Abstract
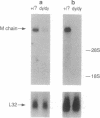
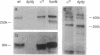
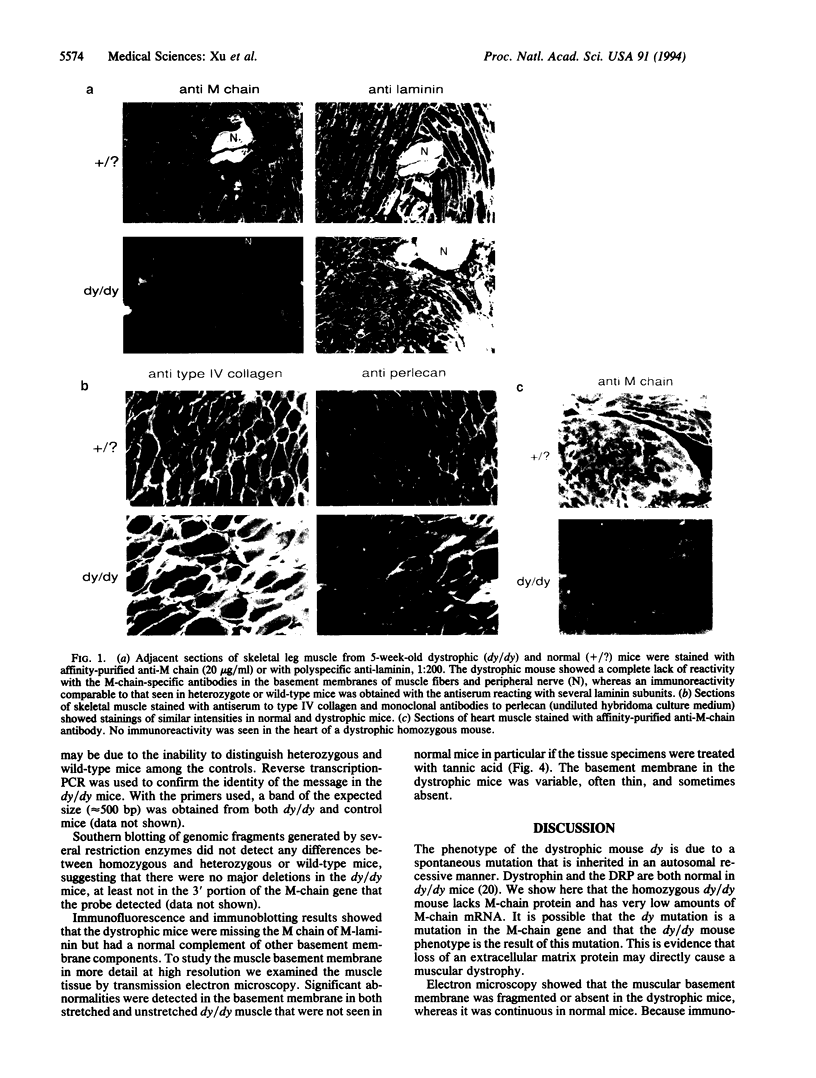
M-laminin is a major member of the laminin family of basement membrane proteins. It is prominently expressed in striated muscle and peripheral nerve. M-laminin is deficient in patients with the autosomal recessive Fukuyama congenital muscular dystrophy but is normal in patients with the sex-linked Duchenne and Becker muscular dystrophies. We have examined M-laminin expression in mice with autosomal recessive muscular dystrophy caused by the mutation dy. The heavy chain of M-laminin was undetectable in skeletal muscle, heart muscle, and peripheral nerve by immunofluorescence and immunoblotting in homozygous dystrophic dy/dy mice but was normal in heterozygous and wild-type nondystrophic mice. Immunofluorescence confirmed the presence of other major basement membrane proteins in the dystrophic mice. Very low levels of M-laminin heavy chain mRNA were detected by Northern blotting of muscle and heart tissue from dy/dy mice, suggesting that M-laminin heavy-chain mRNA may be produced at very low levels or is unstable. Information about the chromosomal localization of the M heavy-chain in human and mouse suggests that a mutation in the M-chain gene causes the muscular dystrophy in dy/dy mice. The dy mouse may provide a model for autosomal muscular dystrophies in humans and facilitate studies of functions of M-laminin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck K., Hunter I., Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990 Feb 1;4(2):148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Buckle V. J., Guenet J. L., Simon-Chazottes D., Love D. R., Davies K. E. Localisation of a dystrophin-related autosomal gene to 6q24 in man, and to mouse chromosome 10 in the region of the dystrophia muscularis (dy) locus. Hum Genet. 1990 Aug;85(3):324–326. doi: 10.1007/BF00206755. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Wadsworth S., Coligan J. E. Expression of merosin in the thymus and its interaction with thymocytes. J Immunol. 1993 Aug 15;151(4):1789–1801. [PubMed] [Google Scholar]
- Ehrig K., Leivo I., Argraves W. S., Ruoslahti E., Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990 May;87(9):3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Laminins and other strange proteins. Biochemistry. 1992 Nov 10;31(44):10643–10651. doi: 10.1021/bi00159a001. [DOI] [PubMed] [Google Scholar]
- Engvall E., Bell M. L., Carlsson R. N., Miller E. J., Ruoslahti E. Nonhelical, fibronectin-binding basement-membrane collagen from endodermal cell culture. Cell. 1982 Jun;29(2):475–482. doi: 10.1016/0092-8674(82)90164-7. [DOI] [PubMed] [Google Scholar]
- Engvall E., Earwicker D., Day A., Muir D., Manthorpe M., Paulsson M. Merosin promotes cell attachment and neurite outgrowth and is a component of the neurite-promoting factor of RN22 schwannoma cells. Exp Cell Res. 1992 Jan;198(1):115–123. doi: 10.1016/0014-4827(92)90156-3. [DOI] [PubMed] [Google Scholar]
- Engvall E., Earwicker D., Haaparanta T., Ruoslahti E., Sanes J. R. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990 Sep;1(10):731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Krusius T., Wewer U., Ruoslahti E. Laminin from rat yolk sac tumor: isolation, partial characterization, and comparison with mouse laminin. Arch Biochem Biophys. 1983 Apr 15;222(2):649–656. doi: 10.1016/0003-9861(83)90562-3. [DOI] [PubMed] [Google Scholar]
- Engvall E. Laminin variants: why, where and when? Kidney Int. 1993 Jan;43(1):2–6. doi: 10.1038/ki.1993.2. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993 Aug;122(4):809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y., Osawa M., Suzuki H. Congenital progressive muscular dystrophy of the Fukuyama type - clinical, genetic and pathological considerations. Brain Dev. 1981;3(1):1–29. doi: 10.1016/s0387-7604(81)80002-2. [DOI] [PubMed] [Google Scholar]
- Hagg T., Muir D., Engvall E., Varon S., Manthorpe M. Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 1989 Dec;3(6):721–732. doi: 10.1016/0896-6273(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Hayashi Y. K., Engvall E., Arikawa-Hirasawa E., Goto K., Koga R., Nonaka I., Sugita H., Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci. 1993 Oct;119(1):53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hunter D. D., Shah V., Merlie J. P., Sanes J. R. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989 Mar 16;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. R., Morris G. E., Ellis J. M., Fairbrother U., Marsden R. F., Bloomfield J. F., Edwards Y. H., Slater C. P., Parry D. J., Davies K. E. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3243–3247. doi: 10.1073/pnas.88.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R. E., Jaros E., Cullen M. J., Bradley W. G. Genetically determined defect of Schwann cell basement membrane in dystrophic mouse. Nature. 1975 Sep 25;257(5524):319–321. doi: 10.1038/257319a0. [DOI] [PubMed] [Google Scholar]
- Marinkovich M. P., Lunstrum G. P., Keene D. R., Burgeson R. E. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992 Nov;119(3):695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Collin H., Azibi K., Chaouch M., Kaplan J. C., Fardeau M., Campbell K. P. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992 Sep 24;359(6393):320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Matsumura K., Kahl S. D., Leveille C. J., Campbell K. P. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991 Sep;7(3):499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Saladin K., Engvall E. Structure of laminin variants. The 300-kDa chains of murine and bovine heart laminin are related to the human placenta merosin heavy chain and replace the a chain in some laminin variants. J Biol Chem. 1991 Sep 15;266(26):17545–17551. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Segawa M., Nomura Y., Nonaka I., Masuda K., Ishihara T., Sakai M., Tomita I., Origuchi Y., Suzuki M [corrected to Sakai M. ]. Localization of a gene for Fukuyama type congenital muscular dystrophy to chromosome 9q31-33. Nat Genet. 1993 Nov;5(3):283–286. doi: 10.1038/ng1193-283. [DOI] [PubMed] [Google Scholar]
- Verrando P., Blanchet-Bardon C., Pisani A., Thomas L., Cambazard F., Eady R. A., Schofield O., Ortonne J. P. Monoclonal antibody GB3 defines a widespread defect of several basement membranes and a keratinocyte dysfunction in patients with lethal junctional epidermolysis bullosa. Lab Invest. 1991 Jan;64(1):85–92. [PubMed] [Google Scholar]
- Vuolteenaho R., Nissinen M., Sainio K., Byers M., Eddy R., Hirvonen H., Shows T. B., Sariola H., Engvall E., Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994 Feb;124(3):381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Engvall E., Paulsson M., Yamada Y., Albrechtsen R. Laminin A, B1, B2, S and M subunits in the postnatal rat liver development and after partial hepatectomy. Lab Invest. 1992 Mar;66(3):378–389. [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]