Abstract
Surface coatings are important components of Magnetic Particle Imaging (MPI) tracers – they preserve their key properties responsible for optimum tracer performance in physiological environments. In vivo, surface coatings form a physical barrier between the hydrophobic SPION cores and the physiological environment, and their design dictates the blood half-life and biodistribution of MPI tracers. Here we show the effect of tuning poly(ethylene glycol) (PEG)-based surface coatings on both in vitro and in vivo (mouse model) MPI performance of SPIONs. Our results showed that varying PEG molecular weight had a profound impact on colloidal stability, characterized using Dynamic Light Scattering (DLS), and the m’(H) response of SPIONs, measured in a 25 kHz/20 mTμ0−1max Magnetic Particle Spectrometer (MPS). Increasing PEG molecular weight from 5 kDa to 20 kDa preserved colloidal stability and m’(H) response of ~25 nm SPIONs – the optimum core diameter for MPI – in serum-rich cell culture medium for up to 24 hours. Furthermore, we compared the in vivo circulation time of SPIONs as a function of hydrodynamic diameter and showed that clustered SPIONs can adversely affect blood half-life; critically, SPIONs with clusters had 5 times shorter blood half-life than individually coated SPIONs. We anticipate that the development of MPI SPION tracers with long blood half-lives have potential not only in vascular imaging applications, but also enable opportunities in molecular targeting and imaging – a critical step towards early cancer detection using the new MPI modality.
Keywords: Blood half-life, Magnetic Particle Imaging, Superparamagnetic Iron Oxide Nanoparticles
I. Introduction
Magnetic particle imaging (MPI) tracers designed for in vivo applications comprise two fundamental components: (1) the superparamagnetic iron oxide nanoparticle (SPION) cores, which are the source of MPI signal, and (2) the surface coatings that render SPION cores soluble in biologically relevant media. SPION cores with a long history of development for a variety of biomedical applications [1], can also be carefully optimized for MPI [2], [3] – recent results from our group show that tuning the core diameter of nearly monodisperse SPIONs to ~25 nm results in nearly 3-fold gain in sensitivity and ~30% improvement in spatial resolution compared to Resovist® when measured under typical MPI field conditions (25 kHz; 18 mTμ0−1max) [4]. While tailoring SPION core size and size distribution ensures optimum MPI performance, surface coatings ensure the optimized core performance translates effectively to relevant in vivo systems. For applications in cardiovascular imaging, surface coatings must prevent rapid clearance of SPIONs from the blood to enable both first-pass cardiovascular and steady state blood pool imaging.
In aqueous environments, the hydrodynamic diameter of SPIONs includes the core diameter, surface coating thickness and any hydration or ion-diffusion layer coupled with the surface coating. Typically, a smaller hydrodynamic diameter results in longer blood half-life, however, it must be no less than ~15 nm to prevent rapid clearance through kidney fenestrae [5], [6]. On the other end, SPIONs with hydrodynamic diameter bigger than the inter-endothelial slits in the spleen (~200-500 nm) will be retained in the red pulp and eventually cleared by resident macrophage cells [7], [8]. In addition to hydrodynamic size, surface charge also plays a critical role in the clearance and immunogenicity of SPIONs; SPIONs with a positive or negative charge attract opsonins – a class of proteins in blood plasma that enable recognition and uptake by macrophages in the mononuclear phagocytic system [9]-[11]. Thus, coatings with a neutral surface charge are preferred, in which case colloidal stability of SPIONs must rely primarily on steric repulsion rather than electrostatic repulsion. Unlike hydrodynamic size, surface charge is often a sole consequence of the coating; for instance, coatings terminated with protonated amines or deprotonated carboxylates result in either a net positive or negative charge, respectively.
Non-ionic (neutral charge) poly(ethylene glycol) (PEG) coatings, such as methoxy-terminated PEG (m-PEG), are highly biocompatible and often used to prolong vascular circulation of large antibodies and nanoparticle systems [12]. In addition to the PEG molecular weight, which can range from 1 kDa–50 kDa and modulate the nanoparticle hydrodynamic size accordingly, the surface density of PEG coatings is a critical parameter that can influence circulation time in nanoparticle systems [9], [13]. Thus, both the molecular weight and surface density of PEG coatings must be tuned to optimize the circulation time of SPIONs.
In this work, we present experimental studies that highlight surface coating parameters that can have an impact on MPI performance and blood half-life of SPIONs. In order to study the effect of surface density and hydrodynamic size, we coated SPIONs that featured similar MPI performance with either a different amount or molecular weight of m-PEG polymer. MPI performance was measured using our home-built 25 kHz (Hmax = 18 mTμ0−1) magnetic particle spectrometer (MPS). The corresponding effects on colloidal stability and MPS signal – defined as the mass susceptibility (m’(H); m3 kgFe−1) – were evaluated in serum-rich cell culture medium. For practical relevance to MPI systems, the blood half-life of SPIONs – measured in female CD-1 mice – was determined using our 25 kHz MPS. Concurrent development of size-optimized SPION cores and surface coatings will ultimately enable the preclinical and clinical translation of MPI tracers.
II. Procedures
A. Synthesis and characterization of PEG-coated SPIONs
Oleic acid coated SPIONs were synthesized according to a previously published method [14]. The as-synthesized hydrophobic SPIONs were phase transferred to the aqueous phase using poly(maleic anhydride-alt-1-octadecene) (PMAO: Sigma Aldrich) grafted with either a 20 or 30 molar excess of 5kDa m-PEG (Sigma Aldrich); additionally, a 20kDa m-PEG was grafted to PMAO at PEG/PMAO of 30. For brevity, hereafter polymers grafted with either a 5kDa or 20kDa PEG will be referred to as PMAO-5kPEG[30] and PMAO-20kPEG[30], respectively, where the numbers in the brackets represent the PEG/PMAO molar ratio. Details of the polymer synthesis and subsequent phase transfer of SPIONs to aqueous phase are published previously [14]. For determination of SPION core size, m(H) curves of SPIONs dispersed in DI water (100μl in polycarbonate capsule) were measured using a vibrating sample magnetometer (VSM; Lake shore Cryotronics). The core size was determined from fitting m(H) response to the Langevin function according to Chantrell’s method [5], which assumes a single lognormal size distribution. Dynamic light scattering (DLS; Malvern Instruments) was used to characterize the hydrodynamic diameter in deionized (DI) water and serum-rich cell culture medium.
B. In vivo blood half-life evaluation
The University of Washington’s Institutional Animal Care and Use Committee (IACUC) approved all animal protocols. Blood half-life of PEG-coated SPIONs was evaluated in 8-10 week old female CD-1 mice (Charles River laboratories). The details of the animal protocol are published previously [15]; briefly, approximately 7 mgFe/kg PEG-coated SPIONs were injected in the tail veins of mice. Approximately 0.2 ml of blood samples were collected at various time points via retro-orbital bleeding and collected in EDTA-coated tubes to prevent clotting. In order to account for statistical differences between animal weights and metabolic rates, each time point was repeated in three mice and the average value was used. For m’(H) measurement in our 25 kHz MPS, 0.1 ml of blood was transferred to a 0.6 ml microcentrifuge tube and measured in triplicates. The average peak intensity of the m’(H) curves was used to determine SPION concentration. A series of SPION standards with known concentrations (concentration determined using inductively coupled plasma-optical emission spectrophotometer; Perkin Elmer Optima 8300) were used to construct a calibration curve that was used to quantify the corresponding MPS signal from blood samples.
III. Results and Discussion
A. SPION characterization and stability in serum-rich cell culture medium
Size characteristics of SPION samples coated with PMAO-5kPEG[20], PMAO-5kPEG[30] or PMAO-20kPEG[30] are summarized in Table I. All measurements were performed in DI water. Note that despite the 4-fold higher molecular weight, the hydrodynamic diameter (dH) of SPIONs coated with 20kDa PEG was only 38% larger than SPIONs coated with 5kDa PEG. It is likely that the polymer chains are not fully extended in water and are in some form of folded or ‘mushroom’ configuration [13]. MPS measurements in DI water showed that the peak intensity and full width at half maximum (FWHM) of m’(H) for all three tracers were within 7-12% due to relatively similar core diameters (dc). For comparison, characteristics of Resovist® SPIONs are also shown – in MPS measurements, our SPIONs provide an average of 6.5 times more signal and 50% narrower FWHM.
TABLE I.
SPION characteristics in deionized water
| Polymer: PMAO- |
dc nm (σ) |
dH nm (PDI) |
m’(H) × 10−2: m3kgFe−1 |
FWHM: mTμ0−1 |
|---|---|---|---|---|
| 5kPEG[20] | 25 (0.14) | 44 (0.13) | 3.5 | 5.8 |
| 5kPEG[30] | 23 (0.22) | 46 (0.13) | 3.1 | 6.7 |
| 20kPEG[30] | 25 (0.17) | 62 (0.06) | 3.2 | 6.2 |
| Resovist® | 14 (0.47) | 72 (0.17) | 0.5 | 12.4 |
Core and hydrodynamic diameters were determined from VSM and DLS measurements. The peak intensity and FWHM of m’(H) were measured in our 25 kHz/20mTμ0−1 max home-built MPS. dc – median core diameter; dH – hydrodynamic diameter; σ – standard deviation of the lognormal distribution function; PDI – polydispersity index from DLS measurements.
In order to test the stability of SPIONs in biologically relevant media, samples listed in Table I were dispersed in Roswell Park Memorial Institute (RPMI) cell culture medium containing 10% fetal bovine serum (FBS); corresponding DLS and MPS measurements are shown in Figure 1 and Figure 2, respectively. The DLS data (Figure 1) also includes RPMI+10% FBS cell culture medium as a control (dashed line), which showed a sharp peak at ~8-10 nm corresponding to serum proteins.
Fig. 1.
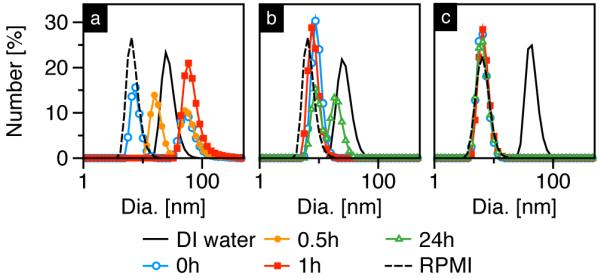
Hydrodynamic size data of SPIONs dispersed in DI water (solid) and its evolution in 10% FBS-RPMI cell culture medium over time. SPIONs were coated with (a) PMAO-5kPEG[20], (b) PMAO-5kPEG[30] and (c) PMAO-20kPEG[30].
Fig. 2.
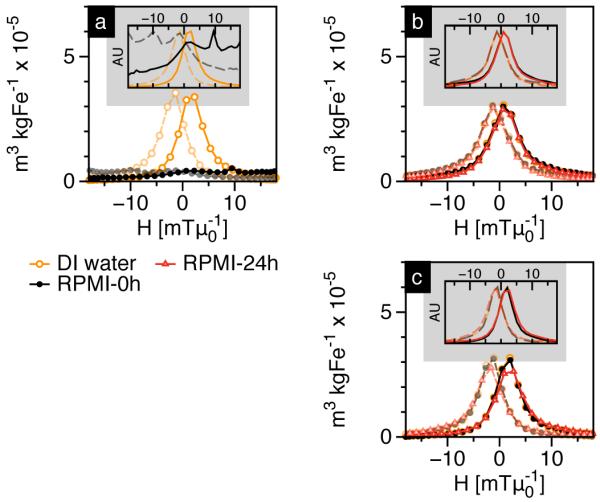
MPS measurements showing mass normalized m’(H) plots of SPIONs coated with (a) PMAO-5kPEG[20], (b) PMAO-5kPEG[30] and (c) PMAO-20kPEG[30], dispersed in 10% FBS-RPMI cell culture medium. Dashed lines indicate the m’(H) plot as the MPS drive field reverses direction. Insets show intensity normalized plots for clearer comparison of peak widths.
SPIONs coated with PMAO-5kPEG[20] showed an increase in dH immediately following dispersion in RPMI+10% FBS (Figure 1a); furthermore, the peak corresponding to serum proteins progressively decreased and eventually disappeared 1 hour post-RPMI, which suggests proteins adsorbed to SPIONs and subsequently induced aggregation. On the other hand, even 24 hours post-dispersion in RPMI+10% FBS, SPIONs coated with either PMAO-5kPEG[30] or PMAO-20kPEG[30] showed little (Figure 1b) or no change (Figure 1c) in hydrodynamic diameter. Note that the peaks corresponding to SPIONs dispersed in DI water (solid lines in (b) and (c)) are not visible after dispersion in RPMI+10% FBS medium because the DLS data shown here is number-weighted – since the total number of proteins is far greater than the total number of SPIONs, the protein peak dominates. The opposite is true in an intensity-weighted distribution (data not shown), which scales as dH6 and thus only shows peaks corresponding to SPIONs or larger aggregates. The above results indicate that at similar surface densities, a 20 kDa PEG preserves the stability of SPIONs in serum-rich medium slightly longer than a 5kDa PEG. In theory, a 20 kDa PEG can form four times the number of hydrogen bonds than a 5 kDa PEG, which can significantly improve SPION solubility in aqueous environments; as a result, the hydrophobic core is better protected from protein adsorption. On the other hand, SPIONs coated with 5 kDa PEG show significant improvement in colloidal stability when the surface density is increased from 20 to 30 PEGs per PMAO, suggesting a higher density coating does a better job of impeding protein adsorption to the SPION core’s hydrophobic surface.
MPS measurements (Figure 2) showed that the relaxation properties of SPIONs are coupled to their colloidal stability. Particularly, SPIONs coated with PMAO-5kPEG[20], which aggregated significantly in RPMI+10% FBS medium, showed a significant loss in MPS (Figure 2a). Our results (inset in Figure 2a) indicate that SPION aggregation broadens the m’(H) response. Aggregated SPIONs can interact magnetically, which broadens the distribution of energy barriers, and consequently the switching field for magnetization reversal [16], [17]. In comparison, SPIONs coated with PMAO-5kPEG[30] and PMAO-20kPEG[30] are relatively stable in RPMI+10% FBS medium (Figure 1b and 1c), which translates to a sustained MPS signal even 24 hours after exposure to the serum-rich environment.
B. Blood half-life
We measured the blood half-life of two SPION formulations (Table II) in 8-10 week old female CD-1 mice. In order to study the effect of hydrodynamic size on blood half-life, we selected two samples coated with PMAO-5kPEG[30], but with significantly different hydrodynamic diameters – sample A’s larger hydrodynamic size was attributed to the presence of SPION clusters formed during the phase transfer process. In order to ensure the characterization of blood half-life was solely a result of the differences in hydrodynamic diameter, the m’(H) properties were characterized in our 25 kHz MPS – both samples showed a nearly identical peak intensity and FWHM; thus, any impact of MPS signal on blood half-life characterization can be neglected.
TABLE II.
Characteristics of SPIONs coated with PMAO-5kPEG[30] in deionized water
| Sample ID | dc nm (σ) | dH nm (PDI) |
m’(H) × 10−2 |
|
|---|---|---|---|---|
| Peak int.: m3kgFe−1 |
FWHM: mTμ0−1 |
|||
| A | 17 (0.20) | 86 (0.18) | 1.3 | 9.9 |
| B | 20 (0.14) | 42 (0.14) | 1.4 | 9.9 |
For practical relevance to MPI, we measured and quantified the m’(H) signal of SPIONs in our MPS. The data gathered over various time points was fit to a one-compartment pharmacokinetic model:
| (1) |
where C(t) is the SPION concentration as a function of time, C0 is the initial concentration, R is a constant that defines the clearance rate of SPIONs, and t is the time elapsed post-injection. The blood half-life is the time (t1/2) when SPION concentration reaches 50% of the initial concentration.
Figure 3 shows that t1/2 of sample B was approximately 5 times longer than sample A. Clustered SPIONs in sample A have a larger hydrodynamic diameter than individually coated SPIONs; as a result, SPIONs in sample A, compared to sample B, were quickly cleared out of circulation, most likely due to opsonization and taken up in the mononuclear phagocytic system. Previously performed biodistribution studies [15] showed that intravenously administered SPIONs were sequestered to the liver and spleen – sites of high mononuclear phagocytic activity.
Fig. 3.
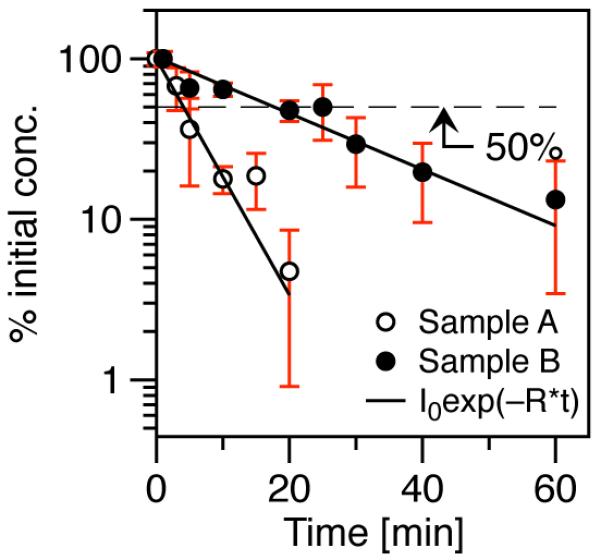
Blood half-life (t1/2) of SPIONs with different hydrodynamic diameters in female CD-1 mice. Sample A (dH = 86 nm): R = 0.17 min−1, t1/2 = 4 min. Sample B (dH = 42 nm): R = 0.036 min−1, t1/2 = 19 min
It should be noted that the blood half-life study presented here is a comparison of SPIONs with similar coatings but different hydrodynamic diameters – the latter arising from formation of clustered SPIONs, which is often a consequence of the phase transfer process. For comparison, TEM analysis of Resovist® SPIONs showed an average core diameter of ~5 nm [18], but DLS data (Table I) showed the presence of large clusters; consequently, Resovist® showed almost immediate clearance from the blood [15]. In order to prolong t1/2 for applications in cardiovascular and blood pool imaging, surface coatings, and the subsequent coating process, must be optimized to eliminate SPION clustering. We have optimized the phase transfer process to eliminate cluster formation. We are currently tuning the PEG surface density and molecular weight of individually coated SPIONs to optimize the blood half-life of MPI tracers. In addition to cardiovascular and blood pool imaging applications, SPIONs with blood half-life values of 1 hour or longer will also improve disease-specific targeting of functionalized MPI tracers.
IV. Conclusions
We studied the role of PEG-based surface coatings on the in vitro and in vivo MPI performance of our monodisperse SPIONs. Characterization of the colloidal stability and m’(H) response of SPIONs dispersed in serum-rich cell culture medium showed that the two are related. Formation of aggregates due to protein adsorption adversely affected the m’(H) response of SPIONs coated with a 5kDa PEG; however, increasing the PEG surface density by 50%, or the PEG molecular weight to 20kDa significantly improved SPION stability and preserved m’(H) response even 24 hours after dispersion in cell culture medium. Circulation studies in female CD-1 mice showed that the presence of clustered SPIONs can dramatically reduce blood half-life.
Acknowledgments
This work was supported by NIH grants 2R42EB013520-02A1 and 1R01EB013689-01/NIBIB
VI. References
- [1].Krishnan KM. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE T. Magn. 2010;46(no. 7):2523–2558. doi: 10.1109/TMAG.2010.2046907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferguson RM, Minard KR, Khandhar AP, Krishnan KM. Optimizing magnetite nanoparticles for mass sensitivity in magnetic particle imaging. Med. Phys. 2011;38(no. 3):1619–1626. doi: 10.1118/1.3554646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferguson RM, Khandhar AP, Arami H, Hua L, Hovorka O, Krishnan KM. Tailoring the magnetic and pharmacokinetic properties of iron oxide magnetic particle imaging tracers. Biomed. Tech. 2013;19:1–15. doi: 10.1515/bmt-2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferguson RM, et al. (in preparation)
- [5].Chantrell RW, Popplewell J, Charles SW. Measurements of Particle Size Distribution Parameters in Ferrofluids. IEEE T. Magn. 1978;14(no. 5):975–978. [Google Scholar]
- [6].Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cesta M. Normal Structure, Function, and Histology of the Spleen. Toxicologic Path. 2006;34(no. 5):455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- [8].MacDonald IC, Ragan DM, Schmidt EE, Groom AC. Kinetics of red blood cell passage through interendothelial slits into venous sinuses in rat spleen, analyzed by in vivo microscopy. Microvascular Research. 1987 doi: 10.1016/0026-2862(87)90011-2. [DOI] [PubMed] [Google Scholar]
- [9].Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in Lipid Research. 2003;42(no. 6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- [10].Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012;14(no. 1):1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- [11].Almeida JPM, Chen AL, Foster A, Drezek R. In vivo biodistribution of nanoparticles. Nanomedicine. 2011;6(no. 5):815–835. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- [12].Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010;49(no. 36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- [13].Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(no. 1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [14].Khandhar AP, Ferguson RM, Simon JA, Krishnan KM. Tailored magnetic nanoparticles for optimizing magnetic fluid hyperthermia. J. Biomed. Mater. Res. 2011;100(no. 3):728–737. doi: 10.1002/jbm.a.34011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khandhar AP, Ferguson RM, Arami H, Krishnan KM. Monodisperse magnetite nanoparticle tracers for in vivo magnetic particle imaging. Biomaterials. 2013;34(no. 15):3837–3845. doi: 10.1016/j.biomaterials.2013.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chantrell RW, Coverdale GN, El Hilo M, O’grady K. Modelling of interaction effects in fine particle systems. J. Magn. Magn. Mater. 1996;157:250–255. [Google Scholar]
- [17].Mørup S, Hansen MF, Frandsen C. Magnetic interactions between nanoparticles. Beilstein J. Nanotechnol. 2010;1:182–190. doi: 10.3762/bjnano.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eberbeck D, Wiekhorst F, Wagner S, Trahms L. How the size distribution of magnetic nanoparticles determines their magnetic particle imaging performance. Appl. Phys. Lett. 2011;98(no. 18):182502. [Google Scholar]


