Abstract
Microdialysis is a powerful sampling technique that enables monitoring of dynamic processes in vitro and in vivo. The combination of microdialysis with chromatographic or electrophoretic methods yields along with selective detection methods yields a “separation-based sensor” capable of monitoring multiple analytes in near real time. Analysis of microdialysis samples requires techniques that are fast (<1 min), have low volume requirements (nL–pL), and, ideally, can be employed on-line. Microchip electrophoresis fulfills these requirements and also permits the possibility of integrating sample preparation and manipulation with detection strategies directly on-chip. Microdialysis coupled to microchip electrophoresis has been employed for monitoring biological events in vivo and in vitro. This review discusses technical considerations for coupling microdialysis sampling and microchip electrophoresis, including various interface designs, and current applications in the field.
Keywords: Microdialysis, Electrophoresis, Microfluidics, Sensor, in vivo monitoring, lab-on-a-chip, electrochemical detection
1. Introduction
Continuous monitoring of biomolecules in living systems is important for the understanding of neurological disorders, evaluation of drug delivery systems, determination of pharmacological responses to drugs and environmental factors, and bioreactor monitoring. Sensors provide a popular approach for monitoring biomolecules in vivo and in vitro, and commercially available sensors have been developed for many bioactive analytes [1-3]. These include sensors for glucose, nitric oxide, glutamate, and dopamine [4]. However, a major drawback of these sensors is that they are generally limited to detection of a single analyte. It is also not possible to monitor a group of structurally related compounds, such as a drug and its metabolites, in a single assay.
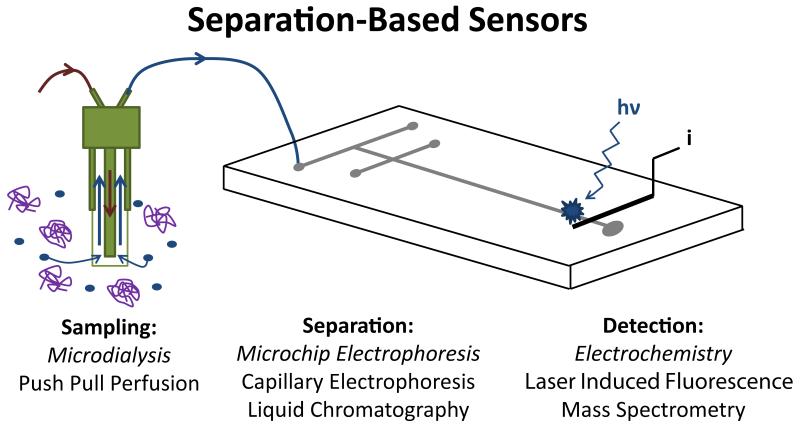
Microdialysis sampling was first introduced by Ungerstedt in 1974 as a method for continuous sampling of the extracellular fluid of the brain [5-9]. This sampling technique has enjoyed wide applicability and has been extensively employed in both research laboratories and clinics [10-17]. Microdialysis sampling is accomplished based on diffusion of molecules across a size-selective membrane. Therefore, microdialysis acts as a “generic” sampling system, and the dialysate includes all the small molecules present in the extracellular fluid of the tissue that is being interrogated. The resulting dialysate is then collected and can be analyzed by a variety of techniques optimized for the compounds of interest. A major advantage of microdialysis sampling is that it makes it possible to monitor multiple analytes simultaneously (within a single analysis) as long as these analytes can be detected individually. Separation-based analytical systems, in particular, can provide the ability to monitor multiple analytes from a single microdialysis sample. These “separation-based sensors” (Fig. 1) have been used to continuously monitor multiple neurotransmitters in the brain, drug metabolism, and biomarkers of disease.
Fig. 1.
Separation-based sensor. Key components are (1) microdialysis probe (2) separation method and (3) detection approach.
Analysis of microdialysis samples can be performed either off-line or on-line. The most common method used for off-line separation-based analysis is liquid chromatography [15,18]. However, over the past twenty years, capillary electrophoresis has become increasingly popular [15,18-20]. An advantage of capillary electrophoresis for off-line analysis is that, because capillary electrophoresis only requires nanoliter amounts of sample, a single 1–10 μL microdialysis sample can be analyzed for several different classes of analytes by multiple capillary electrophoresis methods [21-23]. However, a major drawback of off-line analysis is that fairly large volume samples (1–10 μL) need to be collected to be compatible with the instrumentation and avoid evaporation during sample handling.
In order to avoid the issues with the manipulation and analysis of sub-microliter samples and provide a method for near real-time continuous monitoring, on-line separation-based systems have been developed (Fig. 1). Microdialysis has been coupled to liquid chromatography [14,15,18,24-27] and capillary liquid chromatography [28] for continuous monitoring of drug metabolism and neuropeptides. In 1994, microdialysis was first coupled to capillary electrophoresis and used to monitor the metabolism of an anticancer drug [29]. Later, it was coupled with on-line derivatization to monitor the release of aspartate and glutamate with one minute temporal resolution [30].
Lab-on-a-chip devices were first introduced in the early 1990s as a way to integrate multiple chemical processes into a single device [31]. Microchip electrophoresis was first described by Harrison and Manz in 1992 [32-35], and the first high speed separations were published by Ramsey’s group in 1994 [36,37]. The microchip format has all the advantages of capillary electrophoresis for on-line analysis of microdialysis samples, including efficient separations and ease of fluid handling, as well as the unique ability to integrate components such as mixers and detection directly on-chip. The first report of microdialysis coupled to microchip electrophoresis was demonstrated for monitoring an enzyme reaction in 2004 [38]. Since that time, there have been many papers describing new approaches and applications of this technique for monitoring biomolecules in vivo and in vitro. This review will describe the different approaches that have been developed for coupling microdialysis to microchip electrophoresis, as well as applications of this approach for on-line monitoring.
2. Microdialysis sampling
The key system components required to perform microdialysis (MD) sampling include connecting tubing, the sampling probe, and a perfusion pump. The probe consists of a semipermeable membrane that is attached to inlet and outlet tubing. In the case of animal studies, the probe is surgically placed into the tissue or organ of interest and perfusate is pumped through the tubing and into the probe. In most cases, the composition of the perfusate is as similar as possible to that of the extracellular fluid in the area of interest so as not to disrupt the biological system being interrogated. Compounds outside the probe diffuse across the semipermeable membrane based on their concentration gradient and are pumped to a fraction collector or on-line analysis system (Fig. 2). There are many membrane materials available for the fabrication of microdialysis probes, including polyacrylonitrile (PAN), polyarylethersulfone (PAES), cuprafan (CUP), and polyethersulfone (PES) [39]. These materials differ in charge and hydrophobicity and, therefore, impart some selectivity in the sampling process. The probes are also manufactured with a specific molecular weight cut-off (MWCO), which allows only molecules smaller than the cut-off to diffuse across the membrane. Commercially available probes have molecular weight cutoffs from 6–100 kDa (Table 1).
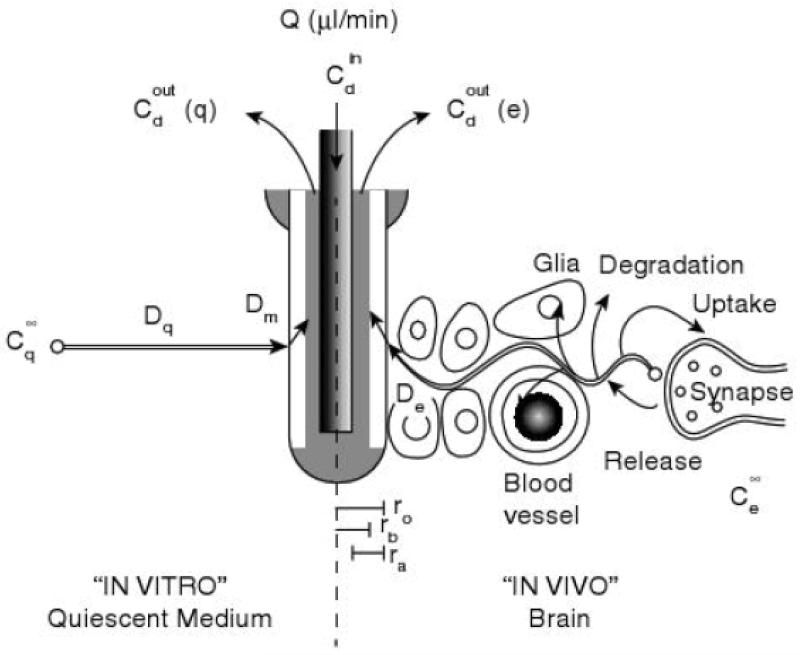
Fig 2.
Microdialysis sampling. Microdialysis sampling considerations in vitro (left) and in vivo (right). Reprinted with permission from Kehr [51] Copyright 1993 Elsevier.
Table 1.
Commercially available microdialysis probes
| Probe Type | Selected areas sampled | Membrane (MWCO) |
|---|---|---|
| Linear | in vitro, homogenous tissue | CUP (6 kDa), PES (55 kDa), PAN (30 kDa), |
| Ridged cannula | Brain | PAES (20 kDa), PES (100 kDa), CUP (6 kDa), PAN (30 kDa), Cellulosic (38 kDa) |
| Flexible cannula | Vasculature, soft tissue | PAES (20 kDa), PES (100 kDa), PAN (30 kDa) |
| Shunt | Bile | PAN (30 kDa) |
Microdialysis sampling is an ideal “generic” sampling system for on-line separation-based sensors. Because only small molecules pass through the dialysis membrane, it is not necessary to remove proteins or other macromolecules, and the sample can be directly injected into the analysis system. In addition, because analytes migrate into and/or out of the probe via diffusion, there is no net fluid loss, making this technique amenable to long-term in vivo monitoring. Additionally, it is possible to monitor drug metabolism in specific tissues by adding the drug to the perfusate. The drug will then diffuse into the tissue based on its concentration gradient and metabolites will diffuse in the opposite direction into the probe. This sampling procedure creates a continual flow of sample, in which the analyte concentrations change over time corresponding to concentration changes occurring in vivo. Multiple compounds are sampled simultaneously, increasing the ability to understand complex biological processes.
2.1 Theory and considerations
2.1.1 Probe designs
It is important to choose the appropriate probe design for the tissue or sample that is being interrogated. Several different designs are available, and the choice of probe is dependent on the specific application. Key parameters include the heterogeneity and flexibility of the sample or tissue as well as the recovery of the analyte of interest across the probe membrane. The most common probe designs are discussed below. More detailed discussions of the different probe designs are available [18,40], and some of the commercially available options are highlighted in Table 1.
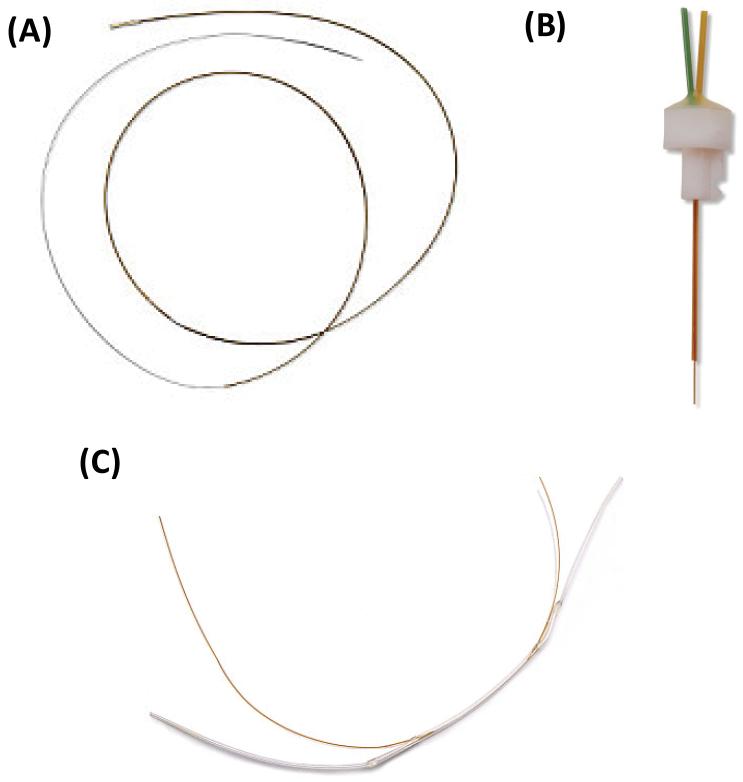
Linear probes are most often used for the interrogation of homogenous tissues, such as skin [41,42], muscle [43], and liver [44], as well as bioreactor monitoring [45,46]. The linear probe consists of a dialysis membrane suspended between two pieces of capillary tubing (Fig. 3A). The membrane is typically up to 10 mm in length, although longer membranes (1–5 cm) are commercially available. This probe is threaded through the tissue of interest or can be placed directly into a bioreactor. These probes are flexible, enabling their use in the peripheral tissue of awake, freely-moving animals. In addition, because the membrane length can be relatively long compared to the other types of probes, recoveries of analyte using this probe are generally higher than in other designs. Shunt probes are a modified version of the linear probe for the sampling of flowing streams that are high in salt concentration (Fig. 3C). The first application of this probe design was reported by Scott and Lunte for sampling bile in rats without altering its normal flow [47]. These probes have also been used extensively for desalting sample streams prior to mass spectrometric analysis [48].
Fig. 3.
Microdialysis probe types. (A) Linear probe design for sampling in homogenous tissues. (B) Rigid cannula probe design for sampling within the brain. (C) Shunt probe design for sampling from bile fluid. Images reproduced with permission from BASi Bioanalytical Systems, Inc. Further reproduction prohibited without permission. Copyright 2014.
Cannula-type probes typically provide better spatial resolution than linear probes but are much more rigid (Fig. 3B). The probe body is generally made of stainless steel, and the probe membrane has a diameter of 220–500 μm and a length of 1–4 mm. These types of probes are used extensively to sample brain tissue due to their high degree of heterogeneity [5,7]. A guide cannula can be used to immobilize the probe on the skull of the experimental animal to ensure that it stays in place throughout the experiment and allow for easy probe removal after experimentation. The MetaQuant probe, a specific type of rigid cannula microdialysis probe, provides two separate flow streams, one for ultra-low flow rate sampling and another make-up flow to increase total collected volume [49]. Due to its rigid nature, this type of probe is not generally used for sampling soft tissue because it can cause tissue damage. For soft tissue and blood sampling, a flexible cannula-type microdialysis probe has been described [50]. This probe is constructed from fused silica or polyimide tubing and can be used to perform intravenous sampling in awake, freely-moving animals [50].
2.1.2 Recovery and calibrations
Because microdialysis sampling is based on diffusion of an analyte to and across a semipermeable membrane, there are many factors that can affect extraction efficiency and analyte recovery. Extraction efficiency of a microdialysis system can be defined by the following equation, where Cperfusate, Cdialysate, and Csample is the concentration of analyte in the perfusate, collected dialysate, and sample (or tissue), respectively.
For many experiments, the perfusate contains no analyte (Cperfusate = 0), therefore, the extraction efficiency equation simplifies to the recovery expression below:
There are many experimental variables that affect the extraction efficiency. These include temperature (of the perfusate and tissue), type of tissue and tissue perfusion, metabolism and degradation of the analyte, pH, probe membrane composition and molecular weight cut-off, flow rate, and physical and chemical characteristics of the analyte (Fig. 2) [51, 52, 53]. In particular, flow rate has a very large impact on extraction efficiency. At high flow rates, perfusate is constantly being pushed through the microdialysis probe, giving analytes little time to diffuse across the probe and reach equilibrium; therefore, relative recovery is low under these conditions. In contrast, very low flow rates give much higher relative recoveries, with flow rates of 0.1 μL/min approaching 100% recovery [54]. These extremely low flow rates can be problematic, however, due to the extremely low sample volumes that are generated and the long time necessary to generate a sample with enough volume to be analyzed with traditional analytical methods. For example, at 0.1 μL/min it would take 100 minutes to acquire a 10 μL sample. Theoretically, this factor could be exploited when employing microchip electrophoresis, as very low volumes are required (nL–pL); however, most researchers performing microdialysis coupled to microchip electrophoresis currently use flow rates of ~ 1.0 μL/min.
Calibration of microdialysis probes for each experiment is important for quantitation because recovery is highly dependent on a number of factors, as discussed above. There are four main methods for microdialysis probe calibration: 1) determining recovery in vitro and assuming that it is the same in vivo, 2) delivering an internal standard in the perfusate and accounting for its loss [55-57], 3) using the no-net-flux and dynamic no-net-flux methods [58-61], and 4) employing ultra-low flow rates (~0.1 μL/min) [54] or the MetaQuant probe [49,62] and assuming 100% recovery. For addition information on these methods, the reader is directed to excellent reviews on the topic [52,63].
2.1.3 Spatial resolution
Temporal and spatial resolution are two important considerations when developing a separation-based sensor using microdialysis sampling. The significance of these two parameters is dependent on the tissue or organ that is being interrogated and the biological process under study.
Spatial resolution, or the region of tissue that is addressed by the probe, is affected by the length and diameter of the microdialysis probe membrane. For homogenous tissues, relatively large probe membranes are used since they permit higher recoveries and high spatial resolution is not necessary. However, when sampling heterogeneous tissues, such as the brain, spatial resolution is extremely important. While typical commercial brain cannula probes are 220–500 μm in diameter, smaller probes for both push-pull perfusion [64-66] and microdialysis [67] are currently being developed. These probes produce less tissue damage and provide better spatial resolution than conventional push-pull perfusion and microdialysis probes. Microfabricated microdialysis probes that contain nanoporous membranes produced in silicon have recently been reported by Kennedy’s group [67]. These new probes will make it possible to take advantage of the positive attributes of microdialysis sampling (exclusion of larger molecules, no net fluid loss, etc.) while significantly improving the spatial resolution.
2.1.4 Temporal resolution
An important consideration when designing an on-line microdialysis-microchip electrophoresis system is determining the optimal temporal resolution or the frequency at which the data are collected and analyzed for the application of interest [68]. For example, to monitor in vivo neurotransmitter release, a temporal resolution of seconds to milliseconds is often desirable. However, for other applications such as drug metabolism, environmental monitoring, and bioreactor sampling, a temporal resolution of several minutes to hours is adequate. Table 2 provides additional applications and the temporal resolution that they require. For on-line applications of microdialysis sampling with a separation method, temporal resolution is dependent on three interrelated parameters. These are 1) the detection limits of the analytical method for the analyte of interest, 2) the time required for analysis, and 3) the zone dispersion that occurs within the probe and connecting tubing.
Table 2.
Temporal resolution necessary for various applications
| Application/ compound | Temporal resolution |
|---|---|
| Neurotransmitters | Milliseconds to seconds |
| Drug transport and metabolism | Minutes to hours |
| Energy biomarkers (glucose, lactate, etc.) | Minutes to hours |
| Peptides | Minutes |
| Bioreactor monitoring | Minutes to hours |
| Reactive oxygen and nitrogen species | Minutes |
| Antioxidants (glutathione, ascorbic acid, etc.) | Minutes to hours |
| Environmental monitoring | Hours to days |
The detection limits, or mass sensitivity, of the analytical method is an important parameter for defining temporal resolution in both on-line and off-line systems [69,70]. Since analyte recovery through the microdialysis probe is usually much less than 100%, the detection limits of the method must be significantly lower than the predicted extracellular concentration of the analyte of interest. The mass of analyte that is collected through the probe, as well as the concentration of analyte in the dialysate, is dependent on the flow rate used for sampling as well as the membrane type and tissue being sampled (Table 3). At high flow rates, there is high absolute recovery of analyte, but the sample concentrations are low. The volume collected per unit time is also higher in this case. On the other hand, at very low flow rates, extraction efficiency can approach 100% but the sample volume per unit time is much smaller, necessitating a low sample volume analytical method such as capillary electrophoresis or microchip electrophoresis.
Table 3.
Factors affecting extraction efficiency, temporal resolution, and lag time in MD-ME
| Extraction efficiency | Temporal Resolution | Lag Time |
|---|---|---|
| Diffusion coefficient in probe membrane | Limits of detection | Dead volume of probe |
| Diffusion coefficient in tissue (in vivo) | Sample volume requirements | Flow rate |
| Flow rate | Diffusion within probe and tubing | Volume of tubing to sample collection/analysis system |
| Tissue damage/scar formation | Analysis time (on-line) | |
| Stirred or unstirred system (in vitro) | Sample preparation steps, e.g., derivatization (on-line) |
|
| Perfusion of tissue (in vivo) | ||
| Metabolism or receptor binding | ||
| Probe membrane area |
The combination of analyte recovery and detection limits of the analytical method defines the smallest volume that must be collected for analysis and, hence, the temporal resolution. However, in cases where the concentration of an analyte is significantly higher than the detection limits of the method, very small sample volumes can be analyzed and the temporal resolution is then dependent on the analysis time for on-line systems. Examples include the determination of amino acids, such as glutamate and aspartate, in brain dialysates. These compounds are present at micromolar concentrations in the extracellular fluid of the brain while their fluorescent derivatives can be detected at low nanomolar to picomolar concentrations. Therefore, micro- to nanoliter volumes of dialysate can be employed for analysis since there is more than enough sensitivity to detect the analytes of interest. Temporal resolution for these two analytes using on-line systems has been reported as high as 12 seconds for capillary electrophoresis [71] and 35 seconds for microchip electrophoresis [72]. In contrast, neuropeptides are present at low picomolar concentrations in the extracelluar fluid of the brain. Therefore, longer sampling times are required to obtain enough mass to detect them [73].
In cases where the detection limits of the analytical method are sufficient and very fast (subminute) separations can be achieved, Taylor dispersion [74] of the analyte zone in the dead volume of the probe and associated tubing can be the defining parameter for temporal resolution [68]. Lada et al. demonstrated that the limiting factor for the response time of a microdialysis probe with the minimal amount of connecting tubing was 16 seconds at a flow rate of 1 μL/min. This increased to 85 seconds for a flow rate of 0.2 μL/min [11,71]. An additional consideration is that zone dispersion is a bigger problem when working with freely-moving rather than anesthesized animals, since additional tubing must be employed to allow for movement in the awake animal system. To mitigate zone diffusion within the connecting tubing, segmented flow has been employed [75]. In this approach, the perfusate flow stream is broken into nanoliter droplets that are separated by oil, and diffusion is restricted to the droplet volume (section 5.3).
An additional parameter that must be considered for on-line systems is the delay between the sampling step and the analytical readout. This “lag time” depends on the length and inner diameter of the connecting tubing between the probe and the analytical system as well as the flow rate used for microdialysis sampling. Ideally, to minimize lag time between the event and the analytical signal, high flow rates and very short lengths of small diameter tubing would be used for the sampling process. As mentioned above, this is most easily accomplished when performing experiments with anesthetized animals, where the instrument can be placed in very close proximity to the animal. However, for freely-moving animal experiments using the Raturn® or a similar set-up, the length of the tubing between the animal and the swivel and then the analytical system can be significant, leading to a long delay between the event and the signal. The additional tubing can also lead to an increase in response time due to Taylor dispersion of the analyte. For large animal sampling, it is possible to place the analytical system on-animal and minimize the amount of tubing that is needed to connect the probe to the analytical system.
2.2 On-line/off-line sample analysis
Microdialysis samples can be analyzed on- or off-line. While there are many conventional separation-based analytical methods that lend themselves to off-line analysis, most of these methods require microliter sample volumes and costly instrumentation. They also usually require manual sample manipulation steps that can lead to fluid loss due to surface tension and evaporation. Separations can take several minutes to an hour and, therefore, samples must be stored prior to analysis (or a refrigerated autosampler must be employed). On-line methods negate the need for sample storage and handling and make it possible to analyze sub-microliter samples; however, the separation method must be fast enough to keep up with sample generation to preserve temporal resolution. For on-line analysis using methods such as capillary and microchip electrophoresis in which the sample volume requirement is low (nL-to-pL), temporal resolution is ideally limited only by the time needed for the separation. On-line methods also allow near real-time analysis, which can be useful to experimenters and clinicians who want to continuously monitor a situation on-site.
3. Microchip electrophoresis
Microchip electrophoresis (ME) is a separation technique that is ideally suited to on-line analysis of microdialysis samples due to its high separation efficiencies, low sample volume requirements (nL), and fast separation times [18,20,76,77]. This technique has become increasingly popular since it was first reported in 1992 [32-37,78]. Additionally, the microchip platform provides the ability to integrate sampling, separation, and detection on chip.
3.1 Separation considerations
Microchip electrophoresis is a liquid-phase separation method in which analytes are separated based on the ratio of their charge to hydrodynamic radii. When performing a typical free-zone electrophoresis experiment, a small channel with charged walls is filled with a background electrolyte followed by a sample plug, and a voltage is applied across the channel. When this voltage is applied, analytes in the channel migrate based on both their innate electrophoretic mobilities and the electroosmotic flow. The electrophoretic mobility of an individual analyte depends on its charge and hydrodynamic radius, with small, highly charged analytes moving the fastest and large analytes moving more slowly due to frictional drag forces. The electroosmotic flow, or EOF, is a bulk flow generated by the electric double layer at the charged walls of the channels [79]. In polydimethylsiloxane (PDMS)-based microchips and others without natively charged walls, a surfactant (for example, sodium dodecyl sulfate (SDS) in normal polarity and tetradecyltrimethylammonium bromide (TTAB) in reverse polarity) is added to help wet the channels and establish an EOF [80].
When analyzing high ionic strength microdialysis samples using microchip electrophoresis, an important consideration is Joule heating in the microchip. Joule heating is a part of any electrophoresis experiment, and occurs due to the current passing through the fluid in the channels releasing heat [76,81]. In electrophoresis, better separation efficiencies and faster analysis times are obtained with higher electric fields. Therefore, Joule heating can be an important consideration in microchip electrophoresis, where the channel lengths are small and applied voltage is large [76,81,82]. Care must be taken in choosing buffer type and concentration that is used for the separation, as many buffer systems are high in ionic strength. Additionally, microdialysis samples need to be high in ionic strength to maintain proper osmolality in the tissue being sampled. This can create a situation where Joule heating readily becomes problematic if the sample is manipulated in the chip electrokinetically. Depending on the microchip material and its thermal conductivity, Joule heating can cause a decrease in separation efficiencies, bubbling or boiling of the liquid in the channels, and/or actual damage to the channels themselves. Injection strategies that couple microdialysis sampling to microchip electrophoresis attempt to overcome this problem by limiting the amount of electrokinetic manipulation to which the sample is subjected; this is discussed in the interface design section of this review (section 5).
The compatibility of the separation buffer with the microdialysis perfusate is also important. If the ionic strength of the sample is much higher than that of the separation buffer, analyte dispersion or destacking can occur in the separation channel due to the lower voltage drop over the injection plug compared to the channel [83]. Destacking results in band broadening, leading to decreased efficiencies and higher limits of detection. Most microdialysis samples obtained from in vivo studies are high ionic strength (~150 mM salt concentration). Therefore, it can sometimes be difficult to match this ionic strength in the separation buffer without substantial Joule heating. On the other hand, the composition of the perfusate can lead to isotachophoretic concentration enhancement of analyte as has been observed for the detection of nitrite using a phosphate-buffered saline as a perfusate [84].
3.2 Microchip substrates
An important consideration using microchip electrophoresis for microdialysis sampling is the type of material that is used to create the device. Many different pure [85,86] and modified materials [87] have been used for microchip electrophoresis; however, we will discuss only those that have been implemented in microdialysis-microchip electrophoresis devices. A summary of the advantages and disadvantages of various microchip materials for MD-ME are highlighted in Table 4.
Table 4.
Material advantages and disadvantages for microchip electrophoresis
| Material | Advantages | Disadvantages |
|---|---|---|
| PDMS |
|
|
|
| ||
| Glass |
|
|
3.2.1 Polydimethylsiloxane microchips
Polydimethylsiloxane (PDMS) has been commonly used for the construction of microchip devices due to its low cost and ease of fabrication. To fabricate a simple all-PDMS device, classic photolithography is used to produce a silicon master with raised features [88]. PDMS and curing agent are mixed together and poured onto the silicon master, allowed to cure, and peeled away. This creates a PDMS substrate containing channels that correspond in dimension to those of the raised features of the silicon master. This substrate can then be placed on a flat substrate (glass, PDMS, etc.) to create a complete device. PDMS is flexible, which makes it forgiving in device fabrication, allowing easier integration of ports for microdialysis coupling and electrodes for electrochemical detection. For on-line MD-ME, it is important to create an irreversible bond between the two substrates (PDMS or PDMS/glass) so that the device can withstand the pressures created by the hydrodynamic flow in microdialysis without leaking. To generate the irreversible bond, researchers have used both plasma oxidation [38,88-90] and semi-curing methods [90-92].
While creating microchip electrophoresis devices using PDMS is a simple, low cost approach, there are some disadvantages. Channels produced in native PDMS do not possess the high, uniform charge that is characteristic of glass devices, and this can prove challenging when attempting to generate a reproducible and strong EOF [80,93]. A strong EOF is necessary to move all analytes toward the detector as well as to electrokinetically manipulate fluid flow. Another problem is the adsorption of hydrophobic analytes into native PDMS, creating inconsistencies in migration time or complete disappearance of analyte. To mitigate these problems, the surface of the PDMS can be either dynamically or permanently modified. Modification schemes for PDMS have been reviewed [94] and include dynamic coatings such as SDS [80,95] or TTAB [96], oxidation of channels or PDMS surfaces with plasma [97] or corona [98,99], and covalent modifications [100]. Overall, PDMS-based devices are an excellent prototyping tool for researchers due to their ease of fabrication and use, low cost, and compatibility with electrochemical detection; however, care must be taken to achieve good, reproducible separations.
3.2.2 Glass microchips
Glass is the most popular substrate for microchip electrophoresis because it has properties most similar to that of the fused silica capillaries that are used in capillary electrophoresis and it is optically transparent. However, all-glass microchip electrophoresis devices are more difficult to fabricate than PDMS. Glass channels are usually created using classical photolithography techniques followed by wet-etching with hydrofluoric acid (HF) [101-104]. This process creates anisotropic channels, as areas near the surface of the substrate encounter the HF longer than those in the bottom of the channel. Once channels are created, the glass substrate containing channels is bonded to another glass substrate to create the complete device. Glass chips are also commercially available, and several vendors sell custom-made and prefabricated glass devices for microchip electrophoresis.
Most applications of glass microchips employ fluorescence detection since the laser light can be focused directly on the channel. Electrochemical detection is also employed when using glass microchips; however, the tolerances and the necessity for high-temperature bonding can make electrode integration for amperometric detection challenging. Alternative bonding methods that do not require such high temperatures have been reported, including using UV-curable adhesives, HF and high pressure, and vacuum hot press systems [105-108], or employing prebonding steps that will make electrode integration easier [105-109].
All-glass chips do have the important benefit of a fast, reproducible EOF (3.90 ± 0.08 × 10−4 cm2/V·s compared to 1.12 ± 0.10 × 10−4 cm2/V·s in native PDMS) [93]. The surface chemistry of the glass channels is well characterized, and analyte adsorption to the channels is low in these devices (with the exception of basic proteins and peptides). Glass devices are very rugged, and under optimal running and storage conditions can last for months or even years with normal use. Additionally, glass exhibits optical clarity over a wide range of wavelengths, making it ideal for optical detection methods such as fluorescence. Metal electrodes have also been integrated into glass chips for electrochemical detection [101,102,104,110]. All-glass microchips are incredibly powerful devices for microdialysis-microchip electrophoresis, provided one has the necessary equipment and expertise to design, fabricate, and integrate into these devices. Alternatively, all-glass chips can be purchased from various vendors, if fabrication in-house is not viable.
3.2.3 Hybrid and other microchip materials
PDMS/glass hybrid design microchips have also been used for microdialysis-microchip electrophoresis applications [90,111]. These devices use channels that have been fabricated in either PDMS or glass with the opposite material used as a base substrate. Hybrid devices are easier to fabricate than all-glass, as irreversible bonding can be accomplished using plasma oxidation or semi-curing methods, thus avoiding high-temperature glass-bonding procedures. Additionally, in PDMS/glass hybrid devices, the separation becomes much more reproducible than in all-PDMS devices due to the stabilizing presence of one (or three) walls of glass; however, efficiencies are lower due to differences in EOF between glass and PDMS, causing band broadening [93].
Other materials have been employed for use in microchip electrophoresis, including poly(methyl methacrylate), polycarbonate, paper, and polyester toner [85,86]. To date, these materials have not been used for on-line microdiaysis-microchip electrophoresis. In the future, it will be interesting to watch the development of these materials for their potential use in MD-ME devices.
4. Detection strategies
4.1 Laser-induced fluorescence
Laser-induced fluorescence (LIF) is the most commonly used detection strategy in microchip electrophoresis, due to the low limits of detection obtainable and the wide range of potentially detectable analytes after derivatization. Generally, confocal microscopes are used to focus the light going into the micron-sized channel where analytes are excited; the resulting fluorescence is then directed to the detector. An advantage of fluorescence detection is that the laser beam can be focused anywhere in the channel so the effective separation length can be varied by simply moving the focal point of the laser. The laser also can be tightly focused so that very narrow bands can be detected. The power of ME-LIF was first demonstrated by Jacobson et al. for the separation of two fluorescent dyes in less than 150 ms [36].
Very few biological analytes exhibit native fluorescence, so analytes usually need to be derivatized to render them fluorescent. Many different commercially available reagents react with specific functional groups, including amines, thiols and carboxylic acids [112]. Several of these have been specifically developed to be compatible with commercially available lasers. The derivatization reagent will also change the electrophoretic mobility of the analytes, which can make resolving analytes more difficult as they become more similar in their charge to size ratio.
Most laser-based fluorescence detectors for microchip electrophoresis are fairly large, which limits their portability and applicability for on-animal sensors or point-of-care diagnostics. Over the past ten years, diode lasers and integrated optics have made it easier to miniaturize the instrumentation needed for LIF detection. In 2005, Culbertson et al. developed a stand-alone ME-LIF device for the detection of amino acids [113]. More recently, Peter Willis’ lab has developed a microchip electrophoresis system with LIF detection for deployment to other planets [114-116]. Additionally, our lab is currently developing a portable miniature LIF detection system for use with on-line microdialysis-microchip electrophoresis [117].
4.2 Electrochemistry
Electrochemistry (EC) has long been employed as a detection strategy for microchip electrophoresis. When a potential is applied to a working electrode, electroactive analytes are oxidized (or reduced) as they flow past the electrode. This oxidation generates electrons, or a measureable current response [118]. In contrast to spectroscopic methods, electrochemical detection does not suffer from path-length reduction of signal when miniaturized. In fact, with microelectrodes, the noise decreases faster than the signal, leading to an overall improvement in signal-to-noise ratio until the signal is undetectable [118-120]. Additionally, because many biologically relevant molecules are natively electroactive, there is no derivatization requirement as there is in most applications of fluorescence detection. In general, fabrication of electrodes through classic photolithography techniques allows direct integration into microfluidic devices. Electrochemistry coupled to microchip electrophoresis has been previously reviewed [121-124].
Highly sensitive and selective analyses can be accomplished through judicious choice of electrode type and applied potential. The electrode material that is used is dependent on the fabrication methods available, ease of integration, and electroactivity of the analytes of interest. Many different types of metals have employed, including platinum, gold, copper, and palladium, which are easily integrated using lithography and metal sputtering or evaporative techniques. Additionally, many different types of carbon electrodes are utilized with this technique, including carbon fiber, pyrolyzed photoresist film, carbon paste, and carbon ink. Using carbon as an electrode material offers many advantages, and many biologically relevant (organic) analytes generate good responses on carbon-based electrodes.
A variety of different options exist for working electrodes used for electrochemical detection in microchip electrophoresis. Many groups are experimenting with nanoelectrodes [125], multiple electrodes [126-128], and 3D electrodes [104] to enhance selectivity and/or sensitivity. In the future, these increases in sensitivity and selectivity will permit better detection of biologically relevant molecules in MD-ME-EC.
Working electrode placement is important when performing microchip electrophoresis with electrochemical detection. Due to the high voltages used for electrophoresis, care must be taken to isolate the separation voltage from the electrochemical detector. Many problems can arise for the researcher and equipment if the separation voltage grounds through the potentiostat, which will occur if these two are not isolated from one another. There are three general methods for isolating the potentiostat from the separation voltage: end-channel detection, off-channel detection using a decoupler, and in-channel detection using an electrically isolated potentiostat [129]. In the end-channel configuration, the working electrode is placed a few microns from the end of the channel in a detection reservoir. The spacing between the working electrode and the channel end allows the separation field to dissipate so that it has very little effect on the electrode and potentiostat. However, this configuration leads to lower separation efficiencies and decreased resolution compared to the other two approaches, due to the diffusion of the analyte into the detection reservoir prior to electrochemical detection [129].
The other two electrode configurations attempt to mitigate these effects by placing the working electrode within the channel. In the case of the off-channel configuration, a band of metal is placed upstream of the working electrode and connected to ground to act as a decoupler. This method relies on the EOF to push the analyte from the decoupler to the detection electrode; therefore, band broadening can occur in low EOF situations. The decoupler must also adsorb the gas generated at the ground electrode (H2 or O2) so that bubbles do not form in the channel. Platinum [130] and palladium [131] have been successfully employed as decouplers with normal polarity separations due to their ability to adsorb H2. However, this configuration cannot be employed in reverse polarity separations, because platinum and palladium do not adsorb the O2 generated at the anode.
Alternatively, isolated or “floating” potentiostats can be employed for in-channel electrochemical detection. In this configuration, the working electrode is usually placed as close to the end of the channel as possible. Because the working electrode is kept within the channel, higher separation efficiencies can be achieved as there is no band-broadening due to diffusion. In these systems, the electric field due to the separation voltage does interact with the working electrode, shifting the applied potential to more positive or negative values in reverse and normal polarity, respectively [132]. As the electrochemical detector is not grounded, this configuration does not destroy the electronics.
5. Microdialysis-microchip electrophoresis interface designs
One of the major challenges associated with the implementation of on-line microdialysis-microchip electrophoresis is the development of a robust interface. Due to the pressure-driven flows used for microdialysis sampling, the interface between the microchip and microdialysis flow stream must be strong to avoid chip delamination. Once the hydrodynamic microdialysis sample flow has been integrated into the chip, care must be taken regarding the manner in which the fluid is manipulated. Because of the high ionic strength perfusate used for most in vivo microdialysis sampling experiments, using only electroosmotically driven flow to manipulate the high ionic strength samples within the microchip is not ideal due to Joule heating, as discussed in section 3.1. Additionally, because microdialysis sampling creates a continuous flow stream, one of the main hurdles to overcome is the ability to inject discrete samples from this stream. There are three main methods that have been used to introduce discrete volumes of microdialysis samples into a microchip electrophoresis system. These are flow-gated injection, pneumatic valving, and segmented flow; their development and use are summarized in Table 5. Previous reviews have outlined some methods of integrating hydrodynamic injections with microchip electrophoresis [133,134].
Table 5.
Microdialysis-Microchip Electrophoresis Devices
| Interface | Region | Analytes | On- or Off-line | Material(s) | Detection | Comments | Reference |
|---|---|---|---|---|---|---|---|
| Flow-gated | in vitro | Enzamatic reaction monitoring fluorescein and FMG |
On-line | Glass | LIF | First report of MD-ME | [38] |
| Flow-gated | Brain (striatum) |
Effect of PDC on glutamate |
On-line | Glass | LIF (OPA derivatized) | [137] | |
| Pneumatic valves |
in vitro | Fluorescein, dichlorofluorescein |
On-line | PDMS/glass | LIF | First report of MD-ME with pneumatic valving interface |
[90] |
| Flow-gated | in vitro | Amino acids | On-line | Glass | LIF (NDA/2-ME derivatized) | [89] | |
| Pneumatic valves |
in vitro | Dopamine from PC12 cells |
On-line | PDMS/glass | EC (carbon ink electrodes with Pd decoupler) |
First report of MD-ME-EC | [111] |
| Segmented flow |
Brain (striatum) |
Effect of PDC on amino acids |
On-line | Glass | LIF (NDA/CN derivatized) | First report of segmented flow MD-ME |
[143] |
| Flow-gated | Brain (striatum) |
Fluorescein and amino acids |
Pseudo-on-line | PDMS | LIF (NDA/CN derivatized | [91] | |
| Pneumatic valves |
in vitro | Catecholamines | On-line | PDMS/glass | EC (carbon ink microarray with Pd decoupler) |
[127] | |
| Segmented flow |
Brain (striatum) |
Effect of PDC or high K+ on amino acids |
Off-line | Glass | LIF (NDA/CN derivatized) | Droplets formed at MD headpiece |
[144] |
| Segmented flow |
Brain (striatum) |
Effect of K+ microinjections on amino acids |
Off-line | Glass | LIF (NDA/CN derivatized) | [145] | |
| Pneumatic valves |
in vitro | Catecholamines | On-line | PDMS/epoxy | EC (Pt electrode and Pd decoupled imbedded in epoxy) |
[126] | |
| Flow-gated | in vitro | Amino acids and an internal standard |
On-line | PDMS | LIF (NDA/CN derivatized) | [92] | |
| Flow-gated | in vitro | Enzymatic reaction monitoring hydrogen peroxide |
On-line | Glass | EC (Pt electrodes) | [110] | |
| Flow-gated | in vitro | Catecholamines | On-line | PDMS/glass | EC (PPF electode) | [146] | |
| Flow-gated | Subcutaneous | Nitrite | On-line | Glass | EC (Pt electrodes) | On-animal (sheep) | [84] |
5.1 Flow-gated injection schemes
Flow-gated injection schemes attempt to balance the pressure-driven hydrodynamic flow of the microdialysis probe with the electrophoretic flow of the separation using a double-t design. In this design, sample flows into the microchip in the larger, top “sampling” channel, which allows hydrodynamic microdialysis pressures without chip delamination. A voltage is applied at the buffer reservoir, and the sample and buffer wastes are held at ground. These voltages create a stable gate at the sample/buffer intersection. When the applied voltage is allowed to float, the sample enters the intersection and can be separated upon re-application of the separation voltage.
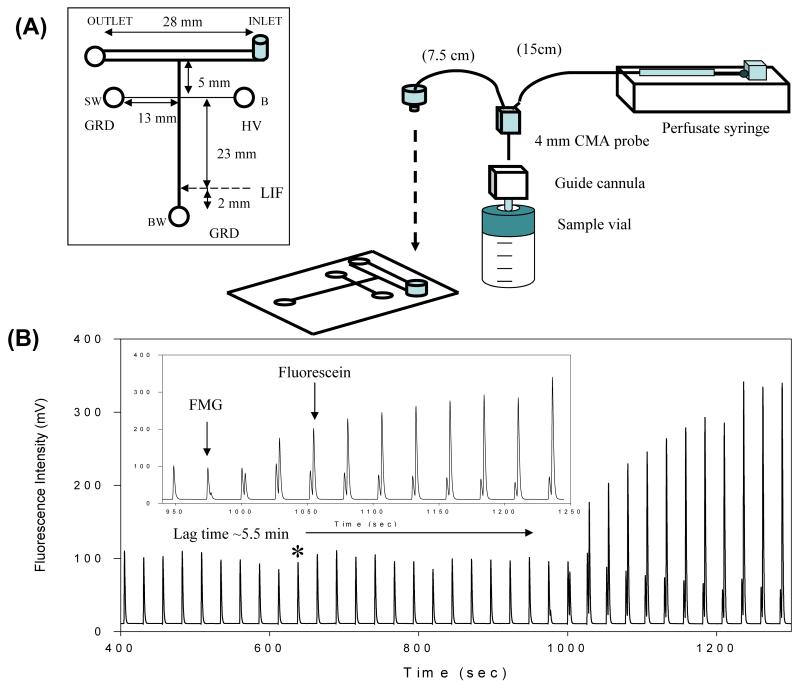
The first report of microdialysis sampling coupled directly to microchip electrophoresis used this flow-gated design in an all-glass device [38]. Huynh et al. based their design on previous reports by the Harrison [135] and Chen [136] groups, who used wide “sample introduction channels” to couple hydrodynamic flows with microchip electrophoresis. Using this approach, Huynh and coworkers demonstrated the double-t design with LIF detection to monitor the fluorescence product of an enzymatic reaction in vitro (Fig. 4). Later, this same design was used to separate and detect primary amines following in-channel derivatization with naphthalene-2,3-dicarboxaldehyde/2-mercaptoethanol (NDA/2-ME) [89]. Placing the derivatization reagents in the buffer reservoir allowed dynamic on-channel derivatization and separation after sample injection.
Fig. 4.
Flow-gated injection scheme. (A) Microchip design and experimental set-up. (B) On-line in vitro monitoring of an enzymatic reaction. Reprinted (adapted) with permission from Huynh et al. [38]. Copyright 2004 American Chemical Society.
There have been several modifications and improvements of this original design, permitting more controlled derivatization and the incorporation of electrodes for electrochemical detection. Kennedy’s group employed a flow-gated injection scheme and on-line derivatization in an all-glass device to monitor amino acids [137]. Nandi et al. developed a PDMS device that was capable of on-chip derivatization and sample injection following accumulation of analyte in a reservoir [91]. Later, this design was further improved by including pre-channel mixing for on-line derivatization of primary amines with NDA/CN− (Fig. 5) [92]. Our group has recently developed an all-glass double-t microchip with in-channel electrochemical detection at integrated platinum electrodes for MD-ME [110]. This device was used to monitor the production of hydrogen peroxide from the reaction of glucose with glucose oxidase using microdialysis sampling. Later, it was employed to monitor, subcutaneously on-animal, the production of nitrite from nitroglycerin administered using retrodialysis [84].
Fig. 5.
On-chip derivatization and flow-gated sample injection. (A) Microchip design. (B) Mixing profiles for various locations in microchip. Reprinted with permission from Nandi et al. [92]. Copyright 2013 Wiley.
The flow-gated injection scheme is a simple way of introducing pressure-driven microdialysis flow to a microchip and injecting discrete samples from the continuous flow stream into the separation channel. The benefits of this system are in its simplicity; fluid flow is manipulated solely by the pressure from the microdialysate and an applied voltage. However, there are some challenges associated with this injection scheme. Channel dimensions, flow rates, and applied voltages must all be optimized concurrently to establish a stable gate. Because there is an electrokinetic influence on the injection, some bias may occur during sample injections. In some cases, this can be helpful by eliminating interferences. In addition, the amount of sample injected will depend on the applied field strength and ionic strength of the sample.
5.2 Pneumatic valves
Pneumatically driven valves have also been used to couple microdialysis sampling to microchip electrophoresis. These devices are fabricated in two layers (valve and flow layer) using flexible polymers such as PDMS [138,139]. In these devices, flow is controlled using pneumatically driven valves that allow discrete sample plugs to enter the electrophoretic channel, which is placed at a right angle to the sample introduction channel.
The first report of the use of pneumatic valves as an interface between microdialysis sampling and microchip electrophoresis was by Li et al. [90]. In their design, a pushback channel was incorporated to eliminate sample diffusion into the separation channel between injections. These researchers demonstrated the device using fluorescein as a model compound, sampled in vitro.
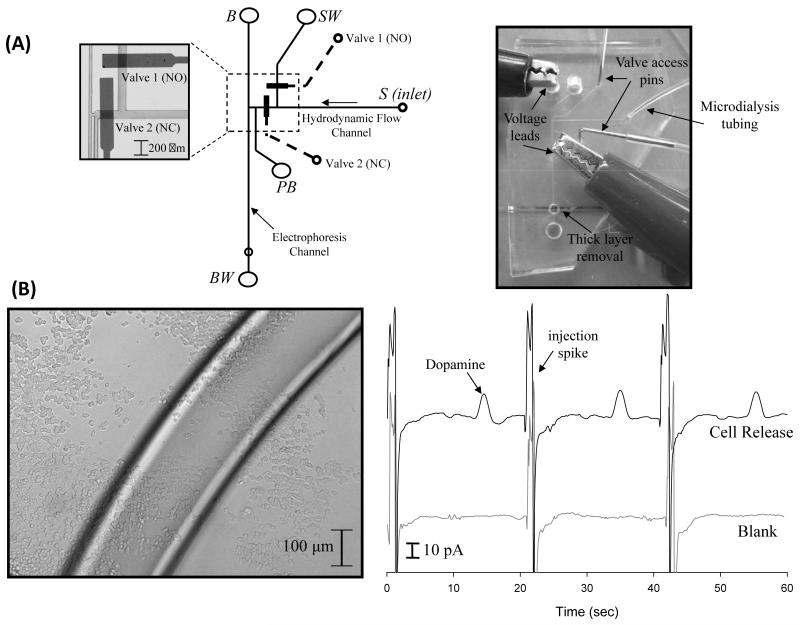
Expanding their original design, Mecker and Martin incorporated valving with electrochemical detection for monitoring dopamine release from PC 12 cells using microdialysis sampling (Fig. 6) [111]. This was the first report of the incorporation of electrochemical detection with MD-ME. The electrochemical part of the system used a palladium decoupler and carbon ink microelectrodes. The chip was produced using reversible bonding, and the electrodes were placed in the chip using an access hole punched into the PDMS layers. This device was able to monitor stimulated dopamine release from preloaded PC 12 cells. Later, the same group incorporated carbon-ink microelectrode arrays [127] and epoxy imbedded electrodes [126] into this design.
Fig. 6.
Pneumatic valve injection scheme. (A) Microchip design and operation. (B) Micrograph of PC 12 cells and on-line detection of stimulated dopamine release from preloaded PC 12 cells. Reprinted (adapted) with permission from Mecker and Martin [111]. Copyright 2008 American Chemical Society.
These pneumatic valve-based devices offer several advantages when coupling microdialysis sampling to microchip electrophoresis. As the Martin group has shown, many different electrode materials can be easily incorporated to the device, expanding the range of analytes that can be studied. Additionally, unlike the flow-gated devices, a larger range of field strengths may be used, as there is no compromise to establish a gate. There is also no electrokinetic injection bias in these devices. However, these devices do require more technical experience to fabricate. Possibly the largest disadvantage of pneumatic valve-based devices is the limitation in miniaturization and portability of the entire system, due to the bulky gas tanks needed to actuate the device valves unless micropumps are utilized. This severely inhibits the development of any point-of-care or on-animal systems using this configuration.
5.3 Segmented flow
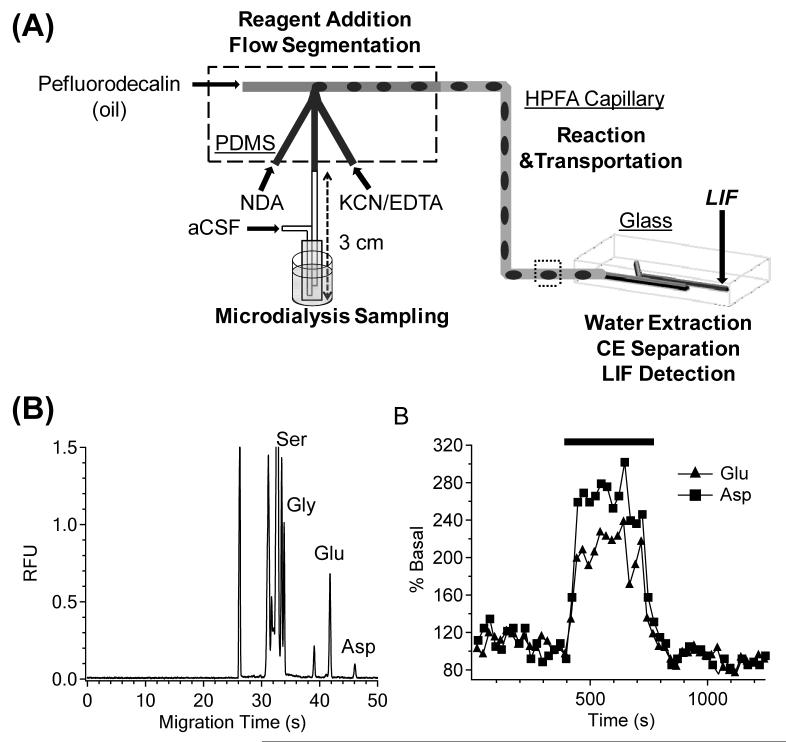
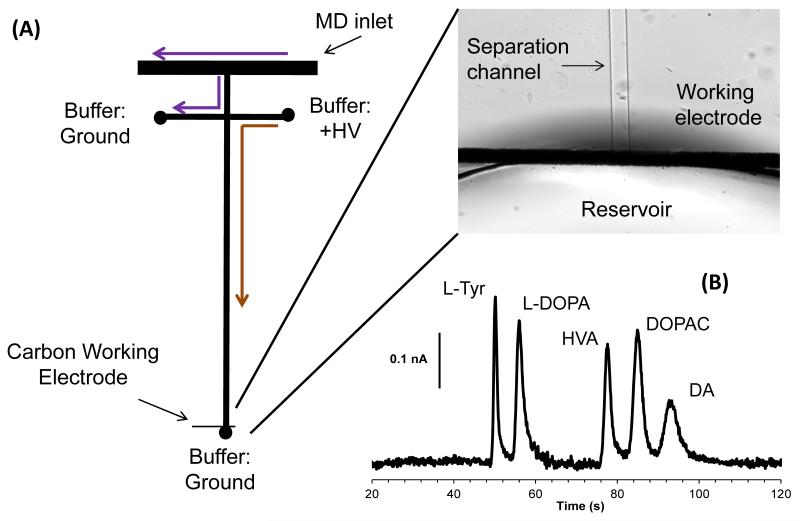
To overcome losses in temporal resolution due to diffusion of analyte within connecting tubing, segmented flow-based devices have been developed. These devices segment aqueous sample as droplets within a water-immiscible stream. The size and frequency of these droplets depends on the flow of the sample and water-immiscible stream, two parameters that can be optimized to give ideal droplet sizes.
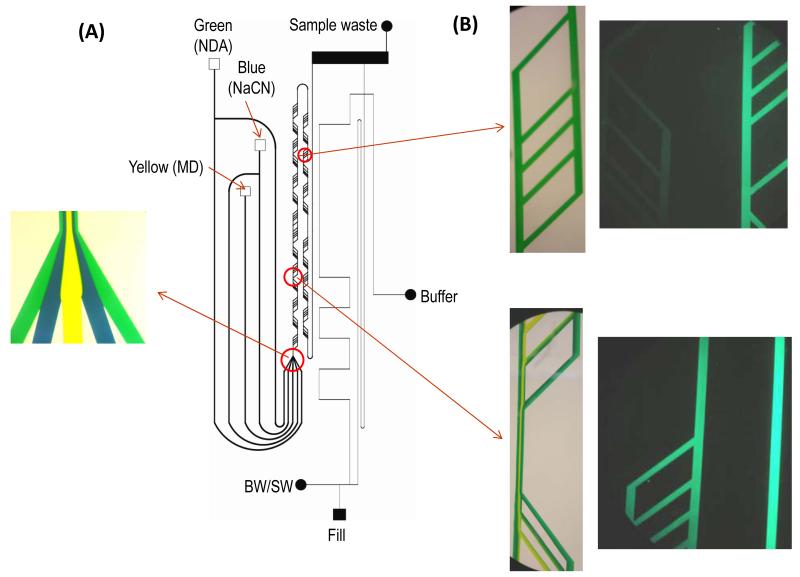
Building on previous work with segmenting microdialysis flow on a PDMS microchip [140,141] and work by Roman et al. on desegmenting droplets prior to injection and electrophoretic separation [142], Kennedy’s group efficiently coupled segmented flow to microchip electrophoresis for in vivo monitoring of amino acids from brain microdialysate [143]. In this work, two microchips were employed. A PDMS microchip was used both to segment the microdialysis flow stream and to react the dialysate with NDA/CN−. This was coupled to a glass chip where the flow was desegmented, the sample injected, and the analytes separated by electrophoresis and detected by LIF (Fig. 7). Using this device, separation efficiencies of over 200,000 theoretical plates and a temporal resolution of 40 s were obtained [143]. The temporal resolution was controlled by limits imposed by the separation time; faster separation times may decrease this resolution.
Fig. 7.
Segmented flow-based device. (A) Overall design of flow segmentation and desegmentation for on-line monitoring. (B) in vivo monitoring of serine (Ser), glycine (Gly), glutamine (Glu), and aspartate (Asp) using on-line device. Reprinted (adapted) with permission from Wang et al. [143]. Copyright 2009 American Chemical Society.
More recently, the same group fabricated a device that segments the continuous microdialysis stream into droplets as it is exiting the animal, eliminating any dead volume in connectors that were previously between the animal and segmenting chip. They used this device to sample, create droplets from the microdialysis brain perfusate in an anesthetized rat, and derivatize amino acids in the droplets [144]. After droplet creation on rat, the droplets were collected and immediately analyzed off-line for amino acids using microchip electrophoresis with LIF detection. In a later publication, they reported that droplets created with this device during MD sampling in awake animals were stored in HPFA collection tubing in a buffer jar filled with hexane at −80°C for up to four days and again analyzed off-line. This process resulted in a temporal resolution of 2 seconds [145]. Theoretically, this device could be directly coupled to on-line ME for near real-time monitoring, provided the separation time is sufficiently fast.
By segmenting the microdialysis flow into discrete droplets, enhanced temporal resolution can be achieved. Each droplet contains a small sample plug corresponding to a short time period. Because the sample is trapped in the droplet, there is limited analyte dispersion, and temporal resolution is preserved. This interface design does add an additional degree of complexity to the device, which could limit its use in point-of-care or on-animal settings.
6. Applications
Fully integrated, portable, and miniaturized systems using on-line microdialysis-microchip electrophoresis will provide researchers with a very valuable tool for monitoring biological events. While many of these devices are still in the development stage, others are currently being employed in some interesting in vitro and in vivo applications.
6.1 in vitro monitoring
Microdialysis coupled to microchip electrophoresis is an excellent tool for the in vitro monitoring of cells or bioreactions. Many have employed microdialysis sampling of a mixture of amino acids [89,92] or fluorescein [90] in vitro as a method of device characterization to show device response to concentration changes, lag and rise time, and/or separation efficiencies.
The Lunte group reported the first coupling of microdialysis sampling to microchip electrophoresis with laser-induced fluorescence detection to monitor the in vitro hydrolysis of fluorescein mono-β-D-galactopyranoside into fluorescein and galactose by β-D-glactosidase [38]. Because both fluorescein and fluorescein mono-β-D-glactopyranoside are fluorescent, it was possible to monitor the disappearance of the substrate and appearance of the product simultaneously and in near real-time with their flow-gated device. The production of hydrogen peroxide from the enzymatic reaction of glucose peroxidase with glucose was also monitored, using an on-line all-glass flow-gated device with integrated platinum electrodes [110].
Another application of microdialysis-microchip electrophoresis results from its ability to monitor cellular events. Martin’s group monitored preloaded PC 12 cells and used on-line microdialysis-microchip electrophoresis with electrochemical detection with a pneumatic valve injection scheme to monitor the release of dopamine after stimulation by a high K+ solution in cells grown within a petri dish [111].
6.2 in vivo monitoring
Perhaps the most exciting applications of microdialysis-microchip electrophoresis concern the continuous on-line monitoring of biological events in vivo. More specifically, many researchers have used the devices that they developed for in vivo monitoring of amino acids from brain microdialysates.
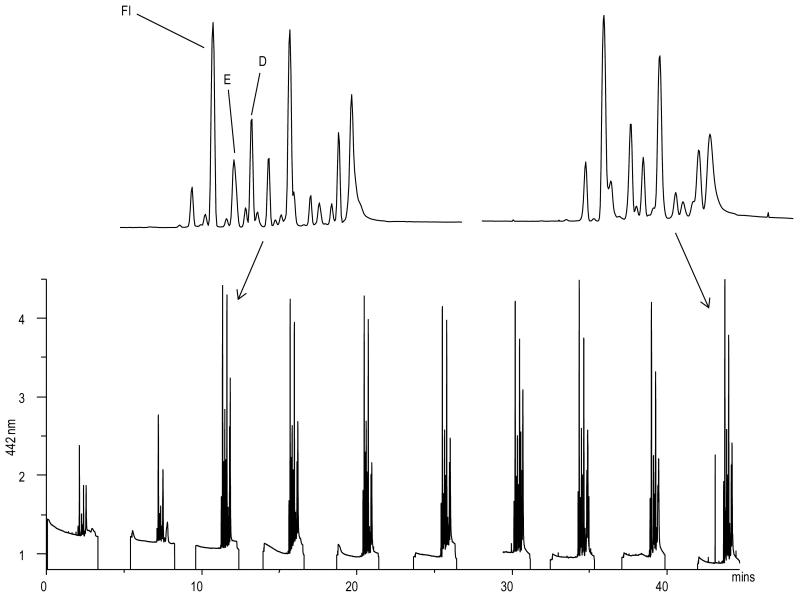
Nandi et al. used their flow-gated injection device to monitor endogenous levels of amino acids after a pseudo on-line derivatization [91]. In addition to the derivatized amino acids, they also investigated blood-brain barrier permeability by injecting fluorescein peripherally and monitoring its concentration in the brain via MD-ME-LIF (Fig. 8). They saw the appearance of fluorescein in the brain dialysate 5 min after the injection, and watched its clearance from the brain over a period of 90 min.
Fig. 8.
in vivo monitoring of amino acid neurotransmitters and fluorescein. Fl represents fluorescein, which was used as a marker of blood-brain barrier permeability. E represents glutamate and D represents aspartate. Reprinted with permission from Nandi et al. [91] Copyright 2010 Wiley.
The Kennedy group employed their flow-gated [137] and segmented flow devices [143] to monitor select amino acids in the brain following delivery of a glutamate uptake inhibitor, L-trans-pyrrolidine-2,4,-dicarboxylic acid, through the microdialysis probe. Upon administration of the inhibitor, they witnessed an increase in glutamate, and glutamate and aspartate with the flow-gated and segmented flow devices, respectively. Microchip electrophoresis with fluorescence detection was also used for off-line analysis of droplets generated by segmented flow for excitatory amino acids following microinjections of either L-trans-pyrrolidine-2,4,-dicarboxylic acid or K+. Concentrations of amino acids (glutamate, aspartate, taurine, glycine, and GABA) were monitored using this device with both stimulation procedures [144]. Lastly, microdialysis samples were segmented and derivativized shortly after their collection and stored for up to four days before being analyzed for amino acids by microchip electrophoresis with LIF detection [145].
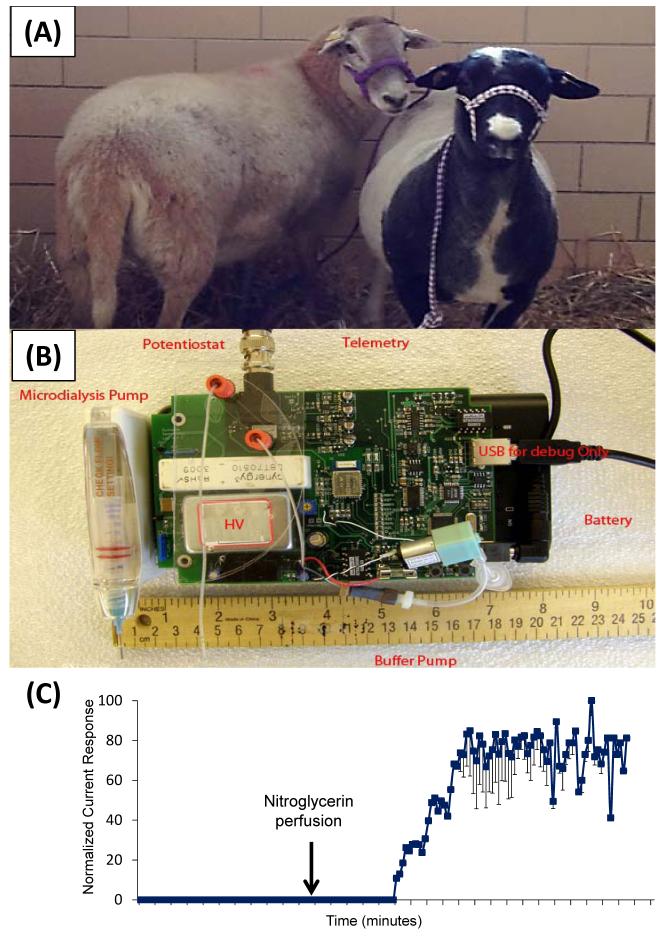
Recently, our group has employed microdialysis-microchip electrophoresis with integrated electrochemical detection at platinum electrodes for on-animal monitoring [84]. To achieve on-animal sensing, all associated equipment was miniaturized and placed in a backpack on a sheep (Fig. 9). In this study, the production of nitrite after a nitroglycerin perfusion was monitored on-animal using subcutaneous microdialysis sampling coupled to the device detailed in reference [110]. Additionally, we are currently developing an on-line microdialysis-microchip electrophoresis flow-gated device for the easy integration of carbon electrodes [146]. This device will be employed to monitor catecholamines in freely-roaming, untethered sheep and measure correlations between neurotransmitters and behavior (Fig. 10).
Fig. 9.
On-animal sensing. (A) Freely-roaming sheep for behavioral studies. (B) Prototype of miniaturized high voltage power supply, microdialysis pump, and potentiostat for on-animal monitoring. (C) On-line monitoring of nitrite following nitroglycerin perfusion.
Fig. 10.
On-line monitoring of catecholamines. (A) Flow-gated injection scheme. (B) Separation of standards of analytes in the dopamine metabolic pathway.
7. Conclusions and Future Directions
Microdialysis sampling coupled to microchip electrophoresis offers a powerful method for monitoring biological events, both in vivo and in vitro. The coupling of these two methods yields a separation-based sensor that can be customized for specific applications. Up to this point, MD-ME has been used primarily for monitoring release of neurochemicals, specifically, amino acid neurotransmitters and catecholamines. In the future, the use of this approach to monitor other analytes of interest, including markers of oxidative stress, neuropeptides, energy biomarkers, and drug metabolites will be investigated. The coupling of this methodology with new sensitive detection methods such as electrogenerated chemiluminescence, mass spectrometry, enzyme based sensors, and on-line immunoassays will increase the applicability of this approach to analytes that are not electroactive or amenable to derivatization with a fluorescence tag. In addition, the integration of sample preparation and preconcentration steps into the microfluidic format will make it possible to detect analytes that are currently below the LODs of the detection formats described in this review.
There are many possible future applications of the separation-based sensors using on-line microdialysis microchip electrophoresis. The small footprint of the microfluidic chips and associated instrumentation make them amenable to on-site and on-animal analysis. Portable analysis systems using sensors coupled to microdialysis sampling for monitoring biomarkers of tissue injury in traumatic brain injury are currently under development and some are commercially available [1,3,16]. However, these methods are limited by the biosensors that are available for specific analytes such as glucose, lactate, and glutamate. The separation-based sensor approach would make it possible to develop integrated systems to separate and detect biomarkers that are not currently monitored, such as catecholamines, amino acid neurotransmitters, antioxidants, neuropeptides, and markers of oxidative stress. On-site analysis would also be useful for monitoring oxidative stress in peripheral tissues, such as target tissue concentrations of antibiotics and markers of inflammation during sepsis [147,148].
In addition to clinical applications, on-site environmental monitoring of lakes, streams and soil could be accomplished with these devices, provided the sensor is optimized for the particular analytes of interest [149]. Monitoring of nutrients and metabolic products in bioreactors is another potential application for the on-line analysis systems [45,46]. Additionally, applications based on monitoring cell culture systems in vitro will continue to grow [150]. In all of these applications, chips can be customized for the analytes of interest and can be disposable.
The simultaneous monitoring of neurochemistry or drug metabolism and behavior in awake, freely-roaming animals is an important future application of this technology. As the microchips and probes become smaller, using telemetric control it will be possible to place devices on freely-roaming animals [84]. This will make it possible to observe the natural behavior of an animal and correlate it with biochemical events in the brain or other tissues. Such a device could be employed to study the effects of neuroactive drugs as well as to better understand the biochemical basis of social behavior and addiction.
Highlights.
On-line systems allow for near-real time monitoring of biological events
Systems can be customized with specific probes, separation, and detection methods
Several interface designs are presented for coupling MD to ME
Factors that affect temporal resolution in MD-ME are discussed
Applications of MD-ME for monitoring biological events both in vivo and in vitro
Acknowledgements
The authors would like to thank Drs. Craig Lunte and Sara Thomas for helpful discussions on microdialysis sampling and Nancy Harmony for editorial assistance in preparation of this manuscript. This research was supported by a research grant from the National Institutes of Health (grant R01 NS042929).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jones DA, Parkin MC, Langemann H, Landolt H, Hopwood SE, Strong AJ, Boutelle MG. On-line monitoring in neurointensive care Enzyme-based electrochemical assay for simultaneous, continuous monitoring of glucose and lactate from critical care patients. J. Electroanal. Chem. 2002;538-539:243–252. [Google Scholar]
- [2].Rogers ML, Brennan PA, Leong CL, Gowers SAN, Aldridge T, Mellor TK, Boutelle MG. Online rapid sampling microdialysis (rsMD) using enzyme-based electroanalysis for dynamic detection of ischaemia during free flap reconstructive surgery. Anal. Bioanal. Chem. 2013;405:3881–3888. doi: 10.1007/s00216-013-6770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rogers ML, Feuerstein D, Leong CL, Takagaki M, Niu X, Graf R, Boutelle MG. Continuous online microdialysis using microfluidic sensors: Dynamic neurometabolic changes during spreading depolarization. ACS Chem. Neurosci. 2013;4:799–807. doi: 10.1021/cn400047x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilson GS, Johnson MA. In-vivo electrochemistry: what can we learn about living systems? Chem Rev. 2008;108:2462–2481. doi: 10.1021/cr068082i. [DOI] [PubMed] [Google Scholar]
- [5].Ungerstedt U. Introduction to intracerebral microdialysis. Tech. Behav. Neural Sci. 1991;7:3–22. [Google Scholar]
- [6].Ungerstedt U. Microdialysis--principles and applications for studies in animals and man. J Intern Med. 1991;230:365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- [7].Ungerstedt U, Hallstrom A. In vivo microdialysis--a new approach to the analysis of neurotransmitters in the brain. Life Sci. 1987;41:861–864. doi: 10.1016/0024-3205(87)90181-0. [DOI] [PubMed] [Google Scholar]
- [8].Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweiz Akad Med Wiss. 1974;30:44–55. [PubMed] [Google Scholar]
- [9].Sharp T, Zetterstrom T. In: Handbook of Microdialysis: Methods, Applications and Clinical Aspects. Westerink BHC, Cremers TIFH, editors. Elsevier; New York: 2007. pp. 5–16. [Google Scholar]
- [10].Rogers ML, Boutelle MG. Real-time clinical monitoring of biomolecules. Annu. Rev. Anal. Chem. 2013;6:427–453. doi: 10.1146/annurev.anchem.111808.073648. [DOI] [PubMed] [Google Scholar]
- [11].Schultz KN, Kennedy RT. Time-resolved microdialysis for in vivo neurochemical measurements and other applications. Annu. Rev. Anal. Chem. 2008;1:627–661. doi: 10.1146/annurev.anchem.1.031207.113047. [DOI] [PubMed] [Google Scholar]
- [12].Kennedy RT. Emerging trends in in vivo neurochemical monitoring by microdialysis. Curr. Opin. Chem. Biol. 2013;17:860–867. doi: 10.1016/j.cbpa.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sloan CDK, Nandi P, Linz TH, Aldrich JV, Audus KL, Lunte SM. Analytical and biological methods for probing the blood-brain barrier. Annu. Rev. Anal. Chem. 2012;5:505–531. doi: 10.1146/annurev-anchem-062011-143002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Westerink BHC, Cremers TIFH. Handbook of Microdialysis: Methods, Applications and Clincal Aspects. Elsevier; New York: 2007. [Google Scholar]
- [15].Tsai T-H. Applications of Microdialysis in Pharmaceutical Science. John Wiley & Sons, Inc.; Hoboken, NJ: 2011. [Google Scholar]
- [16].Shannon RJ, Carpenter KLH, Guilfoyle MR, Helmy A, Hutchinson PJ. Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications. J. Pharmacokinet. Pharmacodyn. 2013;40:343–358. doi: 10.1007/s10928-013-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Azeredo FJ, Dalla Costa T, Derendorf H. Role of Microdialysis in Pharmacokinetics and Pharmacodynamics: Current Status and Future Directions. Clin. Pharmacokinet. 2014;53:205–212. doi: 10.1007/s40262-014-0131-8. [DOI] [PubMed] [Google Scholar]
- [18].Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal. Chim. Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guihen E, O’Connor WT. Current separation and detection methods in microdialysis the drive towards sensitivity and speed. Electrophoresis. 2009;30:2062–2075. doi: 10.1002/elps.200900039. [DOI] [PubMed] [Google Scholar]
- [20].Guihen E, O’Connor WT. Capillary and microchip electrophoresis in microdialysis: Recent applications. Electrophoresis. 2010;31:55–64. doi: 10.1002/elps.200900467. [DOI] [PubMed] [Google Scholar]
- [21].Cooley JC, Lunte CE. Detection of malondialdehyde in vivo using microdialysis sampling with CE-fluorescence. Electrophoresis. 2011;32:2994–2999. doi: 10.1002/elps.201100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crick EW, Osorio I, Frei M, Mayer AP, Lunte CE. Correlation of 3-mercaptopropionic acid induced seizures and changes in striatal neurotransmitters monitored by microdialysis. Eur. J. Pharm. Sci. 2014;57:25–33. doi: 10.1016/j.ejps.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kaul S, Faiman MD, Lunte CE. Determination of GABA, glutamate and carbamathione in brain microdialysis samples by capillary electrophoresis with fluorescence detection. Electrophoresis. 2011;32:284–291. doi: 10.1002/elps.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Steele KM, Lunte CE. Microdialysis sampling coupled to online microbore liquid chromatography for pharmacokinetic studies. J. Pharm. Biomed. Anal. 1995;13:149–154. doi: 10.1016/0731-7085(94)00135-o. [DOI] [PubMed] [Google Scholar]
- [25].Chaurasia CS, Chen C-E, Ashby CR., Jr. In vivo online HPLC-microdialysis: simultaneous detection of monoamines and their metabolites in awake freely-moving rats. J. Pharm. Biomed. Anal. 1999;19:413–422. doi: 10.1016/s0731-7085(98)00182-4. [DOI] [PubMed] [Google Scholar]
- [26].Mathy F-X, Vroman B, Ntivunwa D, De Winne AJ, Verbeeck RK, Preat V. On-line determination of fluconazole in blood and dermal rat microdialysates by microbore high-performance liquid chromatography. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2003;787:323–331. doi: 10.1016/s1570-0232(02)00961-3. [DOI] [PubMed] [Google Scholar]
- [27].Zhang J, Jaquins-Gerstl A, Nesbitt KM, Rutan SC, Michael AC, Weber SG. in vivo monitoring of serotonin in the striatum of freely moving rats with one minute temporal resolution by online microdialysis-capillary high-performance liquid chromatography at elevated temperature and pressure. Anal. Chem. 2013;85:9889–9897. doi: 10.1021/ac4023605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shackman HM, Shou M, Cellar NA, Watson CJ, Kennedy RT. Microdialysis coupled on-line to capillary liquid chromatography with tandem mass spectrometry for monitoring acetylcholine in vivo. J. Neurosci. Methods. 2006;159:86–92. doi: 10.1016/j.jneumeth.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [29].Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. Online Coupling of in vivo Microdialysis Sampling with Capillary Electrophoresis. Anal. Chem. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- [30].Zhou SY, Zuo H, Stobaugh JF, Lunte CE, Lunte SM. Continuous in vivo monitoring of amino acid neurotransmitters by microdialysis sampling with online derivatization and capillary electrophoresis separation. Anal. Chem. 1995;67:594–599. doi: 10.1021/ac00099a017. [DOI] [PubMed] [Google Scholar]
- [31].Manz A, Graber N, Widmer HM. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens. Actuators, B. 1990;1:244–248. [Google Scholar]
- [32].Harrison DJ, Manz A, Fan Z, Luedi H, Widmer HM. Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal. Chem. 1992;64:1926–1932. [Google Scholar]
- [33].Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Luedi H, Widmer HM. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems. Capillary electrophoresis on a chip. J. Chromatogr. 1992;593:253–258. [Google Scholar]
- [34].Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science. 1993;261:895–897. doi: 10.1126/science.261.5123.895. [DOI] [PubMed] [Google Scholar]
- [35].Seiler K, Harrison DJ, Manz A. Planar glass chips for capillary electrophoresis: repetitive sample injection, quantitation, and separation efficiency. Anal. Chem. 1993;65:1481–1488. [Google Scholar]
- [36].Jacobson SC, Hergenroder R, Koutny LB, Ramsey JM. High-speed separations on a microchip. Anal. Chem. 1994;66:1114–1118. [Google Scholar]
- [37].Jacobson SC, Hergenroder R, Koutny LB, Warmack RJ, Ramsey JM. Effects of injection schemes and column geometry on the performance of microchip electrophoresis devices. Anal. Chem. 1994;66:1107–1113. [Google Scholar]
- [38].Huynh BH, Fogarty BA, Martin RS, Lunte SM. On-line coupling of microdialysis sampling with microchip-based capillary electrophoresis. Anal. Chem. 2004;76:6440–6447. doi: 10.1021/ac049365i. [DOI] [PubMed] [Google Scholar]
- [39].Sun L, Stenken JA. Improving microdialysis extraction efficiency of lipophilic eicosanoids. J. Pharm. Biomed. Anal. 2003;33:1059–1071. doi: 10.1016/s0731-7085(03)00363-7. [DOI] [PubMed] [Google Scholar]
- [40].Davies MI, Cooper JD, Desmond SS, Lunte CE, Lunte SM. Analytical considerations for microdialysis sampling. Adv. Drug Delivery Rev. 2000;45:169–188. doi: 10.1016/s0169-409x(00)00114-9. [DOI] [PubMed] [Google Scholar]
- [41].Ault JM, Riley CM, Meltzer NM, Lunte CE. Dermal microdialysis sampling in vivo. Pharm. Res. 1994;11:1631–1639. doi: 10.1023/a:1018922123774. [DOI] [PubMed] [Google Scholar]
- [42].Zuo H, Ye M, Davies MI. Monitoring transdermal delivery of nicotine using in vivo microdialysis sampling. Curr. Sep. 1996;15:63–66. [Google Scholar]
- [43].Siaghy EM, Devaux Y, Schroeder H, Sfaksi N, Ungureanu-Longrois D, Zannad F, Villemot JP, Nabet P, Mertes PM. High-performance liquid chromatographic analysis of muscular interstitial arginine and norepinephrine kinetics. A microdialysis study in rats. J Chromatogr B Biomed Sci Appl. 2000;745:279–286. doi: 10.1016/s0378-4347(00)00284-x. [DOI] [PubMed] [Google Scholar]
- [44].Davies MI, Lunte CE. Simultaneous microdialysis sampling from multiple sites in the liver for the study of phenol metabolism. Life Sci. 1996;59:1001–1013. doi: 10.1016/0024-3205(96)00407-9. [DOI] [PubMed] [Google Scholar]
- [45].Torto N, Gorton L, Laurell T, Marko-Varga G. Technical issues of in vitro microdialysis sampling in bioprocess monitoring. TrAC, Trends Anal. Chem. 1999;18:252–260. [Google Scholar]
- [46].Torto N, Laurell T, Gorton L, Marko-Varga G. Recent trends in the application of microdialysis in bioprocesses. Anal. Chim. Acta. 1998;374:111–135. [Google Scholar]
- [47].Scott DO, Lunte CE. In vivo microdialysis sampling in the bile, blood, and liver of rats to study the disposition of phenol. Pharm. Res. 1993;10:335–342. doi: 10.1023/a:1018971818689. [DOI] [PubMed] [Google Scholar]
- [48].Wu Q, Liu C, Smith RD. Online microdialysis desalting for electrospray ionization-mass spectrometry of proteins and peptides. Rapid Commun. Mass Spectrom. 1996;10:835–838. [Google Scholar]
- [49].Cremers TIFH, de Vries MG, Huinink KD, van Loon JP, Hart M. v. d., Ebert B, Westerink BHC, de Lange ECM. Quantitative microdialysis using modified ultraslow microdialysis: direct rapid and reliable determination of free brain concentrations with the MetaQuant technique. J Neurosci Methods. 2009;178:249–254. doi: 10.1016/j.jneumeth.2008.12.010. [DOI] [PubMed] [Google Scholar]
- [50].Telting-Diaz M, Scott DO, Lunte CE. Intravenous microdialysis sampling in awake, freely-moving rats. Anal. Chem. 1992;64:806–810. doi: 10.1021/ac00031a019. [DOI] [PubMed] [Google Scholar]
- [51].Kehr J. A survey on quantitative microdialysis: theoretical models and practical implications. J. Neurosci. Methods. 1993;48:251–261. doi: 10.1016/0165-0270(93)90096-a. [DOI] [PubMed] [Google Scholar]
- [52].Stenken JA. Methods and issues in microdialysis calibration. Anal. Chim. Acta. 1999;379:337–357. [Google Scholar]
- [53].Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- [54].Menacherry S, Hubert W, Justice JB., Jr. In vivo calibration of microdialysis probes for exogenous compounds. Anal. Chem. 1992;64:577–583. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- [55].Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialyzate samples. J. Neurosci. Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- [56].Stahle L. Drug distribution studies with microdialysis: I. Tissue dependent difference in recovery between caffeine and theophylline. Life Sci. 1991;49:1835–1842. doi: 10.1016/0024-3205(91)90486-u. [DOI] [PubMed] [Google Scholar]
- [57].Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: Application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm. Res. 1993;10:1411–1419. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- [58].Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- [59].Olson RJ, Justice JB., Jr. Quantitative microdialysis under transient conditions. Anal. Chem. 1993;65:1017–1022. doi: 10.1021/ac00056a012. [DOI] [PubMed] [Google Scholar]
- [60].Song Y, Lunte CE. Calibration methods for microdialysis sampling in vivo: muscle and adipose tissue. Anal. Chim. Acta. 1999;400:143–152. [Google Scholar]
- [61].Song Y, Lunte CE. Comparison of calibration by delivery versus no net flux for quantitative in vivo microdialysis sampling. Anal. Chim. Acta. 1999;379:251–262. [Google Scholar]
- [62].Sood P, Cole S, Fraier D, Young AMJ. Evaluation of metaquant microdialysis for measurement of absolute concentrations of amphetamine and dopamine in brain: A viable method for assessing pharmacokinetic profile of drugs in the brain. J. Neurosci. Methods. 2009;185:39–44. doi: 10.1016/j.jneumeth.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [63].Nandi P, Kuhnline CD, Lunte SM. In: Applications of Microdialysis in Pharmaceutical Science. Tsai T-H, editor. John Wiley & Sons, Inc.; Hoboken, NJ: 2011. pp. 39–92. [Google Scholar]
- [64].Lee WH, Slaney TR, Hower RW, Kennedy RT. Microfabricated sampling probes for in vivo monitoring of neurotransmitters. Anal. Chem. 2013;85:3828–3831. doi: 10.1021/ac400579x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Patterson EE, II, Pritchett JS, Shippy SA. High temporal resolution coupling of low-flow push-pull perfusion to capillary electrophoresis for ascorbate analysis at the rat vitreoretinal interface. Analyst. 2009;134:401–406. doi: 10.1039/b813887g. [DOI] [PubMed] [Google Scholar]
- [66].Pritchett JS, Pulido JS, Shippy SA. Measurement of region-specific nitrate levels of the posterior chamber of the rat eye using low-flow push-pull perfusion. Anal. Chem. 2008;80:5342–5349. doi: 10.1021/ac800238d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee WH, Slaney TR, Song P, Kennedy RT. Monitoring Molecules in Neuroscience. 2014. p. 5. [Google Scholar]
- [68].Schultz KN, Kennedy RT. Time-resolved microdialysis for in vivo neurochemical measurements and other applications. Annu. Rev. Anal. Chem. 2008;1:627–661. doi: 10.1146/annurev.anchem.1.031207.113047. [DOI] [PubMed] [Google Scholar]
- [69].Davies MI, Lunte CE. Microdialysis sampling coupled online to microseparation techniques. Chem. Soc. Rev. 1997;26:215–222. [Google Scholar]
- [70].Davies MI, Cooper JD, Desmond SS, Lunte CE, Lunte SM. Analytical considerations for microdialysis sampling. Adv. Drug Delivery Rev. 2000;45:169–188. doi: 10.1016/s0169-409x(00)00114-9. [DOI] [PubMed] [Google Scholar]
- [71].Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis online with capillary electrophoresis with laser-induced fluorescence detection. Anal. Chem. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- [72].Wang M, Roman GT, Perry ML, Kennedy RT. Microfluidic chip for high efficiency electrophoretic analysis of segmented flow from a microdialysis probe and in vivo chemical monitoring. Anal. Chem. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li Q, Zubieta J-K, Kennedy RT. Practical aspects of in vivo detection of neuropeptides by microdialysis coupled off-Line to capillary LC with multistage MS. Anal. Chem. 2009;81:2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Taylor G. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. London, Ser. A. 1953;219:186–203. [Google Scholar]
- [75].Chen D, Du W, Liu Y, Liu W, Kuznetsov A, Mendez FE, Philipson LH, Ismagilov RF. The chemistrode: a droplet-based microfluidic device for stimulation and recording with high temporal, spatial, and chemical resolution. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16843–16848. doi: 10.1073/pnas.0807916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wu D, Qin J, Lin B. Electrophoretic separations on microfluidic chips. J. Chromatogr. A. 2008;1184:542–559. doi: 10.1016/j.chroma.2007.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fogarty BA, Nandi P, Lunte SM. In: Capillary and Microchip Electrophoresis and Associated Microtechniques. Landers JP, editor. CRC Press LLC; New York: 2008. pp. 1327–1340. [Google Scholar]
- [78].Culbertson CT, Mickleburgh TG, Stewart-James SA, Sellens KA, Pressnall M. Micro total analysis systems: Fundamental advances and biological applications. Anal. Chem. 2014;86:95–118. doi: 10.1021/ac403688g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jorgenson JW. Electrophoresis. Anal. Chem. 1986;58:743A–744A. 746A, 748A, 750A, 752A, 754A, 756A–758A, 760A. doi: 10.1021/ac00298a001. [DOI] [PubMed] [Google Scholar]
- [80].Roman GT, McDaniel K, Culbertson CT. High efficiency micellar electrokinetic chromatography of hydrophobic analytes on poly(dimethylsiloxane) microchips. Analyst. 2006;131:194–201. doi: 10.1039/b510765b. [DOI] [PubMed] [Google Scholar]
- [81].Wang Y, Lin Q, Mukherjee T. A model for Joule heating-induced dispersion in microchip electrophoresis. Lab Chip. 2004;4:625–631. doi: 10.1039/b406752e. [DOI] [PubMed] [Google Scholar]
- [82].Legendre LA, Ferrance JP, Landers JP. In: Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques. Landers JP, editor. CRC Press; New York: 2008. pp. 335–358. [Google Scholar]
- [83].Weinberger R. Practical Capillary Electrophoresis. Academic Press; New York: 2000. [Google Scholar]
- [84].Scott DE, Willis SD, Gabbert S, Johnson DA, Naylor E, Janle EM, Krichevsky JE, Lunte CE, Lunte SM. Development of an on-animal separation-based sensor for monitoring drug metabolism in freely roaming sheep. Analyst. doi: 10.1039/c4an01928h. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ren K, Zhou J, Wu H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013;46:2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- [86].Nge PN, Rogers CI, Woolley AT. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013;113:2550–2583. doi: 10.1021/cr300337x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dolnik V. Wall coating for capillary electrophoresis on microchips. Electrophoresis. 2004;25:3589–3601. doi: 10.1002/elps.200406113. [DOI] [PubMed] [Google Scholar]
- [88].Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- [89].Huynh BH, Fogarty BA, Nandi P, Lunte SM. A microchip electrophoresis device with on-line microdialysis sampling and on-chip sample derivatization by naphthalene 2,3-dicarboxaldehyde/2-mercaptoethanol for amino acid and peptide analysis. J. Pharm. Biomed. Anal. 2006;42:529–534. doi: 10.1016/j.jpba.2006.05.014. [DOI] [PubMed] [Google Scholar]
- [90].Li MW, Huynh BH, Hulvey MK, Lunte SM, Martin RS. Design and characterization of poly(dimethylsiloxane)-based valves for interfacing continuous-flow sampling to microchip electrophoresis. Anal. Chem. 2006;78:1042–1051. doi: 10.1021/ac051592c. [DOI] [PubMed] [Google Scholar]
- [91].Nandi P, Desai DP, Lunte SM. Development of a PDMS-based microchip electrophoresis device for continuous online in vivo monitoring of microdialysis samples. Electrophoresis. 2010;31:1414–1422. doi: 10.1002/elps.200900612. [DOI] [PubMed] [Google Scholar]
- [92].Nandi P, Scott DE, Desai D, Lunte SM. Development and optimization of an integrated PDMS based-microdialysis microchip electrophoresis device with on-chip derivatization for continuous monitoring of primary amines. Electrophoresis. 2013;34:895–902. doi: 10.1002/elps.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Coltro WKT, Lunte SM, Carrilho E. Comparison of the analytical performance of electrophoresis microchannels fabricated in PDMS, glass, and polyester-toner. Electrophoresis. 2008;29:4928–4937. doi: 10.1002/elps.200700897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Makamba H, Kim JH, Lim K, Park N, Hahn JH. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis. 2003;24:3607–3619. doi: 10.1002/elps.200305627. [DOI] [PubMed] [Google Scholar]
- [95].Ocvirk G, Munroe M, Tang T, Oleschuk R, Westra K, Harrison DJ. Electrokinetic control of fluid flow in native poly(dimethylsiloxane) capillary electrophoresis devices. Electrophoresis. 2000;21:107–115. doi: 10.1002/(SICI)1522-2683(20000101)21:1<107::AID-ELPS107>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- [96].Hulvey MK, Frankenfeld CN, Lunte SM. Separation and detection of peroxynitrite using microchip electrophoresis with amperometric detection. Analytical Chemistry. 2010;82:1608–1611. doi: 10.1021/ac902821v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vickers JA, Caulum MM, Henry CS. Generation of hydrophilic poly(dimethylsiloxane) for high-performance microchip electrophoresis. Anal. Chem. 2006;78:7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- [98].Thorslund S, Nikolajeff F. Instant oxidation of closed microchannels. J. Micromech. Microeng. 2007;17:N16–N21. [Google Scholar]
- [99].Filla LA, Kirkpatrick DC, Martin RS. Use of a corona discharge to selectively pattern a hydrophilic/hydrophobic interface for integrating segmented flow with microchip electrophoresis and electrochemical detection. Anal. Chem. 2011;83:5996–6003. doi: 10.1021/ac201007s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Campos RPS, Yoshida IVP, Silva JAF. Surface modification of PDMS microchips with poly(ethylene glycol) derivatives for μTAS applications. Electrophoresis. 2014;35:2346–2352. doi: 10.1002/elps.201300531. [DOI] [PubMed] [Google Scholar]
- [101].Keynton RS, Roussel TJ, Crain MM, Jackson DJ, Franco DB, Naber JF, Walsh KM, Baldwin RP. Design and development of microfabricated capillary electrophoresis devices with electrochemical detection. Anal. Chim. Acta. 2004;507:95–105. [Google Scholar]
- [102].Baldwin RP, Roussel TJ, Jr., Crain MM, Bathlagunda V, Jackson DJ, Gullapalli J, Conklin JA, Pai R, Naber JF, Walsh KM, Keynton RS. Fully integrated on-chip electrochemical detection for capillary electrophoresis in a microfabricated device. Anal. Chem. 2002;74:3690–3697. doi: 10.1021/ac011188n. [DOI] [PubMed] [Google Scholar]
- [103].Fan ZH, Harrison DJ. Micromachining of capillary electrophoresis injectors and separators on glass chips and evaluation of flow at capillary intersections. Anal. Chem. 1994;66:177–184. [Google Scholar]
- [104].Pai RS, Walsh KM, Crain MM, Roussel TJ, Jr., Jackson DJ, Baldwin RP, Keynton RS, Naber JF. Fully integrated three-dimensional electrodes for electrochemical detection in microchips: Fabrication, characterization, and spplications. Anal. Chem. 2009;81:4762–4769. doi: 10.1021/ac9002529. [DOI] [PubMed] [Google Scholar]
- [105].Carroll S, Crain MM, Naber JF, Keynton RS, Walsh KM, Baldwin RP. Room temperature UV adhesive bonding of capillary electrophoresis devices. Lab Chip. 2008;8:1564–1569. doi: 10.1039/b805554h. [DOI] [PubMed] [Google Scholar]
- [106].Iles A, Oki A, Pamme N. Bonding of soda-lime glass microchips at low temperature. Microfluid. Nanofluid. 2007;3:119–122. [Google Scholar]
- [107].Akiyama Y, Morishima K, Kogi A, Kikutani Y, Tokeshi M, Kitamori T. Rapid bonding of Pyrex glass microchips. Electrophoresis. 2007;28:994–1001. doi: 10.1002/elps.200600437. [DOI] [PubMed] [Google Scholar]
- [108].Lima RS, Carneiro Leao PAG, Monteiro AM, Oliveira Piazzetta MH, Gobbi AL, Mazo LH, Carrilho E. Glass/SU-8 microchip for electrokinetic applications. Electrophoresis. 2013;34:2996–3002. [Google Scholar]
- [109].Allen PB, Chiu DT. Calcium-assisted glass-to-glass bonding for fabrication of glass microfluidic devices. Anal. Chem. 2008;80:7153–7157. doi: 10.1021/ac801059h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Scott DE, Grigsby RJ, Lunte SM. Microdialysis sampling coupled to microchip electrophoresis with integrated amperometric detection on an all-glass substrate. ChemPhysChem. 2013;14:2288–2294. doi: 10.1002/cphc.201300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mecker LC, Martin RS. Integration of microdialysis sampling and microchip electrophoresis with electrochemical detection. Anal. Chem. 2008;80:9257–9264. doi: 10.1021/ac801614r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].The Molecular Probes handbook. A guide to fluorescent probes and labeling technologies. Life Technologies; 2011. [Google Scholar]
- [113].Culbertson CT, Tugnawat Y, Meyer AR, Roman GT, Ramsey JM, Gonda SR. Microchip separations in reduced-gravity and hypergravity environments. Anal. Chem. 2005;77:7933–7940. doi: 10.1021/ac051198e. [DOI] [PubMed] [Google Scholar]
- [114].Mora MF, Greer F, Stockton AM, Bryant S, Willis PA. Toward total automation of microfluidics for extraterrestial in situ analysis. Anal. Chem. 2011;83:8636–8641. doi: 10.1021/ac202095k. [DOI] [PubMed] [Google Scholar]
- [115].Mora MF, Stockton AM, Willis PA. Analysis of thiols by microchip capillary electrophoresis for in situ planetary investigations. Electrophoresis. 2013;34:309–316. doi: 10.1002/elps.201200379. [DOI] [PubMed] [Google Scholar]
- [116].Mora MF, Stockton AM, Willis PA. Microchip capillary electrophoresis instrumentation for in situ analysis in the search for extraterrestrial life. Electrophoresis. 2012;33:2624–2638. doi: 10.1002/elps.201200102. [DOI] [PubMed] [Google Scholar]
- [117].Creamer JS. Department of Pharmaceutical Chemistry, Univeristy of Kansas; 2014. [Google Scholar]
- [118].Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd Edn 2002. [Google Scholar]
- [119].Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal. Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- [120].Wightman RM. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006;311:1570–1574. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]
- [121].Mark JJP, Scholz R, Matysik F-M. Electrochemical methods in conjunction with capillary and microchip electrophoresis. J. Chromatogr. A. 2012;1267:45–64. doi: 10.1016/j.chroma.2012.07.009. [DOI] [PubMed] [Google Scholar]
- [122].Lacher NA, Garrison KE, Martin RS, Lunte SM. Microchip capillary electrophoresis/electrochemistry. Electrophoresis. 2001;22:2526–2536. doi: 10.1002/1522-2683(200107)22:12<2526::AID-ELPS2526>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [123].Vandaveer WRIV, Pasas-Farmer SA, Fischer DJ, Frankenfeld CN, Lunte SM. Recent developments in electrochemical detection for microchip capillary electrophoresis. Electrophoresis. 2004;25:3528–3549. doi: 10.1002/elps.200406115. [DOI] [PubMed] [Google Scholar]
- [124].Kuban P, Hauser PC. Fundamentals of electrochemical detection techniques for CE and MCE. Electrophoresis. 2009;30:3305–3314. doi: 10.1002/elps.200900217. [DOI] [PubMed] [Google Scholar]
- [125].Chen C-P, Teng W, Hahn J-H. Nanoband electrode for high-performance in-channel amperometric detection in dual-channel microchip capillary electrophoresis. Electrophoresis. 2011;32:838–843. doi: 10.1002/elps.201000661. [DOI] [PubMed] [Google Scholar]
- [126].Johnson AS, Selimovic A, Martin RS. Integration of microchip electrophoresis with electrochemical detection using an epoxy-based molding method to embed multiple electrode materials. Electrophoresis. 2011;32:3121–3128. doi: 10.1002/elps.201100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mecker LC, Filla LA, Martin RS. Use of a carbon-ink mcroelectrode array for signal enhancement in microchip electrophoresis with electrochemical detection. Electroanalysis. 2010;22:2141–2146. doi: 10.1002/elan.201000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Vazquez M, Frankenfeld C, Coltro WKT, Carrilho E, Diamond D, Lunte SM. Dual contactless conductivity and amperometric detection on hybrid PDMS/glass electrophoresis microchips. Analyst. 2010;135:96–103. doi: 10.1039/b908985c. [DOI] [PubMed] [Google Scholar]
- [129].Fischer DJ, Hulvey MK, Regel AR, Lunte SM. Amperometric detection in microchip electrophoresis devices: Effect of electrode material and alignment on analytical performance. Electrophoresis. 2009;30:3324–3333. doi: 10.1002/elps.200900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wu C-C, Wu R-G, Huang J-G, Lin Y-C, Chang H-C. Three-electrode electrochemical detector and platinum film decoupler integrated with a capillary electrophoresis microchip for amperometric detection. Anal. Chem. 2003;75:947–952. doi: 10.1021/ac025912t. [DOI] [PubMed] [Google Scholar]
- [131].Lacher NA, Lunte SM, Martin RS. Development of a microfabricated palladium decoupler/electrochemical detector for microchip capillary electrophoresis using a hybrid glass/poly(dimethylsiloxane) device. Anal. Chem. 2004;76:2482–2491. doi: 10.1021/ac030327t. [DOI] [PubMed] [Google Scholar]
- [132].Gunasekara DB, Hulvey MK, Lunte SM. In-channel amperometric detection for microchip electrophoresis using a wireless isolated potentiostat. Electrophoresis. 2011;32:832–837. doi: 10.1002/elps.201000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Saito RM, Coltro WKT, de Jesus DP. Instrumentation design for hydrodynamic sample injection in microchip electrophoresis: A review. Electrophoresis. 2012;33:2614–2623. doi: 10.1002/elps.201200089. [DOI] [PubMed] [Google Scholar]
- [134].Karlinsey JM. Sample introduction techniques for microchip electrophoresis: A review. Anal. Chim. Acta. 2012;725:1–13. doi: 10.1016/j.aca.2012.02.052. [DOI] [PubMed] [Google Scholar]
- [135].Attiya S, Jemere AB, Tang T, Fitzpatrick G, Seiler K, Chiem N, Harrison DJ. Design of an interface to allow microfluidic electrophoresis chips to drink from the fire hose of the external environment. Electrophoresis. 2001;22:318–327. doi: 10.1002/1522-2683(200101)22:2<318::AID-ELPS318>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [136].Lin Y-H, Lee G-B, Li C-W, Huang G-R, Chen S-H. Flow-through sampling for electrophoresis-based microfluidic chips using hydrodynamic pumping. J. Chromatogr. A. 2001;937:115–125. doi: 10.1016/s0021-9673(01)01326-7. [DOI] [PubMed] [Google Scholar]
- [137].Sandlin ZD, Shou M, Shackman JG, Kennedy RT. Microfluidic electrophoresis chip coupled to microdialysis for in vivo monitoring of amino acid neurotransmitters. Anal. Chem. 2005;77:7702–7708. doi: 10.1021/ac051044z. [DOI] [PubMed] [Google Scholar]
- [138].Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- [139].Unger MA, Chou H-P, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- [140].Song H, Tice JD, Ismagilov RF. A microfluidic system for controlling reaction networks in time. Angew. Chem., Int. Ed. 2003;42:768–772. doi: 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- [141].Wang M, Roman GT, Schultz K, Jennings C, Kennedy RT. Improved temporal resolution for in vivo microdialysis by using segmented flow. Anal. Chem. 2008;80:5607–5615. doi: 10.1021/ac800622s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Roman GT, Wang M, Shultz KN, Jennings C, Kennedy RT. Sampling and electrophoretic analysis of segmented flow streams using virtual walls in a microfluidic device. Anal. Chem. 2008;80:8231–8238. doi: 10.1021/ac801317t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Wang M, Roman GT, Perry ML, Kennedy RT. Microfluidic chip for high efficiency electrophoretic analysis of segmented flow from a microdialysis probe and in vivo chemical monitoring. Anal. Chem. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wang M, Slaney T, Mabrouk O, Kennedy RT. Collection of nanoliter microdialysate fractions in plugs for off-line in vivo chemical monitoring with up to 2 s temporal resolution. J. Neurosci. Methods. 2010;190:39–48. doi: 10.1016/j.jneumeth.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Wang M, Hershey ND, Mabrouk OS, Kennedy RT. Collection, storage, and electrophoretic analysis of nanoliter microdialysis samples collected from awake animals in vivo. Anal. Bioanal. Chem. 2011;400:2013–2023. doi: 10.1007/s00216-011-4956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Saylor RA, Lunte SM. Monitoring Molecules in Neuroscience. 2014. p. 6. [Google Scholar]
- [147].Deitchman AN, Derendorf H. Measuring drug distribution in the critically ill patient. Adv. Drug Delivery Rev. 2014 doi: 10.1016/j.addr.2014.08.014. Ahead of Print. [DOI] [PubMed] [Google Scholar]
- [148].de la Pena A, Liu P, Derendorf H. Microdialysis in peripheral tissues. Adv. Drug Delivery Rev. 2000;45:189–216. doi: 10.1016/s0169-409x(00)00106-x. [DOI] [PubMed] [Google Scholar]
- [149].Miro M, Frenzel W. In: Applications of Microdialysis in Pharmaceutical Science. Tsai T-H, editor. John Wiley & Sons, Inc.; Hoboken, NJ: 2011. [Google Scholar]
- [150].Johnson AS, Selimovic A, Martin RS. Microchip-based electrochemical detection for monitoring cellular systems. Anal. Bioanal. Chem. 2013;405:3013–3020. doi: 10.1007/s00216-012-6682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]