25 to <30) | 137 | 34.5 |
| Obese (≥30 to <35) | 95 | 23.9 |
| Severely obese (≥35) | 85 | 21.4 |
| Race/Ethnicity | ||
| Non-Hispanic white | 122 | 30.7 |
| Non-Hispanic black | 21 | 5.3 |
| Hispanic | 226 | 56.9 |
| Asian/Pacific islander | 28 | 7.1 |
| Years of education | ||
| Less than high school | 182 | 45.8 |
| High school graduate | 215 | 54.2 |
| Social support | ||
| Low | 95 | 23.9 |
| High | 302 | 76.1 |
| Site of treatment | ||
| Academic | 289 | 72.8 |
| Non-academic | 108 | 27.2 |
| Hormone receptor status | ||
| ER and/or PR positive | 260 | 65.5 |
| ER and PR negative | 137 | 34.5 |
|
| ||
| Mean | SD | |
|
| ||
| PEPPI (range 0–50) | 37.4 | 12.0 |
ER estrogen receptor, PR progesterone receptor, PEPPI patient efficacy in patient–provider interactions, SD standard deviation
There were no significant racial or ethnic differences in number of comorbid conditions, social support, stage of disease at diagnosis, or type of treatment site (academic vs. non-academic facility).
Bivariate analyses
Most of the patients (86 %) received chemotherapy dose ratios that were over 0.9. Chemotherapy first cycle dose reductions were associated with both clinical and non-clinical factors as shown in Table 2. The percentage of patients receiving reduced chemotherapy doses with the first cycle of chemotherapy was statistically significantly different according to race/ethnicity. A lower percentage (9.3 %) of Hispanic women were treated with reduced chemotherapy doses than non-Hispanic whites (20.5 %), blacks (33.3 %), and Asian/Pacific Islanders (10.7 %) (p < 0.001).
Table 2. Number and percentage of patients given reduced chemotherapy doses for initial cycle of therapy, N = 397.
| N | % | p value | |
|---|---|---|---|
| Age group | |||
| <40 | 6 | 10.2 | 0.270 |
| 40–49 | 15 | 10.9 | |
| 50–59 | 25 | 17.1 | |
| ≥60 | 10 | 18.5 | |
| Number of comorbid conditions | |||
| None | 31 | 10.6 | <0.001 |
| 1 | 12 | 17.9 | |
| 2 or more | 13 | 35.1 | |
| Tumor stage | |||
| I | 11 | 14.3 | 0.502 |
| II | 28 | 12.6 | |
| III | 17 | 17.5 | |
| Hormone receptor status | |||
| ER or PR positive | 33 | 12.7 | 0.265 |
| ER and PR negative | 23 | 16.8 | |
| Body mass index | |||
| Healthy weight (≥18.5 to <25) | 6 | 7.5 | <0.001 |
| Overweight (≥25 to <30) | 6 | 4.4 | |
| Obese (≥30 to <35) | 18 | 19.0 | |
| Severely obese (≥35) | 26 | 30.6 | |
| Race/Ethnicity | |||
| Non-Hispanic white | 25 | 20.5 | <0.001 |
| Non-Hispanic black | 7 | 33.3 | |
| Hispanic | 21 | 9.3 | |
| Asian/Pacific Islander | 3 | 10.7 | |
| Years of education | |||
| Less than high school | 18 | 9.9 | 0.026 |
| High school graduated | 38 | 17.7 | |
| Social support | |||
| Low | 19 | 20.0 | 0.058 |
| High | 37 | 12.3 | |
| Treatment Center | |||
| Non-academic | 48 | 16.6 | 0.019 |
| Academic | 8 | 7.4 | |
|
| |||
| Mean | SD | p value | |
|
| |||
| PEPPI | |||
| Chemotherapy ratio >0.9 | 37.2 | 12.2 | 0.894 |
| Chemotherapy ratio ≤0.9 | 37.4 | 12.0 | |
Bold values indicate statistically significant difference
ER estrogen receptor, PR progesterone receptor, PEPPI patient efficacy in patient–provider interactions, SD standard deviation
A higher number of comorbid conditions (p < 0.001), increasing BMI (p < 0.001), non-Hispanic white and black race/ethnicity (p < 0.001), fewer years of education (p = 0.026), and treatment at a non-academic treatment site (p = 0.019) were all associated with receipt of a first cycle regimen ratio less than 0.9. Age, stage, hormone receptor status, social support, and patient reported self-efficacy in patient–provider interactions were not associated with first cycle chemotherapy dose selection.
Multivariate analyses
In logistic regression (Table 3), only increasing BMI (p < 0.001) and type of treatment site (non-academic vs. academic, p = 0.037) were associated with a first cycle dose ratio under 0.9. We tested for interactions between treatment center and the following variables—age, ethnicity/race, comorbidity, BMI, and education, and no significant interactions were identified.
Table 3. Factors associate with initial dose reductions, logistic regression, N = 397.
| OR | 95 % CI | p value† | |
|---|---|---|---|
| Age | |||
| <60 years | Referent | 0.862 | |
| ≥60 years and older | 0.926 | (0.388, 2.111) | |
| Number of comorbid conditions | |||
| None | Referent | 0.088 | |
| 1 | 1.314 | (0.587, 2.943) | |
| 2 or more | 2.652 | (1.111, 6.327) | |
| Body mass index | |||
| Normal | Referent | <0.001 | |
| Overweight | 0.633 | (0.191, 2.103) | |
| Obese | 2.519 | (0.891, 7.123) | |
| Severely obese | 4.679 | (1.689, 12.961) | |
| Race/Ethnicity | |||
| Non-Hispanic white | Referent | 0.335 | |
| Black | 1.165 | (0.380, 3.573) | |
| Latina | 0.535 | (0.225, 1.166) | |
| Asian | 0.512 | (0.128, 2.242) | |
| Educational attainment | |||
| Less than high school | Referent | 0.233 | |
| More than high school | 1.608 | (0.737, 3.507) | |
| Tumor stage | |||
| I | Referent | 0.918 | |
| II | 0.871 | (0.376, 2.016) | |
| III | 0.991 | (0.388, 2.527) | |
| Hormone receptor status | |||
| ER and PR negative | Referent | 0.120 | |
| ER and/or PR positive | 0.592 | (0.305, 1.147) | |
| Social support | |||
| Low | Referent | 0.258 | |
| High | 0.672 | (0.338,1.337) | |
| Treatment site | |||
| Non-academic center | Referent | 0.030 | |
| Academic center | 0.382 | (0.160, 0.909) | |
| PEPPI | 0.987 | (0.960, 1.014) | 0.327 |
Bold values indicate statistically significant difference
ER estrogen receptor, PR progesterone receptor, PEPPI patient efficacy in patient–provider interactions, CI confidence intervals, OR odds ratio
p values refer to tests for significance of the variables in the adjusted model
Conclusions
In summary, in this uniformly low-income sample receiving care through the BCCTP Act, the majority of patients were treated with chemotherapy doses that were over 90 % of the expected chemotherapy dose. Only obesity status and type of facility were associated with first cycle adjuvant chemotherapy dose selection. No other clinical or social factors were associated with dose selection, and, contrary to our hypothesis, Hispanic ethnicity and self-reported patient efficacy in patient–provider interactions were not associated with chemotherapy dose.
The association between obesity status and first cycle dose selection has been reported in multiple clinical settings [12– 16] even in patients who are participating in clinical trials [10, 11]. Systematic dose reductions in the overweight and obese are most likely the result of lack of awareness of both the importance of using actual body weight when calculating BSA and the lack of excessive toxicity when full weight-based doses are used. The American Society of Clinical Oncology published guidelines in 2012 on chemotherapy dosing in obese patients receiving adjuvant or curative chemotherapy [30]; the patients in our sample were treated well before the publication of the guidelines. It is important to note that the majority of obese patients, approximately 70 % in this sample, were treated with chemotherapy doses over 90 % of what would have been expected had actual body weight been used when calculating BSA.
The association between type of treatment site and the specific process of chemotherapy dose selection is intriguing. Findings in the literature are mixed regarding the association between type of treatment facility and quality of care in women with breast cancer. One study of patients in the Florida Cancer Data System demonstrated that patients treated in non-teaching hospitals were more likely to receive standard therapy than those treated in teaching hospitals [31]. When restricted to just the use of adjuvant chemotherapy for regional disease, however, teaching hospital status was associated with higher use of chemotherapy, particularly among Hispanics [32]. Another study found that, among women over age 65, chemotherapy was given more often in private practice settings than in other settings [33]. Another study identified hospital type as a factor associated with quality of care [7].
In an academic setting, oncologists generally have multiple other oncologists who share responsibility for a group of patients. They may also have other resources available such as mid-level providers, patient navigators, and interpreters to support the oncologist and patient between treatment cycles should toxicity arise. Having a large group of colleagues and the other resources mentioned may allay the concerns of an individual physician about potential toxicity and its management between treatment cycles.
With respect to chemotherapy dose selection among Hispanics, we found no evidence of systematic underdosing in this sample of patients. Furthermore, while patient self-efficacy was lower among the Hispanics in our sample, patient self-efficacy was not associated with chemotherapy dose selection as described above.
The findings that comorbidity, age, and stage were not associated with first cycle chemotherapy dose selection are consistent with previous research among patients with breast cancer receiving adjuvant therapy [13, 14]. These factors are more likely to determine the receipt of chemotherapy rather than the dose selected once chemotherapy is decided upon.
There are several limitations of this study that warrant discussion. The first is that our sample was drawn from a single state. Wide variation in chemotherapy dose selection has been demonstrated according to geographic region in the United States [16]. The findings of our study thus cannot be generalized to other regions. In addition, the patients enrolled in the BCCTP met specific criteria in order to be in the program and are not representative of all low-income patients. The low number of black and Asian women does not allow us to draw any conclusions about the quality of chemotherapy dose selection in these minority groups in our sample. In addition, Hispanics in the United States are heterogeneous, and while most, including in those in California, are of Mexican heritage [34], caution is needed when discussing quality of care across any population as if that population was homogenous. Finally, our measure of social support, which inquired about how often patients were accompanied to physician visits, does not specifically ask whether the patient was accompanied to the initial medical oncology consultation, which is most likely the visit at which dose selection would be determined. We thus have a less precise measure of perceived social support than would have been ideal for the construct we were aiming to capture.
Despite these limitations, this study includes detailed and complete information on chemotherapy dosing, information exceedingly difficult to obtain in a multi-site sample, in low-income women. The inclusion of both patient self-reported data and data from the medical record in a sample of Hispanic women yields a rich dataset that allows us to investigate patterns and correlates of chemotherapy dose selection in a sample hitherto unstudied.
In conclusion, in this sample of low-income women receiving care through a federal- and state-funded cancer treatment program, the doses of adjuvant chemotherapy did not differ according to ethnicity or patient self-efficacy in communicating with medical providers. Only obesity status and type of treatment site were independently associated with chemotherapy dose selection in the adjuvant setting. Our findings, although not conclusive with respect to racial, ethnic, or social disparities in chemotherapy dosing in the United States population at large, indicate that such disparities need not exist. Access to adequate health care allows the vast majority of low-income women with breast cancer to receive high-quality breast cancer chemotherapy. In the face of evidence that access to physician care is declining among Hispanics [35], our study suggests that access to high-quality specialty care must remain a priority to preserve what is an attainable goal among potentially vulnerable minority groups.
Acknowledgments
This study was supported by Grant No. TURSG-02-081 from the American Cancer Society, Grant No. 7PB-0070 from the California Breast Cancer Research Program, Grant No. R01CA119197-0181 from the National Cancer Institute, and by a supplement to R01CA119197. Dr. Maly was further supported by Grant No. 1R01CA140481-01A1 from the National Cancer Institute.
Footnotes
A portion of this work was presented during the 2012 meeting of the American Society of Clinical Oncology Quality Care Symposium, San Diego, November 30–December 1, 2012.
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standards The study complied with the ethical standards set forth for human subjects by the University of California, Los Angeles Human Subjects Protection Committee, and the University of Michigan Institutional Review Board.
Contributor Information
Jennifer J. Griggs, Email: jengrigg@med.umich.edu, jengrigg@umich.edu, University of Michigan Ann Arbor, Michigan 2800 Plymouth Road, Building 16, Ann Arbor, MI 48109, USA.
Yihang Liu, Department of Family Medicine, David Geffen School of Medicine, UCLA, 10880 Wilshire Blvd, Suite 1800, Los Angeles, CA 90095-7087, USA.
Melony E. Sorbero, RAND Corporation, 4570 Fifth Avenue, Suite 600, Pittsburgh, PA 15213, USA
Christina H. Jagielski, University of Alabama at Birmingham, 1720 2nd Avenue South, Birmingham, AL 35294, USA
Rose C. Maly, Department of Family Medicine, David Geffen School of Medicine, UCLA, 10880 Wilshire Blvd, Suite 1800, Los Angeles, CA 90095-7087, USA
References
- 1.Banegas MP, Leng M, Graubard BI, Morales LS. The risk of developing invasive breast cancer in Hispanic women: a look across Hispanic subgroups. Cancer. 2013;119:1373–1380. doi: 10.1002/cncr.27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 3.Campbell RT, Li X, Dolecek TA, Barrett RE, Weaver KE, Warnecke RB. Economic, racial and ethnic disparities in breast cancer in the US: towards a more comprehensive model. Health Place. 2009;15:855–864. doi: 10.1016/j.healthplace.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, Blake-Cerda M, Arce C, Motola-Kuba D, Villarreal-Garza C, Gonzalez-Angulo AM, Bargallo E, Aguilar JL, Mohar A, Arrieta O. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;8:3658–3669. doi: 10.1002/cncr.25961. [DOI] [PubMed] [Google Scholar]
- 6.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, Remington PL, Newcomb PA. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–1551. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed on 29 jan 2014]; http://www.dhcs.ca.gov/services/medi-cal/pages/BCCTP.aspx.
- 9.Malin JL, Diamant AL, Leake B, Liu Y, Thind A, Kahn KL, Schneider EC, Epstein AM, Maly RC. Quality of care for breast cancer for uninsured women in California under the Breast and Cervical Cancer Prevention Treatment Act. J Clin Oncol. 2010;28:3479–3484. doi: 10.1200/JCO.2009.27.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colleoni M, Li S, Gelber RD, Price KN, Coates AS, Castiglione-Gertsch M, Goldhirsch A. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366:1108–1110. doi: 10.1016/S0140-6736(05)67110-3. [DOI] [PubMed] [Google Scholar]
- 11.Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, Schilsky RL. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from Cancer and Leukemia Group B Study 8541. J Clin Oncol. 1996;14:3000–3008. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 12.Madarnas Y, Sawka CA, Franssen E, Bjarnason GA. Are medical oncologists biased in their treatment of the large woman with breast cancer? Breast Cancer Res Treat. 2001;66:123–133. doi: 10.1023/a:1010635328299. [DOI] [PubMed] [Google Scholar]
- 13.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 14.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 15.Shayne M, Crawford J, Dale DC, Culakova E, Lyman GH. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100:255–262. doi: 10.1007/s10549-006-9254-4. [DOI] [PubMed] [Google Scholar]
- 16.Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25:277–284. doi: 10.1200/JCO.2006.08.3063. [DOI] [PubMed] [Google Scholar]
- 17.Doty MM. Hispanic patients' double burden: lack of health insurance and limited english. The commonwealth fund. 2003 www.cmwf.org.
- 18.Morales LS, Cunningham WE, Brown JA, Liu H, Hays RD. Are Latinos less satisfied with communication by health care providers? J Gen Intern Med. 1999;14:409–417. doi: 10.1046/j.1525-1497.1999.06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vargas Bustamante A, Chen J. Physicians cite hurdles ranging from lack of coverage to poor communication in providing high-quality care to Latinos. Health Aff (Millwood) 2011;30:1921–1929. doi: 10.1377/hlthaff.2011.0344. [DOI] [PubMed] [Google Scholar]
- 20.Chen JY, Diamant AL, Thind A, Maly RC. Determinants of breast cancer knowledge among newly diagnosed, low-income, medically underserved women with breast cancer. Cancer. 2008;112:1153–1161. doi: 10.1002/cncr.23262. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed on 29 jan 2014]; http://www.nccn.org/clinical.asp.
- 22.van der Sijs H, Guchelaar HJ. Formulas for calculating body surface area. Ann Pharmacother. 2002;36:345–346. doi: 10.1345/aph.1A134. [DOI] [PubMed] [Google Scholar]
- 23.Ottevanger PB, Verhagen CA, Beex LV. Quality of adjuvant chemotherapy in primary breast cancer in a non-trial setting. A comprehensive cancer centre study. The Breast Cancer Group of the Dutch Comprehensive Cancer Centre East (IKO) Eur J Cancer. 1999;35:386–391. doi: 10.1016/s0959-8049(98)00376-1. [DOI] [PubMed] [Google Scholar]
- 24.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Maly RC, Liu Y, Leake B, Thind A, Diamant AL. Treatment-related symptoms among underserved women with breast cancer: the impact of physician-patient communication. Breast Cancer Res Treat. 2009;119:707–716. doi: 10.1007/s10549-009-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer. 2006;106:957–965. doi: 10.1002/cncr.21680. [DOI] [PubMed] [Google Scholar]
- 27.Maliski SL, Kwan L, Krupski T, Fink A, Orecklin JR, Litwin MS. Confidence in the ability to communicate with physicians among low-income patients with prostate cancer. Urology. 2004;64:329–334. doi: 10.1016/j.urology.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed on 29 jan 2014]; http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 29. [Accessed on 29 jan 2014]; http://www.oshpd.ca.gov/hid/Products/Hospitals/Utilization/Hospital_Utilization.html.
- 30.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, Shayne M, Sparreboom A, Sucheston LE, Lyman GH. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 31.Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006;95:89–95. doi: 10.1007/s10549-005-9050-6. [DOI] [PubMed] [Google Scholar]
- 32.Voti L, Richardson LC, Reis IM, Fleming LE, Mackinnon J, Coebergh JW. Treatment of local breast carcinoma in Florida: the role of the distance to radiation therapy facilities. Cancer. 2006;106:201–207. doi: 10.1002/cncr.21557. [DOI] [PubMed] [Google Scholar]
- 33.Hershman DL, Buono D, McBride RB, Tsai WY, Neugut AI. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115:3848–3857. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. [Accessed on 29 jan 2014]; http://www.pewhispanic.org.
- 35.Mahmoudi E, Jensen GA. Diverging racial and ethnic disparities in access to physician care: comparing 2000 and 2007. Med Care. 2012;50:327–334. doi: 10.1097/MLR.0b013e318245a111. [DOI] [PubMed] [Google Scholar]