Abstract
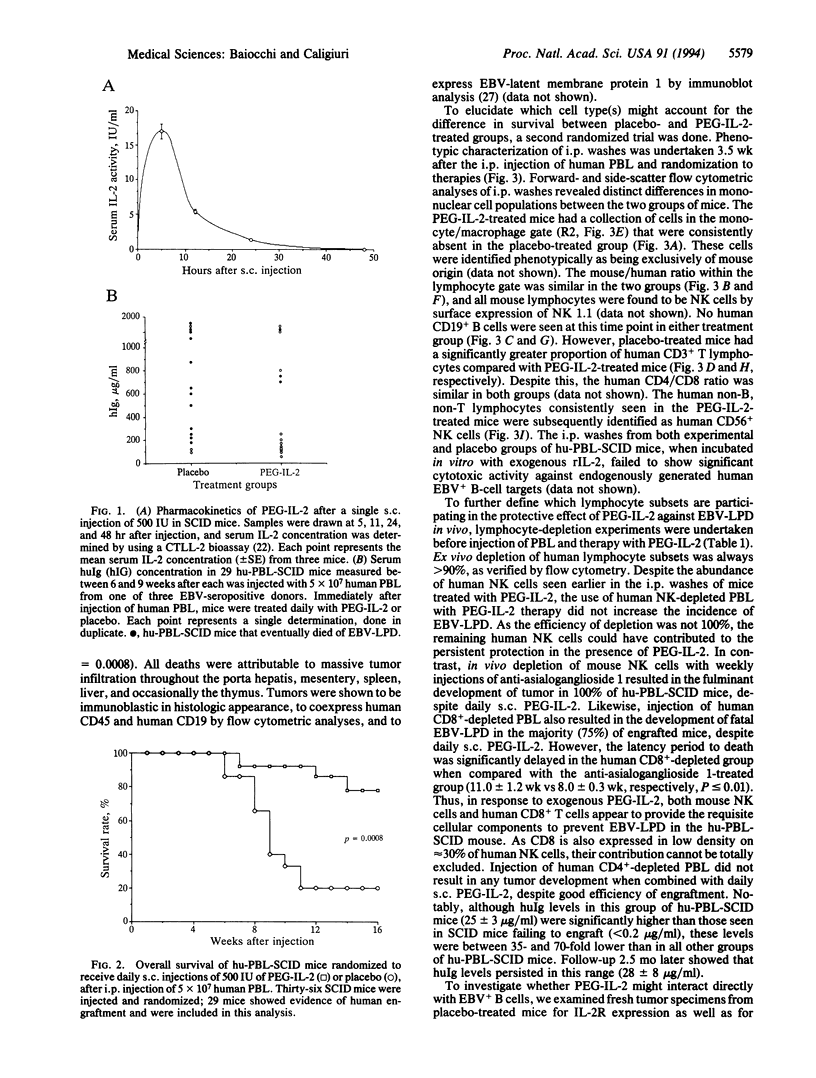
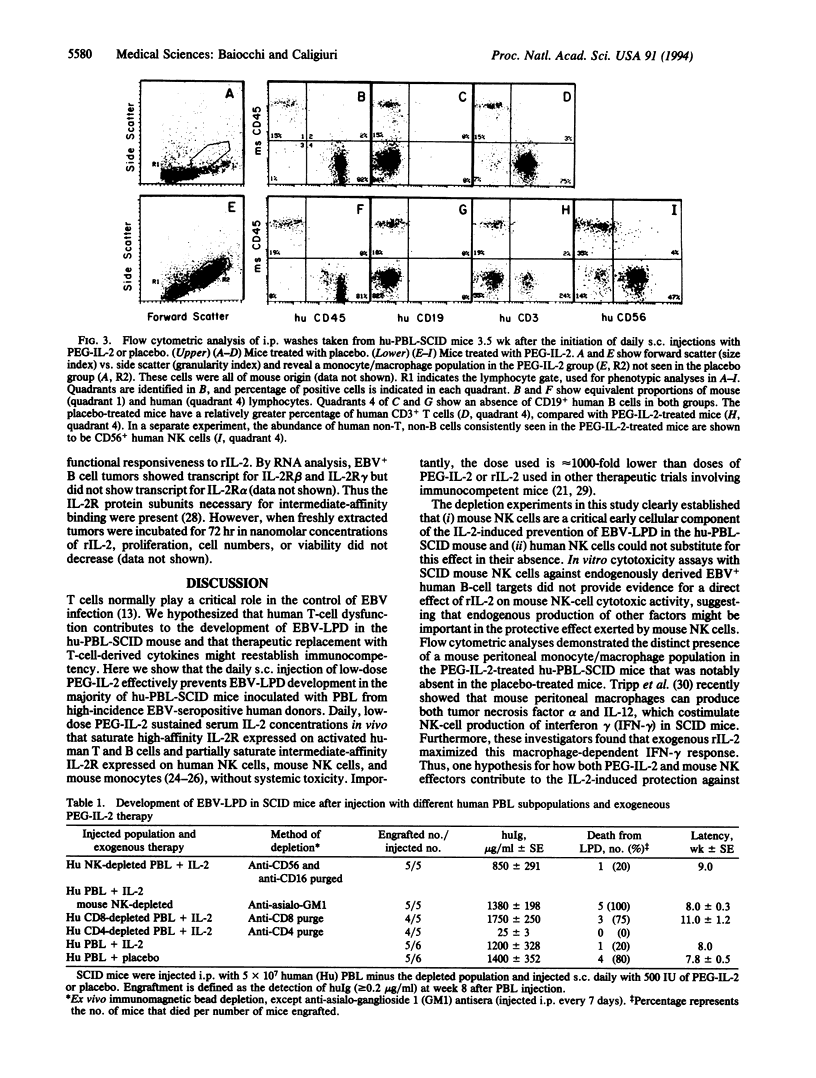
When severe combined immune deficient (SCID) mice undergo i.p. injection with peripheral blood lymphocytes from normal human donors seropositive for EBV, a majority of these mice (hu-PBL-SCID mouse model) subsequently develop a fatal EBV+ lymphoproliferative disease (EBV-LPD) of human B-cell origin. Because T cells normally are critical in the control of EBV infection, we hypothesized that human T-cell dysfunction accounts for EBV-LPD in the hu-PBL-SCID mouse and that systemic administration of T-cell-derived cytokines would reestablish protective immunity against EBV-LPD. We show that the daily s.c. administration of a very low dose (500 international units) of polyethylene glycol-modified recombinant human interleukin 2 (PEG-IL-2) to hu-PBL-SCID mice can prevent the development of fatal EBV-LPD and significantly improves survival (78%), compared with the survival of hu-PBL-SCID mice treated with placebo (20%, P = 0.0008). Additional lymphocyte-depletion experiments showed that mouse natural killer cells and human CD8+ T cells provided cellular immunity necessary for the PEG-IL-2-mediated protective effect, whereas i.p. injection of human peripheral blood lymphocytes depleted of CD4+ T cells had no adverse effect when combined with PEG-IL-2 therapy and may have been beneficial. These data establish that very low-dose PEG-IL-2 therapy can overcome the immune deficiencies that lead to EBV-LPD in the hu-PBL-SCID mouse and point to the usefulness of this model for evaluating cytokine therapies in EBV-LPD. The use of low-dose IL-2 as a preventative immune therapy has potential application in immunocompromised individuals at high risk for EBV-LPD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerstein A., Kedar E., Slavin S. Use of recombinant human interleukin-2 in conjunction with syngeneic bone marrow transplantation in mice as a model for control of minimal residual disease in malignant hematologic disorders. Blood. 1991 Sep 1;78(5):1212–1215. [PubMed] [Google Scholar]
- Bancroft G. J. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993 Aug;5(4):503–510. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- Beral V., Peterman T., Berkelman R., Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991 Apr 6;337(8745):805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. A., Murray C., Soiffer R. J., Klumpp T. R., Seiden M., Cochran K., Cameron C., Ish C., Buchanan L., Perillo D. Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol. 1991 Dec;9(12):2110–2119. doi: 10.1200/JCO.1991.9.12.2110. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Warnke R., Sklar J. Monoclonality of lymphoproliferative lesions in cardiac-transplant recipients. Clonal analysis based on immunoglobulin-gene rearrangements. N Engl J Med. 1984 Feb 23;310(8):477–482. doi: 10.1056/NEJM198402233100801. [DOI] [PubMed] [Google Scholar]
- Gatti R. A., Good R. A. Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer. 1971 Jul;28(1):89–98. doi: 10.1002/1097-0142(197107)28:1<89::aid-cncr2820280117>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Hamilton-Dutoit S. J., Pallesen G., Franzmann M. B., Karkov J., Black F., Skinhøj P., Pedersen C. AIDS-related lymphoma. Histopathology, immunophenotype, and association with Epstein-Barr virus as demonstrated by in situ nucleic acid hybridization. Am J Pathol. 1991 Jan;138(1):149–163. [PMC free article] [PubMed] [Google Scholar]
- Ho M., Jaffe R., Miller G., Breinig M. K., Dummer J. S., Makowka L., Atchison R. W., Karrer F., Nalesnik M. A., Starzl T. E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988 Apr;45(4):719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Kamel-Reid S., Dick J. E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988 Dec 23;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Katre N. V. Immunogenicity of recombinant IL-2 modified by covalent attachment of polyethylene glycol. J Immunol. 1990 Jan 1;144(1):209–213. [PubMed] [Google Scholar]
- Katre N. V., Knauf M. J., Laird W. J. Chemical modification of recombinant interleukin 2 by polyethylene glycol increases its potency in the murine Meth A sarcoma model. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1487–1491. doi: 10.1073/pnas.84.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf M. J., Bell D. P., Hirtzer P., Luo Z. P., Young J. D., Katre N. V. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J Biol Chem. 1988 Oct 15;263(29):15064–15070. [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M. E., Schnier G. S., Beecher M. S., Ashman L. K., William D. E., Caligiuri M. A. Expression of a functional c-kit receptor on a subset of natural killer cells. J Exp Med. 1993 Sep 1;178(3):1079–1084. doi: 10.1084/jem.178.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Pahwa R., Chatila T., Pahwa S., Paradise C., Day N. K., Geha R., Schwartz S. A., Slade H., Oyaizu N., Good R. A. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5069–5073. doi: 10.1073/pnas.86.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchio G. R., Kobayashi R., Kirven M., Baird S. M., Kipps T. J., Mosier D. E. Heterogeneity among Epstein-Barr virus-seropositive donors in the generation of immunoblastic B-cell lymphomas in SCID mice receiving human peripheral blood leukocyte grafts. Cancer Res. 1992 May 1;52(9):2468–2477. [PubMed] [Google Scholar]
- Pluda J. M., Venzon D. J., Tosato G., Lietzau J., Wyvill K., Nelson D. L., Jaffe E. S., Karp J. E., Broder S., Yarchoan R. Parameters affecting the development of non-Hodgkin's lymphoma in patients with severe human immunodeficiency virus infection receiving antiretroviral therapy. J Clin Oncol. 1993 Jun;11(6):1099–1107. doi: 10.1200/JCO.1993.11.6.1099. [DOI] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. Evidence for additional subunits associated to the mouse interleukin 2 receptor p55/p75 complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):11–15. doi: 10.1073/pnas.87.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Thangavelu M., Snyder L., Anastasi J., Le Beau M. M., Kirven M., Picchio G., Mosier D. E., Rowley J. D. Cytogenetic characterization of B-cell lymphomas from severe combined immunodeficiency disease mice given injections of lymphocytes from Epstein-Barr virus-positive donors. Cancer Res. 1992 Sep 1;52(17):4678–4681. [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M. L., Veronesi A., D'Andrea E., Del Mistro A., Indraccolo S., Mazza M. R., Mion M., Zamarchi R., Menin C., Panozzo M. Lymphoproliferative disease in human peripheral blood mononuclear cell-injected SCID mice. I. T lymphocyte requirement for B cell tumor generation. J Exp Med. 1992 Dec 1;176(6):1763–1767. doi: 10.1084/jem.176.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss S. D., Sondel P. M., Robb R. J. Characterization of the interleukin 2 receptors (IL-2R) expressed on human natural killer cells activated in vivo by IL-2: association of the p64 IL-2R gamma chain with the IL-2R beta chain in functional intermediate-affinity IL-2R. J Exp Med. 1992 Aug 1;176(2):531–541. doi: 10.1084/jem.176.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman R. J., Aukerman S. L., Katre N. V., Winkelhake J. L., Young J. D. Schedule dependency of the antitumor activity and toxicity of polyethylene glycol-modified interleukin 2 in murine tumor models. Cancer Res. 1989 Dec 1;49(23):6521–6528. [PubMed] [Google Scholar]



