Abstract
Background
Viral and bacterial infections are the most common causes of chronic obstructive pulmonary disease (COPD) exacerbations. Whether serum inflammatory markers can differentiate bacterial from virus infection in patients with COPD exacerbation requiring emergency department (ED) visits remains controversial.
Methods
Viral culture and polymerase chain reaction (PCR) were used to identify the viruses in the oropharynx of patients with COPD exacerbations. The bacteria were identified by the semiquantitative culture of the expectorated sputum. The peripheral blood white blood cell (WBC) counts, serum C-reactive protein (CRP), procalcitonin (PCT), and clinical symptoms were compared among patients with different types of infections.
Results
Viruses were isolated from 16 (22.2%) of the 72 patients enrolled. The most commonly identified viruses were parainfluenza type 3, influenza A, and rhinovirus. A total of 30 (41.7%) patients had positive bacterial cultures, with the most commonly found bacteria being Haemophilus influenzae and Haemophilus parainfluenzae. Five patients (6.9%) had both positive sputum cultures and virus identification. The WBC, CRP, and PCT levels of the bacteria-positive and bacteria-negative groups were not statistically different. Multivariate analysis showed that patients with increased sputum volumes during the COPD exacerbations had higher risks of recurrent exacerbations in the 1-year period following the first exacerbation.
Conclusion
WBC, CRP, or PCT could not differentiate between bacterial and viral infections in patients with COPD exacerbation requiring ED visits. Those with increased sputum during a COPD exacerbation had higher risks for recurrent exacerbations.
Keywords: chronic obstructive pulmonary disease, bacterial infection, virus, CRP
Introduction
Bacterial colonization and viral respiratory pathogens play important roles in exacerbations of chronic obstructive pulmonary disease (COPD),1,2 especially in patients requiring hospitalization.3 Viral and/or bacterial infections have been detected in up to 78% of patients with COPD exacerbations.4 Although bacteria are considered the major cause of COPD exacerbations and antibiotics commonly used to treat exacerbations,5 the importance of viral infections in COPD exacerbations was mentioned after the introduction and wide use of viral culture and real-time polymerase chain reaction (PCR).6,7 The link of viral infections to COPD has been emphasized in recent studies. COPD patients had more airway inflammation after virus-associated exacerbations.8,9 Seemungal et al found that viral infections caused frequent COPD exacerbations with prolonged symptoms.10 After viral infections, secondary bacterial infections were frequently detected in COPD patients.11
Biomarkers are commonly used to differentiate infective or noninfective causes of COPD exacerbations. A previous study suggested that patients treated with antibiotics according to procalcitonin (PCT) levels for exacerbations of COPD, reduced antibiotics use.12 However, PCT does not differentiate bacterial from viral causes of COPD exacerbations requiring admission to intensive care unit or ward.13,14 Whether serum inflammatory markers, such as C-reactive protein (CRP) or PCT, can distinguish bacterial from viral infection or not in patients with COPD exacerbations requiring emergency department (ED) visits remains controversial.
This study was conducted to clarify peripheral blood white blood cell (WBC) counts, PCT, and CRP levels, and their relationships with viral or bacterial pathogens, in COPD patients requiring ED visits for exacerbations.
Materials and methods
Subjects
COPD patients aged greater than 50 years old who visited the ED of the Linkou Chang-Gung Memorial Hospital due to COPD exacerbations between April 2009 and August 2010 were prospectively enrolled for this study. COPD was diagnosed based on the Global Initiatives for Chronic Obstructive Lung Disease (GOLD) guidelines, and an exacerbation was defined as “a worsening of the patient’s respiratory symptoms that is beyond normal day-to-day variation”.15 All the patients were current smokers or ex-smokers with a smoking history of greater than 20 pack-years. Postbronchodilator spirometry prior to the ED visit that demonstrated a ratio of forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) of <70% was documented. Patients with a history of bronchial asthma or with abnormal chest radiographs indicating other respiratory diseases were excluded. Patients who were hospitalized for cardiovascular disease, acute pulmonary edema, acute pulmonary embolism, pneumothorax, or active pneumonia were also excluded. Active pneumonia was diagnosed by chest radiograph obtained in ED.
Data collection
Medical records were reviewed and analyzed for the following data: age, sex, body mass index (BMI), medications used prior to the ED admission, clinical symptoms (worsened dyspnea, increased sputum volume and purulence, fever, cough, sore throat, and wheeze), family cluster of common cold symptoms, peripheral blood WBC count, serum CRP and PCT levels, spirometry, and hospital days of the current exacerbation. Prior corticosteroid use was defined as having had a corticosteroid prescribed at 10 mg or more of prednisolone per day for more than 30 days within 1 year before the ED visit. The Anthonisen criteria were defined as the presence of one of three symptoms, including increased dyspnea, increased sputum volume, and increased sputum purulence.16 Number of exacerbations in the following years was retrospectively collected.
Specimen processing
Sputum was obtained by spontaneous production, and the specimen was sent to the central laboratory of Linkou Chang-Gung Memorial Hospital for bacterial culture. A positive bacterial culture was defined as moderate to heavy growth of bacteria in the semiquantitative culture. Oropharyngeal swab specimens were collected from these patients for virus identification using both virus isolation and multiplex RT-PCR (MRT-PCR) assays.
Virus isolation
Oropharyngeal swab specimens were inoculated onto MK2, MRC-5, and MDCK cells to isolate the viruses. The cell cultures were incubated at 35°C for 2 weeks. The final identification of the viruses was performed using an immunofluorescence assay (IFA) screening kit (Chemicon Inc., Temecula, CA, USA) for respiratory viruses.
MRT-PCR
The MRT-PCR assay was designed to amplify the conserved regions of 14 viral targets. The MRT-PCR was performed using 0.2 mL thin-walled PCR tubes in a Bio-Rad iCycler or ABI 7900. The RT-PCR mixture used in the assay was the SuperScript® III One-Step RT-PCR kit (Invitrogen; Life Technologies Corp, Carlsbad, CA, USA). Each reaction contained the following kit reagents: 0.2 M probe, 0.4 M primer, 12.5 μL of 2× ABI Master mixture (containing 0.4 mmol/L of each deoxyribonucleotide triphosphate [dNTP] and 2.4 mmol/L MgSO4), and 5 μL of specimen DNA or RNA extract, or control. The final reaction volume was adjusted to 25 μL with PCR-grade water, and the RT-PCR amplification was performed using the following conditions: an initial complementary DNA step of 50°C for 30 minutes, followed by 95°C for 15 minutes and then, 50 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds.
Statistical analysis
All statistical analyses were performed using the SPSS (SPSS 18 for Windows, SPSS Inc., Chicago, IL, USA) statistical package and Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). All values are reported as means ± standard deviation (SD). Differences among subgroups were compared by using the χ2 test or Fisher’s exact test when the expected number of events was less than 5. The significance level (α) for all statistical tests was set at 0.05, and P<0.05 was considered statistically significant.
Ethics
This study was conducted in accordance with the amended Declaration of Helsinki. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, and written informed consent was obtained from all patients.
Results
In all, 72 COPD patients with exacerbations that required ED visits were included in the study period. None of the patients were included twice. No patients received long-term oxygen therapy in this study. The mean FEV1 was 0.86±0.32 L (40.12%±15.70% of predicted value). The baseline characteristics of the 72 COPD patients, including age, sex, BMI, FEV1% predicted, FVC% predicted, GOLD stage, pack-years of tobacco use, previous inhaled or oral medications, peripheral blood WBC counts, and serum CRP and PCT levels are shown in Table 1. A total of 52 patients (72.2%) presented with two or more of the Anthonisen criteria, and 38 patients (52.8%) had recurrent exacerbations that required ED visits in the subsequent 1-year period. A total of 46 patients (63.9%) were admitted to ward and 26 patients discharged from the ED. Four hospitalized patients were admitted to the intensive care unit for acute respiratory failure, three of whom needed mechanical ventilator support, while the last received noninvasive ventilation support. None of the patients died.
Table 1.
Baseline characteristics of 72 COPD exacerbation patients requiring emergency department admissions
| Baseline characteristics (n=72) | |
|---|---|
| Age, year | 75.17±7.94 |
| Male, n (%) | 72 (100) |
| BMI, kg/m2 | 21.98±4.57 |
| FEV1/FVC ratio, % | 55.34±9.86 |
| FEV1 % predicted normal, % | 40.12±15.70 |
| GOLD 1, n (%) | 1 (1.39) |
| GOLD 2, n (%) | 13 (18.06) |
| GOLD 3, n (%) | 43 (59.72) |
| GOLD 4, n (%) | 15 (20.83) |
| FVC% predicted normal, % | 50.15±17.10 |
| Tobacco use, pack-years | 61.29±30.69 |
| Current smoker, n (%) | 33 (45.8) |
| Ex-smoker, n (%) | 39 (54.2) |
| ICS + LABA, n (%) | 58 (80.56) |
| Inhaled anticholinergics, n (%) | 56 (77.78) |
| Oral corticosteroid, n (%) | 14 (19.44) |
| Short term oral antibiotics, n (%) | 7 (9.7) |
| Oral methylxanthines, n (%) | 52 (72.22) |
| Anthonisen criteria ≥2, n (%) | 52 (72.22) |
| WBC, 109/L | 10.52±4.19 |
| CRP, mg/L | 34.97±48.02 |
| PCT, ng/mL | 0.2012±0.4315 |
| Exacerbation in the sequential 1 year, n (exacerbation/person/year) | 38 (0.52) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GOLD, Global Initiatives for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting beta-agonists; PCT, procalcitonin; WBC, white blood cell.
Viruses were detected in the oropharynx of 16 (22.2%) of the 72 patients (Table 2). Parainfluenza virus type 3 (PIV3) was the most commonly detected virus (37.5%), followed by influenza A (Inf A) and human rhinovirus. Both adenovirus and PIV3 were identified in one patient. No influenza B or respiratory syncytial virus (RSV) was found. One patient had simultaneous detection of PIV3 and adenovirus. Patients with or without the confirmed presence of viruses were divided into two groups: 16 patients were virus-positive and 56 patients were virus-negative. There were no significant between-group differences in age, BMI, FEV1, FVC, length of hospital days, or number of exacerbation in the subsequent 1 year. Figure 1 shows the laboratory data at the time of ED admission of the 72 COPD exacerbation patients. WBC counts, CRP, and PCT levels were not significantly different between the virus-positive and virus-negative group. A larger proportion of the virus-positive patients had been previously treated with oral corticosteroids than had the virus-negative patients (37.5% versus 14.3%) (P=0.039). The viral-positive groups presented with more sore throat symptoms than did the viral-negative groups (62.5% versus 33.9%) (P=0.04). The family cluster of common cold symptoms was significantly higher for viral-positive groups than viral-negative groups (25% versus 1.8%) (P=0.001).
Table 2.
Pathogens in 72 COPD patients with acute exacerbation requiring an emergency department visit
| Microorganism identified
| |||||
|---|---|---|---|---|---|
| Viruses | n=17 | Bacteria | n=31 | Others | n=3 |
| Parainfluenza virus type 3 | 6 | Haemophilus influenzae | 9 | Chlamydia | 2 |
| Influenza A | 3 | Haemophilus parainfluenzae | 7 | Mycobacterium avium-intracellulare | 1 |
| Human rhinovirus | 3 | Klebsiella pneumoniae | 4 | ||
| Adenovirus | 2 | Pseudomonas aeruginosa | 3 | ||
| Coronavirus-OC43 | 1 | Acinetobacter baumannii | 2 | ||
| Coronavirus-229E | 1 | Streptococcus pneumoniae | 1 | ||
| Human metapneumovirus | 1 | Moraxella catarrhalis | 1 | ||
| Staphylococcus aureus | 1 | ||||
| Escherichia coli | 1 | ||||
| Serratia marcescens | 1 | ||||
| Enterobacter cloacae | 1 | ||||
Abbreviation: COPD, chronic obstructive pulmonary disease.
Figure 1.
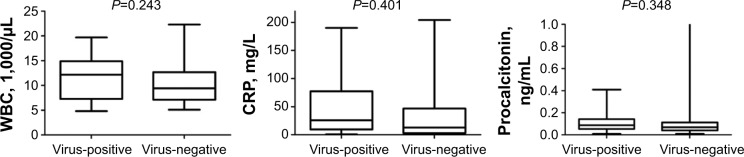
Laboratory data on day 1 at the ED in the virus-positive and virus-negative patients.
Abbreviations: CRP, C-reactive protein; ED, emergency department; WBC, white blood cell.
In all, 30 patients (41.7%) with COPD exacerbations had positive sputum bacterial cultures (Table 2). Haemophilus influenzae was the most commonly identified bacterial strain (30%), followed by Haemophilus parainfluenzae. The sputum bacterial culture of one patient grew Staphylococcus aureus and Acinetobacter baumannii. Chlamydia species were found by MRT-PCR in two cases, but no patients were positive for mycoplasma by PCR. There were no statistically significant differences between the bacteria-positive and bacteria-negative patients in age, BMI, FEV1, FVC, clinical symptoms, medications used prior to ED admission, length of hospital days, or recurrent exacerbations in the subsequent 1 year. WBC, CRP, and PCT levels were not significantly different between the bacteria-positive and bacteria-negative groups (Figure 2). Among the seven patients with PCT >0.5 ng/mL, three (42.9%) had sputum that was positive for bacteria growth, but none of these subjects were positive for viruses. The only significant difference was that the bacteria-positive group had a lower FEV1/FVC ratio than did the bacteria-negative group (52.43% versus 57.41%) (P=0.033). Both virus and bacteria were identified in five patients (6.9%). One had Inf A and Streptococcus pneumoniae, another had adenovirus and H. influenzae, the third had PIV3, adenovirus, and H. influenzae, the fourth had PIV3 and H. parainfluenzae, and the last had Escherichia coli and human metapneumovirus.
Figure 2.
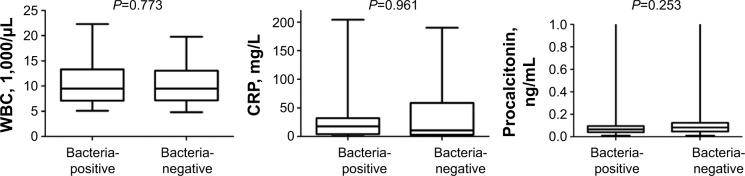
Laboratory data on day 1 at the ED in the bacteria-positive and bacteria-negative patients.
Abbreviations: CRP, C-reactive protein; ED, emergency department; WBC, white blood cell.
Table 3 shows the results of univariate and multivariate analysis of variables associated with recurrent exacerbations in the subsequent 1-year period. The results indicated that an increased sputum volume during the COPD exacerbation that required the ED visit was independently associated with a higher risk of a recurrent exacerbation during the subsequent year.
Table 3.
Univariate and multivariate logistic regression analysis of clinical variables associated with recurrent exacerbations in the subsequent 1 year, in 72 COPD exacerbation patients
| Parameter | Odds ratios | 95% CI | P-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.023 | 0.964–1.085 | 0.456 |
| BMI | 0.998 | 0.885–1.102 | 0.824 |
| Worsened dyspnea | 2.312 | 0.2–26.708 | 0.502 |
| Increase sputum | 4.219 | 1.506–11.822 | 0.006 |
| Sputum purulence | 1.795 | 0.701–4.596 | 0.223 |
| Virus-positive | 2.363 | 0.726–7.689 | 0.153 |
| Bacteria-positive | 2.091 | 0.801–5.458 | 0.132 |
| FEV1 % predicted | 1.005 | 0.975–1.035 | 0.756 |
| Multivariate analysis | |||
| Increase sputum | 3.687 | 1.092–12.448 | 0.036 |
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second.
The percentage of patients free from readmission to the EDs in 1 year, analyzed by Kaplan–Meier curves and evaluated with the log-rank test, was not significantly different between the bacteria-positive and bacteria-negative groups. The curves for patients who were virus-positive and virus-negative also did not differ significantly (Figure 3). When the outcomes were analyzed specifically by PCT and CRP levels, the percentage of patients free from readmission to the EDs also showed no statistically significant differences (Figure 4).
Figure 3.
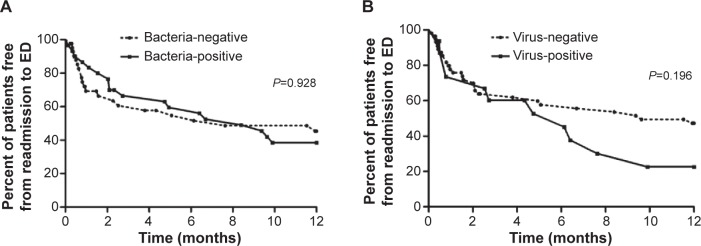
Kaplan–Meier survival curves (A) for the patients with bacteria positive and bacteria negative, and (B) for the patients with virus positive and virus negative.
Abbreviation: ED, emergency department.
Figure 4.
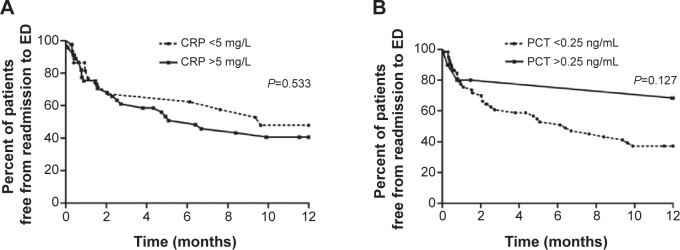
Kaplan–Meier survival curves (A) for the patients with high CRP levels and low CRP levels, and (B) for the patients with high PCT levels and low PCT levels.
Abbreviations: CRP, C-reactive protein; ED, emergency department; PCT, procalcitonin.
Discussion
Our study demonstrated that viruses were identified in the oropharynx of 22.2% and bacterial infections were confirmed in 41.7% of the patients with COPD exacerbations requiring ED visits. The most commonly identified virus was PIV3, and the most commonly found bacterium was H. influenzae. Higher percentages of the virus-positive patients presented with sore throat and with the family cluster of common cold symptoms. The analysis of maintenance medications prescribed before the ED visits showed that the percentage of patients who had oral corticosteroid treatment prior to the ED admission was higher in the virus-positive group. Multivariate analysis for evaluation of the risk factors for recurrent exacerbations showed a higher rate of recurrent COPD exacerbation in the 1-year period after the original ED visit in those with an increased sputum volume during the first COPD exacerbation. However, the commonly used biomarkers for infection, such as serum CRP or PCT levels, did not aid in differentiating between bacterial or viral infections in patients with COPD exacerbations.
A more rapid decline of lung function is observed in COPD patients with exacerbations.17 The patients with exacerbations also had higher mortality rates and more frequent exacerbations requiring hospital admissions.18 Papi et al found that infective exacerbations were detected in 78% of patients, with bacteria identified in 54.7% and viruses in 48.4%.4 COPD exacerbations in which pathogens were identified were associated with longer hospitalization stays than noninfective exacerbations.4 However, the positive rates of viral identification in patients with COPD exacerbations requiring hospitalization varied in different publications. In the study by Kherad et al in which the samples for virus identification were obtained via nasopharyngeal swabs and the identification was performed using qualitative RT-PCR assays, the overall virus-positive rate was 51%.19 McManus et al showed that 36.8% of patients hospitalized for COPD exacerbations had sputum samples that were positive for viruses.20 In a study of patients with COPD exacerbations requiring mechanical ventilation, viruses were identified in 43% of nasopharyngeal aspirates and posterior pharyngeal swabs.21 In our study, viral pathogens were detected in the oropharyngeal swabs of 16 patients (22.2%). The virus-positive rate of the oropharyngeal swabs was thus lower than in the previous studies. The possible causes of the variation may include seasonal and geographic differences in the etiology of virus-associated COPD exacerbations. In a recent systematic review, the rates of detection of viruses associated with COPD exacerbations were highest in Europe.7 Another possible cause of the differences in the results is the site of the sample collections. In our study, we collected oropharyngeal swabs but not nasopharyngeal swabs or sputum samples, which might result in different rates of viral detection.
In this study, the commonly identified viruses in patients with COPD exacerbations requiring ED visits were PIV3, Inf A virus, and human rhinovirus. This result was similar to the finding of Cameron et al from a study in Australia in which the most frequently detected viral etiologies were Inf A, PIV3, and rhinovirus.21 In a recent review of eight studies, picornavirus was the most common virus in western countries and influenza virus was most common in Asia.7
H. influenzae was the most common bacterial pathogen in COPD exacerbations in a previous study.4 Pseudomonas aeruginosa and Klebsiella pneumoniae were also emphasized in recent studies.22,23 In our study, H. influenzae, H. parainfluenzae, and K. pneumoniae were the most commonly identified bacteria. Our data provided worthwhile epidemiologic results of viral and bacterial etiologies of COPD exacerbation, and this may have important implications for appropriate empirical selection of antibiotics and proper management of COPD exacerbations.
Biomarkers are commonly used to differentiate infective or noninfective causes of COPD exacerbations. We compared WBC, CRP, and PCT levels between the bacteria-positive and bacteria-negative patients, and these biomarkers did not show statistically significant between-group differences in our study. Similarly, for the WBC, CRP, and PCT levels, the differences between the virus-positive and virus-negative patients were not statistically significant. PCT is a biomarker that is more specific for bacterial infection and might be useful as a guide for decisions regarding antibiotic treatment. However, it could not distinguish bacterial from viral and noninfectious causes of COPD exacerbations.13 Daniels et al compared CRP and PCT as markers of clinical outcome in COPD exacerbations and found that PCT was not a good biomarker in COPD exacerbations because patients with low PCT values did benefit from antibiotics.24 The severity of the bacterial infections of the airways in some patients with COPD exacerbations is probably insufficient to induce a significant PCT production.
Sputum purulence has been suggested to be useful in determining the initiation of antibiotic treatment in hospitalized patients with COPD exacerbations.25 However, there was no difference in sputum purulence between the bacteria-positive and bacteria-negative groups in our study. In a retrospective cohort study, up to 85% of patients hospitalized for COPD exacerbations were treated with antibiotics.26 Although the GOLD guidelines suggest “the use of antibiotics in exacerbations remains controversial” and “consider antibiotic when signs of bacterial infection”, the study by Rothberg et al supported antibiotic administration as improving the outcomes among patients hospitalized for COPD exacerbations.27
COPD exacerbation patients in whom oropharyngeal viruses were identified were more likely to have had previous corticosteroid treatment, a sore throat, and a family cluster of common cold symptoms. Clinicians should consider virus-induced COPD exacerbations if the patients present with a sore throat or a family cluster of common cold symptoms.
A total of 52% of our patients had recurrent exacerbations during the subsequent 1-year period after the exacerbation. Hurst et al reported on a cohort of patients with COPD and showed that 39% of patients with stage 2, 52% with stage 3, and 62% with stage 4 COPD had exacerbations during the preceding year.28 Bafadhel et al divided COPD exacerbations to four distinct clusters, including bacterial, viral, eosinophilic predominant, and pauci-inflammatory, by biomarkers such as sputum IL-1β or serum CXCL10.29 We analyzed the clinical variables associated with the 1-year recurrent exacerbation rate, and increased sputum volume during the initial COPD exacerbation was the only independent risk factor for recurrent exacerbations in the subsequent 1-year period. Our study provides additional information for further investigation about COPD phenotypes of recurrent exacerbations.
There are some limitations in this study, which should be mentioned. First, the techniques used in this study cannot distinguish acute infection from colonization. Therefore, we selected patients requiring ED visits rather than only symptom-based exacerbations. Second, although this study was conducted in a 3,300-bed tertiary teaching hospital, it was still a single-center, not a multicenter, study, and our results were obtained for a very low number of patients. Third, whether these patients had prior influenza or pneumococcal vaccinations had not been recorded. We could not clarify the influence of vaccinations in COPD exacerbations from this study. Further, virus was assessed both by cultures and by PCR, but bacteria were identified only by culture. Finally, our virus identification was obtained from oropharyngeal swab specimens. We did not obtain specimens from nasopharyngeal swabs or the lower respiratory tract for virus identification. The actual rates of virus identification in different sampling sites were unknown.
In conclusion, viruses and bacteria both play important roles in COPD exacerbations. WBC, PCT, and CRP levels are not good indicators for bacterial infections in COPD exacerbations. Those with increased sputum volume in COPD exacerbation had a higher rate of recurrent exacerbations in the subsequent 1-year period. Understanding the etiologies of bacteria and viruses in COPD exacerbations might aid in the appropriate management of COPD exacerbations.
Acknowledgments
This study was sponsored by the Chang-Gung Research Program, grant number CMRPG360781.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 2.Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renom F, Yáñez A, Garau M, et al. Prognosis of COPD patients requiring frequent hospitalization: role of airway infection. Respir Med. 2010;104(6):840–848. doi: 10.1016/j.rmed.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 5.Albert RK, Connett J, Bailey WC, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. New Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varkey JB, Varkey B. Viral infections in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14(2):89–94. doi: 10.1097/MCP.0b013e3282f4a99f. [DOI] [PubMed] [Google Scholar]
- 7.Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15(3):536–542. doi: 10.1111/j.1440-1843.2010.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde G, Borg I, Wiethege A, et al. Inflammatory response in acute viral exacerbations of COPD. Infection. 2008;36(5):427–433. doi: 10.1007/s15010-008-7327-5. [DOI] [PubMed] [Google Scholar]
- 10.Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 11.Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(11):1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 13.Falsey AR, Becker KL, Swinburne AJ, et al. Utility of serum procalcitonin values in patients with acute exacerbations of chronic obstructive pulmonary disease: a cautionary note. Int J Chron Obstruct Pulmon Dis. 2012;7:127–135. doi: 10.2147/COPD.S29149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daubin C, Parienti JJ, Vabret A, et al. Procalcitonin levels in acute exacerbation of COPD admitted in ICU: a prospective cohort study. BMC Infect Dis. 2008;8:145. doi: 10.1186/1471-2334-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 16.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kherad O, Kaiser L, Bridevaux PO, et al. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron RJ, de Wit D, Welsh TN, Ferguson J, Grissell TV, Rye PJ. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32(7):1022–1029. doi: 10.1007/s00134-006-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SH, Kuo PH, Hsueh PR, Yang PC, Kuo SH. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12(1):81–87. doi: 10.1111/j.1440-1843.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J. 2009;34(5):1072–1078. doi: 10.1183/09031936.00003309. [DOI] [PubMed] [Google Scholar]
- 24.Daniels JM, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138(5):1108–1115. doi: 10.1378/chest.09-2927. [DOI] [PubMed] [Google Scholar]
- 25.Soler N, Esperatti M, Ewig S, Huerta A, Agustí C, Torres A. Sputum purulence-guided antibiotic use in hospitalised patients with exacerbations of COPD. Eur Respir J. 2012;40(6):1344–1353. doi: 10.1183/09031936.00150211. [DOI] [PubMed] [Google Scholar]
- 26.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 28.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 29.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]


