Abstract
Hypothesis
Widespread use of computed tomography and ultrasound has led to the identification of increasing numbers of patients with asymptomatic cystic lesions of the pancreas.
Design
Retrospective case series of patients with pancreatic cystic lesions.
Setting
University-affiliated tertiary care referral center.
Patients
Two hundred twelve patients with pancreatic cystic lesions seen in our surgical practice during 5 years (April 1997-March 2002).
Main Outcome Measures
Presence or absence of symptoms, cyst size and location, cytologic or pathologic diagnosis, surgical treatment, and outcome.
Results
Seventy-eight (36.7%) of 212 patients were asymptomatic. Incidental cysts were smaller (3.3 ± 1.9 vs 4.6 ± 2.7 cm; P<.001) and were found in older patients (65 ± 13 vs 56 ± 15 years; P<.001). Seventy-eight percent of the asymptomatic patients and 87% of those with symptoms underwent surgery, with a single operative death in the entire group (0.5%). Seventeen percent of asymptomatic cysts were serous cystadenomas; 28%, mucinous cystic neoplasms; 27%, intraductal papillary mucinous neoplasms; and 2.5%, ductal adenocarcinomas. The respective numbers for symptomatic cysts were 7%, 16%, 40%, and 9%. Ten percent of asymptomatic patients had a variety of other cystic lesions, and in 12%, no definitive cytologic or pathologic diagnosis was obtained. Overall, 17% of asymptomatic patients had in situ or invasive cancer, and 42% had a premalignant lesion. When evaluated as a function of size, only 1 (3.5%) of 28 asymptomatic cysts smaller than 2 cm had cancer compared with 13 (26%) of 50 cysts larger than 2 cm (P = .04). The proportion of premalignant lesions, however, remained high in both groups (46% and 38%, respectively). Pseudocysts comprised only 3.8% of asymptomatic cysts compared with 19.4% of symptomatic cysts (P = .003).
Conclusions
Incidental pancreatic cysts are common, occur in older patients, are smaller than symptomatic cysts, and are unlikely to be pseudocysts. More than half of them are either malignant or premalignant lesions and therefore cannot be dismissed.
Ultrasound and computed tomography (CT) may reveal incidental findings within the abdomen when performed for other conditions or situations. Cysts of the liver and kidney are examples of such findings1,2 but are frequently dismissed because they are so common and almost universally benign. Cystic lesions of the pancreas are more unusual, and when present, are generally associated with symptoms. However, as imaging has become more widespread and used to screen individuals with trivial or no symptoms, predictably, a larger number of incidental pancreatic cysts is being found.
In the last 20 years, there has been an increased awareness of cystic tumors of the pancreas.3–7 The distinction of serous cystadenomas from mucinous cystic neoplasms, the recognition of the solid pseudopapillary neoplasms and several other rare pancreatic cystic tumors, and more recently, the emergence of intraductal papillary mucinous tumors (IPMTs), has brought a new dimension to a field that was once only the subject of case reports. The malignant potential of many of these tumors is well accepted, and this concern has led to an increasing number of resections for cystic lesions.8
At present, there are no guidelines for the management of asymptomatic pancreatic cysts. The purpose of this study is to describe the frequency and clinicopathologic characteristics of incidental pancreatic cysts in a cohort of patients with cystic lesions of the pancreas and to compare them with symptomatic patients.
METHODS
The medical records of 212 consecutive patients with pancreatic cystic lesions seen in our surgical practices during a 5-year period (April 1997-March 2002) were retrospectively reviewed. The absence or presence of symptoms and their type were recorded. Patients were considered to have incidental pancreatic cysts if the cysts were discovered during a work-up for a different medical problem, even though some of these patients acknowledged symptoms that could potentially be related to the pancreatic cystic lesion. These symptoms were typically trivial or of low intensity and were not the reason for the imaging study.
The findings and type of imaging and diagnostic modalities employed in the work-up of these lesions were recorded as well as size, location, and cytologic or pathologic diagnosis. Lesions were considered to be malignant whenever carcinoma in situ or invasive cancer was present, potentially malignant when the diagnosis was that of IPMT, mucinous cystic neoplasm, solid pseudopapillary tumor, or neuroendocrine tumor of the pancreas (even if the final diagnosis was that of adenoma or borderline), or benign when the diagnosis was a serous cystadenoma, lymphangioma, pseudocyst, or other nonmalignant condition. Surgical treatment and its outcomes were also recorded. For patients who did not undergo surgery, the presumed diagnosis and long-term follow-up were obtained.
Results are presented as mean ± SD. Comparisons were made between patients with incidental pancreatic cysts and those with symptomatic lesions, and the groups were further divided according to cyst size (≤2 cm vs >2 cm). Statistical methods included the χ2 for discrete variables and the t test for continuous variables. A P value less than .05 was considered statistically significant.
RESULTS
Of the 212 patients, 78 (36.7%) were asymptomatic. Their characteristics and those of patients with symptomatic cysts are presented in Table 1s. There was no difference in sex, but patients with incidental cysts were almost 10 years older, had smaller cysts, and were less likely to have the cyst located in the head of the pancreas.
Table 1.
Characteristics of 212 Patients With Cystic Lesions of the Pancreas
| Characteristic | Symptomatic Cysts | Incidental Cysts | All Patients | P Value* |
|---|---|---|---|---|
| No. of patients | 134 | 78 | 212 | |
| Sex, % female | 63.4 | 64.1 | 63.6 | .96 |
| Age, mean ± SD, y | 56 ± 14.7 | 65 ± 12.9 | 59 ± 14.7 | <.001 |
| Cyst size, mean ± SD, cm | 4.6 ± 2.7 | 3.3 ± 1.9 | 4.2 ± 2.6 | <.001 |
| Cyst location, % | .003† | |||
| Head | 45 | 31 | 40 | |
| Uncinate | 3 | 8 | 5 | |
| Neck/proximal body | 19 | 28 | 22 | |
| Distal pancreas | 29 | 33 | 31 | |
| All pancreas | 4 | 0 | 2 |
P value compares symptomatic vs incidental cysts.
Comparison is among all locations.
MODE OF DETECTION OF INCIDENTAL CYSTS
The 78 incidental pancreatic cysts were identified by CT (68 cysts [77%]), transabdominal ultrasound (13 [17%]), magnetic resonance imaging, or endoscopic ultrasound (EUS) (the latter performed to investigate esophageal or gastric abnormalities). The indications for these studies were varied. A third of the cases had gastrointestinal problems, such as follow-up for colon cancer, cholelithiasis, liver cysts, appendicitis, or constipation. Other common indications stemmed from renal, gynecologic, or vascular problems. In 4 cases, cysts were found in healthy, asymptomatic individuals during medical check-ups that included imaging procedures. Figure 1 and Figure 2 show examples of incidental pancreatic cysts identified by CT.
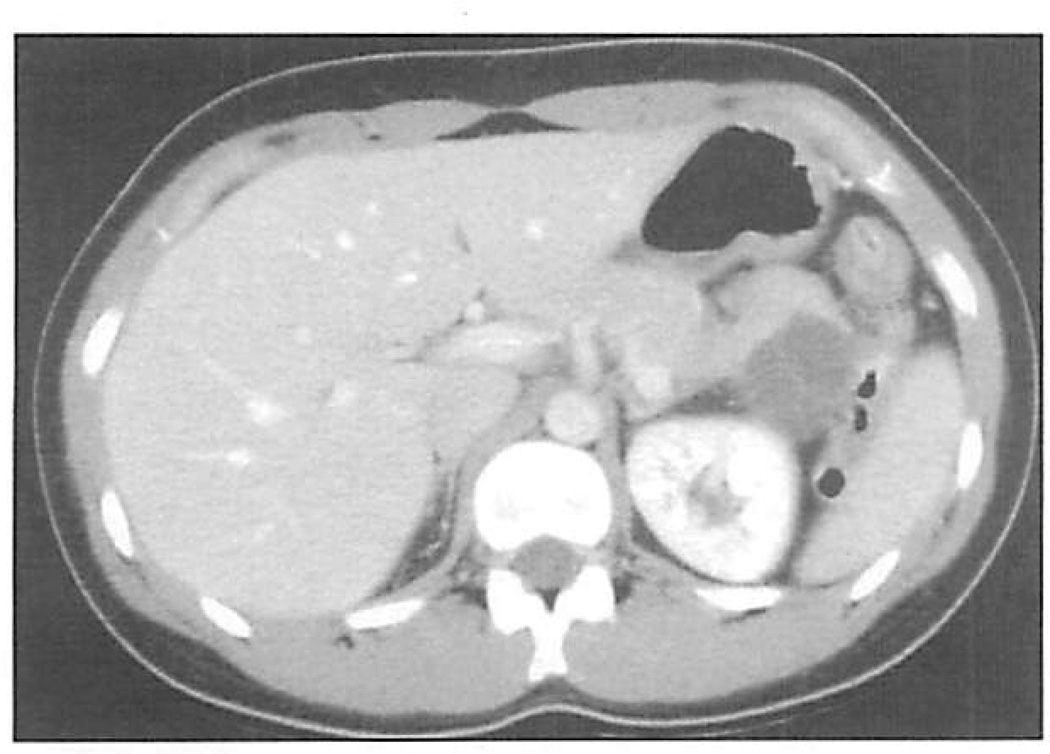
Figure 1.
Computed tomographic (CT) scan of a 24-year-old woman, demonstrating a 4.5-cm septated cyst in the tail of the pancreas. The CT scan was taken for right flank pain related to urolithiasis. She was asymptomatic from a gastrointestinal standpoint. Pathologic testing showed that this was a mucinous cystic neoplasm.
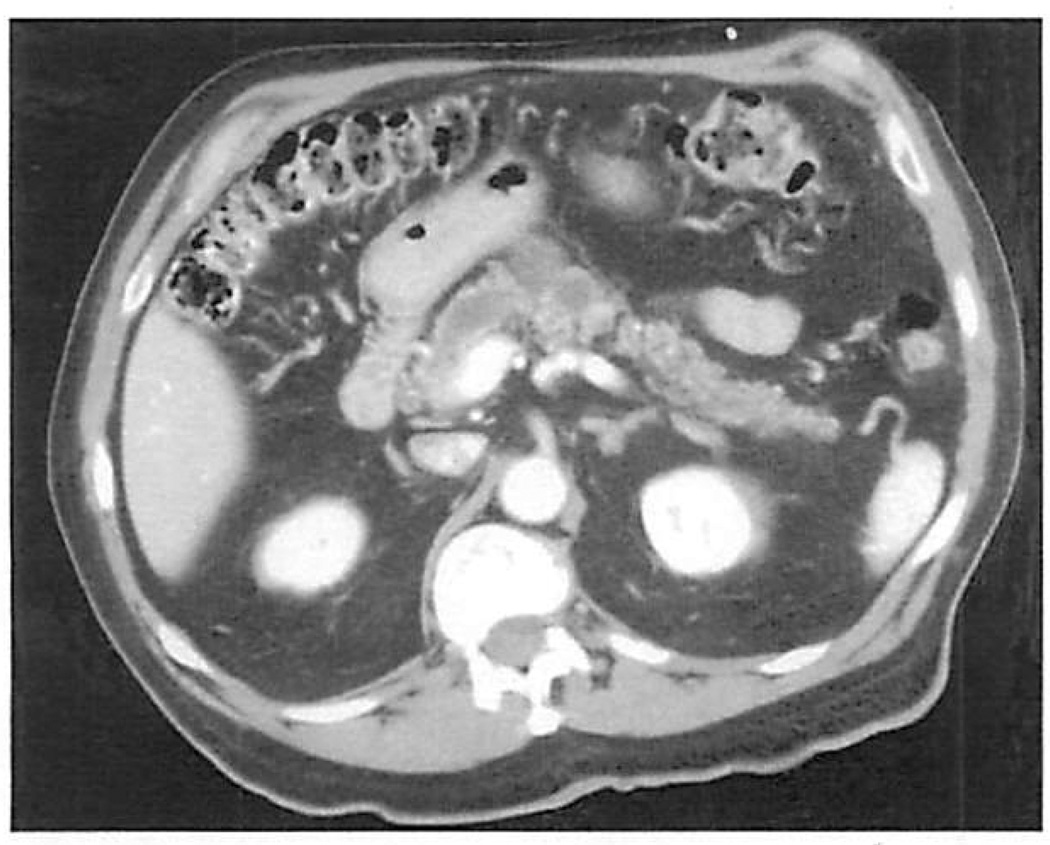
Figure 2.
Computed tomographic scan of a 72-year-old man, demonstrating cystic dilation of the pancreatic duct. The lesion was initially discovered by ultrasound done for intermittent right upper quadrant abdominal pain: he also had gallstones. The lesion was an intraductal papillary mucinous tumor with carcinoma in situ.
SYMPTOMATIC CYSTS
The symptoms experienced by the 134 patients with symptomatic cysts are presented in Table 2. The most common symptoms were abdominal pain and weight loss, although 18% of patients had jaundice, and 5% had a palpable mass. The range of duration of symptoms was from 2 days to more than 15 years, with a median of 12 weeks.
Table 2.
Signs and Symptoms in 134 Patients With Symptomatic Pancreatic Cysts
| Sign/Symptom | Percentage of Patients |
|---|---|
| Abdominal pain | 69 |
| Weight loss | 38 |
| Pancreatitis | 36 |
| Back pain | 18 |
| Jaundice | 18 |
| Palpable mass | 5 |
| Postprandial fullness | 4 |
Forty-eight patients (36%) with symptomatic cysts had a history of pancreatitis; yet, only 23 of them had a final diagnosis of a pseudocyst. The other 25 had neoplastic cysts, mostly IPMTs (18 patients) but also mucinous cystic neoplasms (5 patients), ductal adenocarcinoma (1 patient), and serous cystadenoma (1 patient). Eleven (44%) of those patients who had pancreatitis and a neoplastic cyst were initially diagnosed as having a pseudocyst.
PATHOLOGIC DIAGNOSIS AND SURGICAL TREATMENT
The final diagnosis of the 212 patients is shown in Figure 3. The most common pathologic diagnosis was IPMT, accounting for 36% of all lesions. Of the 75 patients with IPMTs, 40 (53%) had in situ or invasive cancer, 21 (28%) were borderline, and the remaining had adenomas. The second most common diagnosis was mucinous cystic neoplasm. Of the 43 patients with this pathologic finding, 11 (26%) had cystadenocarcinoma (including in situ tumors), 10 (23%) had borderline tumors, and the rest had adenomas. Fourteen patients (6.6%) had ductal pancreatic adenocarcinoma presenting as a cystic lesion. This was either from a retention cyst from pancreatic duct obstruction, or the cystic appearance could have been a result of extensive necrosis within the tumor.
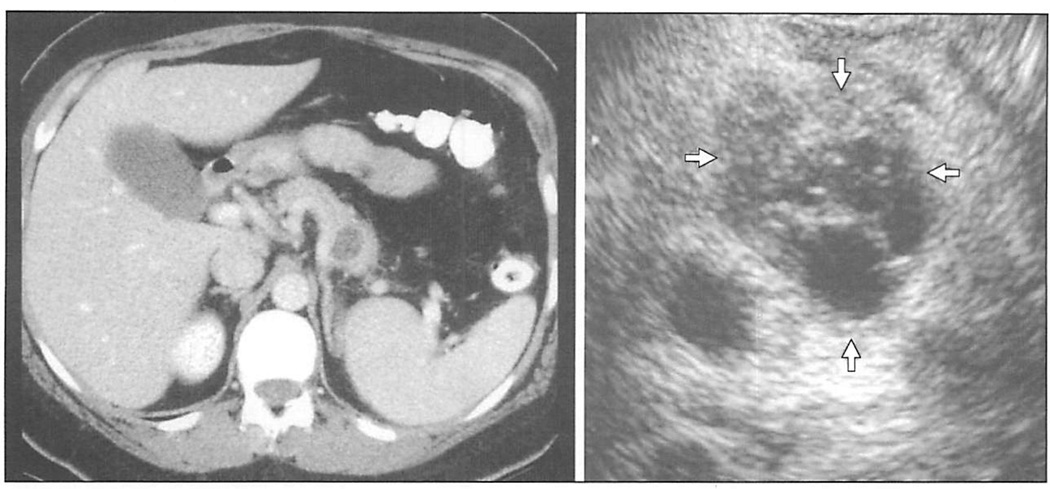
Figure 3.
Computed tomographic scan and endoscopic ultrasound of a 53-year-old woman with an incidentally discovered cyst in the tail of the pancreas. Endoscopic ultrasound provided additional morphologic detail, demonstrating septae within the cyst (arrows). It also allowed for the sampling of cyst fluid, which was positive for mucin, and had an elevated carcinoembryonic antigen level. The lesion was resected and found to be a mucinous cystic neoplasm with borderline features.
Table 3 presents a comparison of pathologic diagnosis and surgical intervention between incidental and symptomatic pancreatic cysts. Overall, both groups had similar distribution of the various cystic lesions, with the exception of pancreatic pseudocysts. These accounted for 19.4% of cystic lesions in symptomatic patients, but only 3.8% of those found incidentally (P = .003). Seventy-eight percent of patients in the incidental group and 87% of those with symptomatic cysts underwent surgery. The only operative mortality (1 [0.5%] of 178) was a patient who died of pulmonary embolism 9 days following pancreatoduodenectomy for a symptomatic serous cystadenoma.
Table 3.
Pathologic Diagnosis and Surgical Intervention in 212 Pancreatic Cysts
| Symptomatic (n = 134) |
Incidental (n = 78) |
P Value |
|
|---|---|---|---|
| Diagnosis | |||
| IPMT | 54 (40) | 21 (27) | .08 |
| With cancer | 31 (57.4) | 9 (42.8) | … |
| Borderline | 14 (25.9) | 7 (33.3) | … |
| Adenoma | 9 (16.6) | 5 (23.8) | … |
| Mucinous cystic tumor | 21 (15.6) | 22 (28) | .04 |
| With cancer | 7 (33.3) | 4 (18) | … |
| Borderline | 3 (14.8) | 7 (31.8) | … |
| Adenoma | 11 (52.3) | 11 (50) | … |
| Serous cystadenoma | 10 (7.4) | 13 (16.6) | .06 |
| Pseudocyst | 26 (19.4) | 3 (3.8) | .003 |
| Ductal adenocarcinoma | 12 (9) | 2 (2.5) | .12 |
| Other* | 9 (6.7) | 8 (10.2) | .52 |
| All potentially malignant | 40 (29.8) | 33 (42.3) | .09 |
| All malignant lesions | 53 (39.5) | 13 (16.6) | <.001 |
| No diagnosis | 2 (1.5) | 9 (11.5) | .004 |
| Surgery | |||
| Whipple | 55 (41) | 25 (32) | … |
| Distal pancreatectomy | 30 (22.3) | 18 (23) | … |
| Middle pancreatectomy | 7 (5.2) | 9 (11.5) | … |
| Total pancreatectomy | 7 (5.2) | 5 (6.4) | … |
| Enucleation | 1 (0.7) | 2 (2.5) | … |
| Pseudocyst drainage | 12 (9) | 0 | … |
| Exploratory laparotomy | 5 (3.7) | 2 (2.5) | … |
| No surgery | 17 (12.6) | 17 (21.8) | .12 |
Abbreviation: IPMT, intraductal papillary mucinous neoplasm.
Simple cyst: 4; endocrine tumor: 4; retention cyst: 2; chronic pancreatitis: 2; solid and pseudopapillary tumor: 2; lymphangioma: 1; lymphoma: 1; and retroperitoneal cyst: 1.
Thirty-four patients did not undergo surgery. Twenty-three of these underwent successful fine-needle aspiration by either EUS or CT guidance. Based on cytologic findings, the final diagnoses were: pseudocyst (7 patients), mucinous cystic neoplasm or IPMT (6), serous cystadenoma (4), ductal adenocarcinoma (4), lymphoma (1), and neuroendocrine tumor (1). Of the remaining 11 patients, the lesion resolved spontaneously in 3, and 8 are currently being followed up without a definite diagnosis. The 4 patients with ductal adenocarcinoma were found to have advanced disease on further work-up; the patient with the neuroendocrine tumor and the 6 with mucinous lesions either refused surgery or their advanced age and comorbidities precluded surgical intervention.
SIZE AND RISK OF MALIGNANCY
Table 4 presents a breakdown of malignant, potentially malignant, and benign symptomatic and incidental cystic lesions as a function of their size. Cysts that were 2 cm or smaller were malignant in 9 (39%) of 23 symptomatic patients but only in 1 (3.5%) of 28 in the incidental group (P<.005). However, 50% of incidental cysts that were 2 cm or smaller were either IPMTs or MCNs, both of which are considered to be premalignant lesions.
Table 4.
Size and Malignant Potential of Symptomatic and Incidental Pancreatic Cysts
| Size of Cyst, cm |
Symptomatic (n = 134) | Incidental (n = 78) | ||||
|---|---|---|---|---|---|---|
| Malignant | Potentially Malignant | Benign | Malignant | Potentially Malignant | Benign | |
| <2 | 9 | 10 | 4 | 1 | 13 | 14 |
| >2 | 43 | 31 | 37 | 13 | 20 | 17 |
| Total | 52 | 41 | 41 | 14 | 33 | 31 |
ROLE OF EUS
Forty-seven (60%) of 78 patients with incidental cysts and 52 (39%) of 134 patients with symptomatic cysts underwent EUS (Figure 4). A final pathologic diagnosis obtained at the time of surgery was available in 78 of these 99 patients who underwent EUS. Based on this final pathologic diagnosis, the sensitivity and specificity of EUS to diagnose a malignant lesion (ie, invasive or in situ cancer) were 69% and 90%, respectively. There were 6 false-positive EUS diagnoses of malignancy, of which only 1 was based on results of cytologic testing alone (the reading indicated adenocarcinoma, and the final diagnosis showed an IPMT with mild atypia); the other 5 were based on the presence of mucin or mucinous epithelium within the aspirate and on morphologic changes seen during EUS that prompted the diagnosis of a malignant tumor with invasion. The sensitivity rose to 81% if the goal was to identify all malignant and premalignant lesions, but the specificity dropped to 58% in this setting.
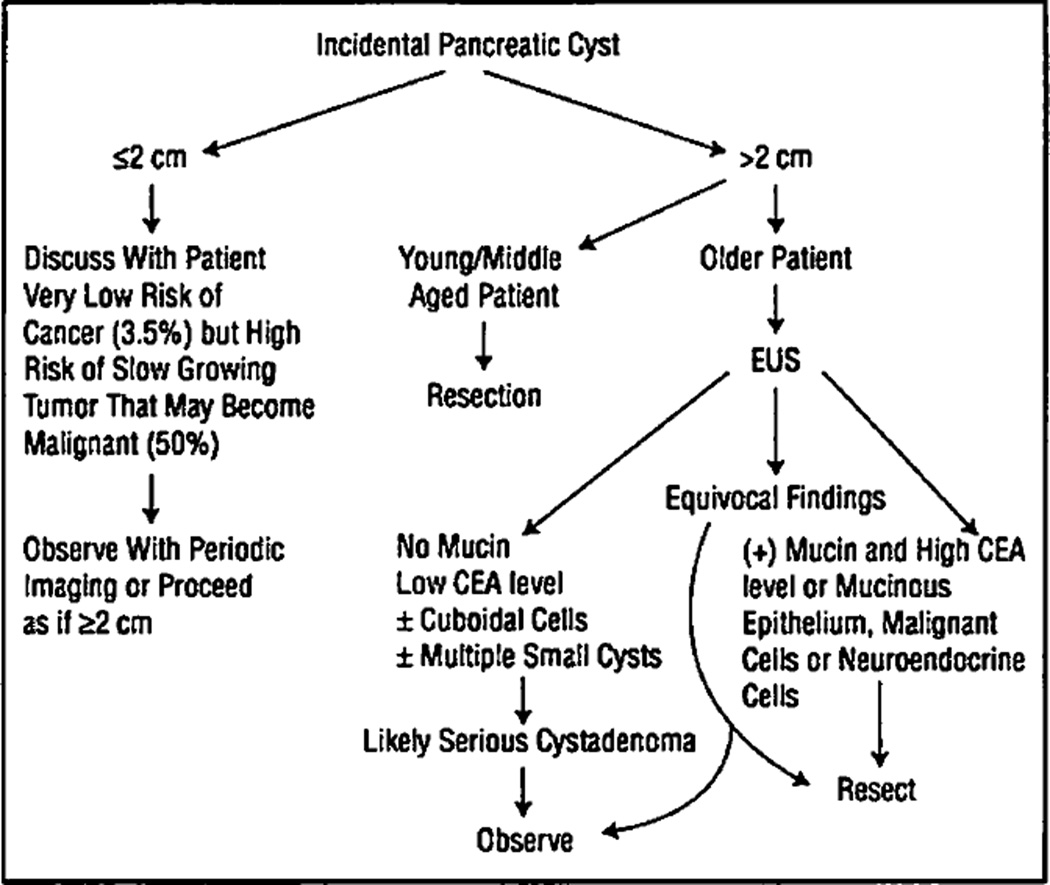
Figure 4.
Algorithm for the management of incidental pancreatic cysts. CEA indicates carcinoembryonic antigen; EUS, endoscopic ultrasound.
Carcinoembryonic antigen in the cystic fluid was available in 72 of the 78 patients who underwent EUS and had a final pathologic diagnosis. At a cutoff level of 20 ng/mL, we found a sensitivity of 82% for the detection of malignant and premalignant lesions, with a specificity of only 30%. The false positives included several pseudocysts, retention cysts, a retroperitoneal cyst, and a single serous cystadenoma. There were 6 false negatives, including 2 mucinous cystadenomas and 4 IPMTs. If the cutoff level is increased to 70 ng/mL, the specificity increases to 64% but the sensitivity decreases to 60%. Another tumor marker, CA 72-2, was available in 39 patients, and the sensitivity and specificity for the detection of malignant or premalignant lesions were 65% and 81%, respectively.
COMMENT
Unexpected findings of diagnostic studies are a source of concern to patients and physicians. This is particularly true when the discovery occurs during a work-up for an unrelated problem, as opposed to during the process of screening. In the latter case, the presence of an abnormality is certainly unsettling but the steps following its discovery are generally well defined (ie, excision following identification of a polyp during colonoscopy, or biopsy when a mammogram shows a suspicious abnormality). In truly incidental findings, however, the subsequent conduct is often unclear. Physicians and/or patients are faced with a decision: they can either ignore the finding or act on it, and this decision-making process is heavily dependent on the available information regarding that particular problem.
To our knowledge, there are no available data on the implications of an incidental pancreatic cyst. While there are several series of cystic tumors of the pancreas reported in the literature (including our own)9–12 and many of them have established that these neoplasms can be asymptomatic, none has addressed the problem from the perspective of the cyst found incidentally. The present study attempted to do so by analyzing a consecutive series of patients from a surgical practice found to have pancreatic cystic lesions. The fact that all of these patients were referred for a surgical opinion has an implicit bias but nonetheless offers insight into the nature of this problem.
More than one third of the 212 patients in this series were asymptomatic. Predictably, cysts in the incidental group were smaller and more commonly located in the uncinate process, neck, or distal pancreas, where symptoms are less likely to occur. The distribution of pathologic diagnoses was not equal. The most marked difference was the diagnosis of pseudocyst in nearly 20% of patients in the symptomatic group compared with only 4% of the asymptomatic patients. Patients with incidental cysts also had a significantly larger proportion of mucinous cystic neoplasms and fewer IPMTs, probably because of the predilection of mucinous cystic neoplasms for the tail of the pancreas. However, the combined numbers of both types of mucin-producing tumors of the pancreas was 55% in both groups. We have no explanation for the finding that patients with incidental pancreatic cysts were nearly 10 years older than their symptomatic counterparts. Even if we had excluded patients with pseudocysts (who were much younger than those with neoplastic cysts), a significant difference would remain.
In an attempt to establish guidelines for the management of incidental cysts, we analyzed pathologic diagnosis as a function of size and presence or absence of symptoms. Even though the subgroup of patients with incidental cysts measuring 2 cm or smaller had a very low risk of cancer (1 [3.5%] of 28), the overall proportion of premalignant lesions was 50% or greater in all subgroups, regardless of size and mode of presentation.
It has been frequently quoted that cystic neoplasms correspond to 10% of all pancreatic cystic lesions, with pseudocysts accounting for the bulk of the reminder.13,14 This study shows almost the opposite, since pseudocysts comprised only 13.6% of cystic lesions and 4% of asymptomatic, incidentally discovered cystic lesions. While referral bias could make our series non-representative of the experience of gastroenterologists or surgeons in other institutions, it is unlikely to account for a big difference. With the current widespread use of radiologic imaging and the detection of increasing numbers of incidental pancreatic cysts, most of them will be neoplastic at the time of detection.
Forty-eight patients in this series had a history of pancreatitis. Conventional teaching states that the presence of a cystic lesion in a patient with a history of pancreatitis suggests a pancreatic pseudocyst. This was not the case in our experience since less than 50% of pancreatitis patients (23 patients) had pseudocyst as their final diagnosis, and the remaining 25 had a neoplasm, mostly IPMT. Recent awareness of IPMT has resulted in increasing numbers of reported cases throughout the world, and this was the most common diagnosis in our series (36%). Misdiagnosing cystic neoplasms for pseudocysts is not uncommon,15 and a history of pancreatitis increases this likelihood. Ten (44%) of the 25 patients with a cystic tumor and prior pancreatitis were initially diagnosed as having pancreatic pseudocysts.
Endoscopic ultrasound has emerged as a very useful tool in the evaluation of pancreatic cystic lesions.16,17 It provides fine detail on the characteristics of the cyst, including the presence of septae, nodules, debris, and thickness of the wall, and allows for the sampling of both its fluid and solid components. It can also identify other lesions in the remaining pancreas, the presence of lymphadenopathy, and vascular involvement. Endoscopic ultrasound was used in nearly 47% of the patients in this series. As expected, a larger proportion of patients in the incidental compared with the symptomatic group underwent EUS (60% vs 39%). The purpose of this study was not to evaluate the role of EUS in cystic lesions, since this is currently being done in a prospective fashion within a multi-institutional effort. Nonetheless, we were able to determine the diagnostic accuracy of EUS-guided biopsy in the 78 patients who had a final pathologic diagnosis, and found that the sensitivity and specificity for the diagnosis of cancer within a cystic lesion were 69% and 90%, respectively.
The physician is more in need of a study that determines if IPMT or a mucinous cystic neoplasm is present rather than if it has become malignant, because based on current thinking, we know that all of these lesions are likely to progress to invasive cancer with time and therefore should be removed if the patient is an acceptable surgical candidate. Cytologic analysis alone does not provide a full answer because the sensitivity and specificity to detect all premalignant lesions is only 56% and 81%, respectively. Fluid can also be examined for amylase and a variety of tumor markers, and we and others have shown that cyst fluid analysis is a useful adjunct in the differential diagnosis of cystic lesions of the pancreas.18–20 At present, CEA is the only tumor marker in cyst fluid that we use on a routine basis for clinical decision-making. Although intermediate levels (20–100 ng/mL) can occasionally be present in pseudocysts, higher levels are almost exclusively seen in mucin-producing lesions, and serous cystadenomas, as a general rule, have low (<20 ng/mL) CEA values. The only serous cystadenoma we have seen with a high CEA level is the one described in this series, where a level higher than 1000 ng/mL was found. We are not aware of any other report in the literature describing such a finding. Unfortunately, almost 20% of mucin-producing lesions will not have an elevated CEA level; thus, there is currently no tumor marker that is accurate enough to distinguish benign from potentially malignant or malignant cysts, and CEA level should only be used in conjunction with results of cytologic testing, EUS, and CT, as well as clinical findings.
Endoscopic ultrasound was also useful in the evaluation of 21 patients who did not undergo surgery. Based on cytologic findings and fluid examination, we were able to establish the diagnosis and nonoperatively manage 7 patients with pseudocysts, 4 with serous cystadenoma, and 1 with a lymphangioma. In addition, the diagnosis of either IPMT or mucinous cystic neoplasm was established in 6 patients and that of ductal adenocarcinoma in 3. These patients either refused surgery or were not operated on because of advanced disease or high surgical risk.
Our current methods of evaluation are not perfect. Nearly 8% of the patients with incidental cysts had non-neoplastic cysts that were removed. These “simple” cysts, or “retention” cysts, were causing no symptoms, and there is no reason to think they would do so in the future. They were removed out of concern that they could represent a malignant or premalignant lesion. In addition, there were 13 serous cystadenomas resected in asymptomatic patients. Again, some of them were removed because a mucinous lesion could not be excluded, but several others were suspected preoperatively of being serous cystadenomas on the basis of morphologic or cytologic findings. The issue of removal of serous cystadenomas is a matter of debate. Even though the risk of malignancy in these tumors is negligible,21,22 and at least one study has shown that development of symptoms does not occur over a 3-year follow-up,9 there is no question that serous cystadenomas can reach very large dimensions and can cause symptoms at a later date. Early resection is therefore advisable in patients with a long life expectancy and good surgical risk.
A recent study found that positron emission tomography was very accurate in discriminating between malignant and benign cystic lesions.23 All but 1 of 17 patients with malignant cystic lesions demonstrated 18-fluorodeoxyglucose uptake, and 38 of 39 patients with benign lesions had no uptake of the tracer. However, more than a third of benign lesions were either mucinous cystic neoplasms or IPMTs, which are considered to be pre-malignant. We have no experience with this modality in cystic lesions of the pancreas, but perhaps this and further refinements in fluid cyst analysis may allow for a better differential diagnosis in the future. For now, treatment of incidental pancreatic cysts needs to be carefully individualized. The treatment of a 24-year-old woman with a 5-cm septated cyst in the tail of the pancreas cannot be the same as that of a 75-year-old with a 2-cm cyst in the head of the pancreas. Whereas in the first case, the surgeon can recommend a distal pancreatectomy with a very high certainty that the lesion is neoplastic and most likely premalignant, in the latter, further evaluation with EUS to rule out a mucin-producing lesion may be appropriate. Figure 4 shows a proposed algorithm for the management of asymptomatic pancreatic cysts. The recommendations assume that pancreatic resection can be done with low morbidity and mortality.
In summary, this study shows that incidental pancreatic cysts currently comprise more than a third of cystic lesions seen in a surgical practice and are very unlikely to be pancreatic pseudocysts. They are significantly smaller than their symptomatic counterparts, and are found in older patients, yet they cannot be dismissed because more than 60% of them will be either malignant or premalignant.
DISCUSSION
Mark Callery, MD, Boston, Mass: This is very, very practical information for the doctors who have to look after these patients. The decisions to recommend and to undergo a pancreatic resection for a solid tumor of the pancreas is usually straight-forward but we have lacked clear information up until this study, in particular, to help patients understand why they need to have such a significant procedure, and you showed one patient who is such a young woman, 28 years old.
From a practical standpoint, not in the operating room but in the outpatient clinical setting, how do you go about explaining to the patient what the lesion means and why they should undergo this operation? This data is practical because it decreases the chance that an uncertain patient has an encounter with an uncertain surgeon which is not a good combination. Why do you think the fine needle cytology for a mucinous lesion still hovers only around 50%?
Dana Andersen, MD, Worcester, Mass: Understanding the natural history and the pathologic implications of these lesions is important. I agree with you, their incidence is dramatically increased over that which was previously understood.
I have 2 questions for you, one of which is the role of endoscopic ultrasound. We have been very impressed that this is indeed perhaps the most helpful technique to image and to aspirate these lesions, and if I understood your data correctly, this was performed in about 60% of your patients. I would ask you specifically if a patient came to you with a small lesion and had had no endoscopic ultrasound, would that be your procedure of choice to further evaluate the lesion?
The second question concerns the issue of size. We have also struggled with the issue of whether a 1- or a 1.5-cm simple unilocular lesion should be resected, particularly when it resides in the head of the pancreas and we have struggled with a size criterion for these otherwise benign-appearing lesions. Your data would suggest that you are using 2 cm in some way to make a decision about these lesions and I would ask you if that is truly a decision point for you.
And then finally the question concerns the cystic lesion nestled in the head of the pancreas. I noticed on your list of operations that no duodenum-preserving resections were listed. We have found this to be a major help for lesions in the head of the pancreas to avoid the morbidity of the Whipple procedure and would ask whether your are considering using those procedures now.
James Hebert, MD, Burlington, Vt: I have a question about using serum tumor markers. I know you all have found in previous work that CA19-9 from the cyst is not helpful in predicting mucinous tumors but other authors and we found this as well, that the serum CA19-9 is elevated in most of these mucinous cystic tumors, even small ones. Could you comment on the value of that in this study?
Nick Perencevich, MD, Concord, NH: In the management of someone who seems to have a classic case of pancreatitis followed by a pseudocyst, is your workup now different? Is that patient going to be getting an endoscopic biopsy and CEA level also?
Dr Fernández-del Castillo: When to operate? Should we operate? Should we do a further workup? That still remains an art and needs to be individualized. If a 24-year-old woman, which is the case I presented, has a 4-cm septated cystic lesion in the tail, that is a neoplasm. If she has no history of pancreatitis, no history of pancreatic trauma, doing an endoscopic ultrasound is a superfluous exercise. She will need to have an operation even though she is incidentally found. This of course will need to be discussed with the patient, and if she feels comfortable with it, that is what we ought to do. Some patients will put more pressure to get more confirmation that this really needs to come out, but there is really nothing in a 24-year-old patient that looks like that in the tail of the pancreas that can stay there and we can be comfortable with, whereas if you see this in a 78-year-old woman and it is in the head of the pancreas and it is smaller, well that is a lesion that you really want to be sure is a mucinous lesion before you go ahead and do a Whipple on a patient with advanced age. That patient is a very good candidate for endoscopic ultrasound to further characterize with more detail what this is. Our methods are not perfect Even endoscopic ultrasound—you know cytology can fail, the markers can fail, the mucin, the amylase. At times we do not reach a diagnosis and then we have to say, well, you know, we still cannot be sure if this is a neoplasm or not and if it is a neoplasm it has to come out. Time will tell if we can find a better marker for that.
Indeed, we used endoscopic ultrasound in 60% of the patients of those patients with incidental findings. We find it is a very useful tool to characterize cystic lesions of the pancreas and to obtain the fluid, but as I mentioned, it is not perfect and we still have a lot to work out in finding better markers and to understand some of the data. Sometimes we have encountered contamination from the endoscopic ultrasound as they go through the gastric mucosa or duodenal mucosa. Then the cytologist can read, “I see some mucinous epithelium and this mucinous epithelium really is not from the inside of the cyst but is coming from the wall of the duodenum,” so we have to be very careful how we interpret this in the right clinical context. Yes, in particular for the small lesions, this becomes a very valuable tool. For the larger lesions, when it is obvious that the patient needs an operation, then why even do a further workup.
The size continues to be a problem and I agree with you. I did not mean to give here an impression that 2 cm is our threshold but we are seeing increasing numbers of very small cystic lesions and of course the smaller they get the harder they will be to characterize, and I don’t think we have full information for that. I start to pay attention to them when they are 2 cm or larger. That is a very artificial number. Something that is 2 cm obviously was smaller perhaps a year or 2 before but lesions that look 1 cm, and now CAT scans are getting so good that they are picking up these little things. Endoscopic ultrasound goes in there and we don’t really get any information from that, so I do not have an answer for that question.
What about the duodenum-preserving resection of the head of the pancreas? That is an operation we do not do. It is a much harder operation. It also has a fair amount of complications, and really the advantages of preserving the duodenum in the absence of the head of the pancreas are not that clear-cut and I do not think warrant that exercise. That has been our experience. We do not do this operation.
In terms of serum tumor markers, we have looked at CA19-9 in the cyst fluid and that is absolutely useless. It is all over the place. It is there in benign lesions, in pseudocysts, in mucinous and serous lesions, so CA19-9 in the fluid of the cyst is not useful. In terms of the serum, that is, in the blood of these patients, I do not have data on CA19-9. I am very interested if you really have seen that patients with mucinous cystic neoplasms have high levels of CA19-9. That would be a very interesting finding. We might be able to retrieve some of that data because some of our patients may have this marker done but I am not aware of any other study addressing this.
In terms of symptomatic patients, in the patient with a history of pancreatitis, yes, just like we were taught that the most common cystic lesion in die pancreas was a pseudocyst, we were also taught that patients who have pancreatitis or have a history of pancreatitis and have a history of a cystic lesion, well, this is a pseudocyst. In fact, that is not always the case and we now know that intraductal papillary mucinous tumors and sometimes mucinous cystic neoplasms and even serous cystadenomas can cause pancreatitis, so the clinician needs to be doubly alert because somebody who has pancreatitis could have had the pancreatitis because he has a neoplasm that can pre-dispose him for that. Unless you have a patient with pancreatitis that you have seen from the beginning and in the beginning had no cysts and then as the course of the disease worsened he developed a pseudocyst, I do not think you can be certain that a history of pancreatitis will equate this with a pancreatic pseudocyst. For that, endoscopic ultrasound can be very useful because of course a pseudocyst will have very high amylase levels and should not have tumor markers so you can start to make some clinical correlation.
Footnotes
This study was presented at the meeting of the New England Surgical Society, Dixville Notch, NH, September 27, 2002, and is published after peer review and revision.
REFERENCES
- 1.Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 1989;62:335–337. doi: 10.1259/0007-1285-62-736-335. [DOI] [PubMed] [Google Scholar]
- 2.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002;167:21–23. [PubMed] [Google Scholar]
- 3.Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas: new clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–445. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson LDR, Becker RC, Prygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathological study of 130 cases. Am J Surg Pathol. 1999;23:1–16. doi: 10.1097/00000478-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Loftus EV, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroentemlogy. 1996;110:1909–1918. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- 6.Pyke CM, van Heerden JA, Colby TV, Sarr MG, Weaver AL. The spectrum of serous cystadenoma of the pancreas. Ann Surg. 1992;215:132–139. doi: 10.1097/00000658-199202000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol. 1988;151:1133–1138. doi: 10.2214/ajr.151.6.1133. [DOI] [PubMed] [Google Scholar]
- 8.Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernández-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 9.Le Borgne J, de Calan L, Partensky C the French Surgical Association. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional restrospective study of 398 cases. Ann Surg. 1999;230:152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-del Castillo C, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am. 1995;75:1001–1016. doi: 10.1016/s0039-6109(16)46742-3. [DOI] [PubMed] [Google Scholar]
- 11.Talamini MA, Pitt HA, Hruban RH, Boitnott JK, Coleman JA, Cameron JL. Spectrum of cystic tumors of the pancreas. Am J Surg. 1992;163:117–124. doi: 10.1016/0002-9610(92)90263-q. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Enjoji M. Cystic neoplasms of the pancreas. Gastroenterology. 1987;92:1934–1943. doi: 10.1016/0016-5085(87)90627-5. [DOI] [PubMed] [Google Scholar]
- 13.ReMine SG, Frey 0, Rossi RL, Munson JL, Braasch JW. Cystic neoplasms of the pancreas. Arch Surg. 1887;122:443–446. doi: 10.1001/archsurg.1987.01400160069010. [DOI] [PubMed] [Google Scholar]
- 14.Cubilla AL, Fitzgerald PJ. Tumors of the Exocrine Pancreas: Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1984. 2nd series. Fascicle 19. [Google Scholar]
- 15.Warshaw AL, Rutledge PL. Cystic tumors mistaken for pancreatic pseudocysts. Ann Surg. 1987;205:393–398. doi: 10.1097/00000658-198704000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brugge WR. The role of endoscopic ultrasound in pancreatic disorders. Int J Pancreatol. 1996;20:1–10. doi: 10.1007/BF02787371. [DOI] [PubMed] [Google Scholar]
- 17.Maguchi H, Osanai M, Yanagawa N, et al. Endoscopic ultrasonography diagnosis of pancreatic cystic disease. Endoscopy. 1998;30(suppl):A108–A110. doi: 10.1055/s-2007-1001488. [DOI] [PubMed] [Google Scholar]
- 18.Sperti C, Pasquali C, Guolo P, Polverosi R, Liessi G, Pedrazzoli S. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer. 1996;78:237–243. doi: 10.1002/(SICI)1097-0142(19960715)78:2<237::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Hammel P, Levy P, Voitot H, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology. 1995;108:1230–1235. doi: 10.1016/0016-5085(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 20.Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mutinous cystadenocarcinoma. Ann Surg. 1993;217:41–47. doi: 10.1097/00000658-199301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimi N, Sugie S, Tanaka T, et al. A rare case of serous cystadenocarcinoma of the pancreas. Cancer. 1992;69:2449–2453. doi: 10.1002/1097-0142(19920515)69:10<2449::aid-cncr2820691011>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Abe H, Kubota K, Mori M, et al. Serous cystadenoma of the pancreas with invasive growth: benign or malignant? Am J Gastroenterol. 1998;93:1963–1966. doi: 10.1111/j.1572-0241.1998.00556.x. [DOI] [PubMed] [Google Scholar]
- 23.Sperti C, Pasquali C, Chierichetti F, Liessi G, Ferlin G, Pedrazzoli S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann Surg. 2001;234:675–680. doi: 10.1097/00000658-200111000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]