Abstract
β-thalassemias result from diminished β-globin synthesis and are associated with ineffective erythropoiesis and secondary iron overload caused by inappropriately low levels of the iron regulatory hormone hepcidin. The serine protease TMPRSS6 attenuates hepcidin production in response to iron stores. Hepcidin induction reduces iron overload and mitigates anemia in murine models of β-thalassemia intermedia. To further interrogate the efficacy of an RNAi-therapeutic downregulating Tmprss6, β-thalassemic Hbbth3/+ animals on an iron replete, an iron deficient, or an iron replete diet also containing the iron chelator deferiprone were treated with Tmprss6 siRNA. We demonstrate that the total body iron burden is markedly improved in Hbbth3/+ animals treated with siRNA and chelated with oral deferiprone, representing a significant improvement compared to either compound alone. These data indicate that siRNA suppression of Tmprss6, in conjunction with oral iron chelation therapy, may prove superior for treatment of anemia and secondary iron loading seen in β-thalassemia intermedia. Am. J. Hematol. 90:310–313, 2015. © 2015 The Authors. American Journal of Hematology Published by Wiley Periodicals, Inc.
Introduction
The thalassemias result from a quantitative defect in the production of α- or β-globin chains. β-Thalassemia is caused by the partial or complete lack of β-globin synthesis resulting in a variably severe anemia and/or red blood cell abnormalities. In its most severe form, β-thalassemia major, affected patients have profound secondary iron overload largely due the requirement for chronic red blood cell transfusional support. Patients with β-thalassemia intermedia, a less severe form, do not require chronic transfusions (i.e., nontransfusion dependent thalassemia), but still develop iron overload as a consequence of chronic suppression of the synthesis of the iron regulatory hormone hepcidin by ineffective erythropoiesis [1,2]. In both cases, complications of iron overload, including liver disease, cardiomyopathy, and endocrinopathies, are the most significant contributors to morbidity and mortality in these individuals. A mouse model of β-thalassemia intermedia, Hbbth3/+, has a heterozygous deletion of both the β-minor and β-major hemoglobin genes [3]. Similar to their human counterparts, these animals have moderately severe, transfusion-independent hypochromic, microcytic anemia associated and ineffective erythropoeisis resulting in splenomegaly, hepcidin suppression, and secondary iron overload [4–7].
TMPRSS6 (matriptase-2) is a serine protease expressed predominantly in the liver that down-regulates hepcidin transcription by inhibiting an iron-responsive bone morphogenetic protein-mothers against decapentaplegic (BMP-SMAD) signaling pathway [8]. Germline mutations in TMPRSS6 in both mice and humans result in iron-refractory iron deficiency anemia (IRIDA) due to chronic inappropriate overexpression of hepcidin relative to liver iron stores [9]. Modulating hepcidin expression by targeting the BMP-SMAD signaling pathway in hepatocytes may be an effective modality for treating iron disorders, particularly those associated with a relative hepcidin deficiency or excess. For example, deletion of Tmprss6 in the Hfe−/− mouse model of hereditary hemochromatosis results in elevated expression of hepcidin, leading to an amelioration of the iron overload phenotype [10]. Likewise, induction of hepcidin or iron deficiency in β-thalassemic animals using several different methods not only mitigates the iron overload, but also ameliorates the anemia through an incompletely understood mechanism [11–13]. Most recently, we, and others, have demonstrated that suppression of Tmprss6 mRNA expression with lipid nanoparticle (LNP)-formulated siRNAs or antisense oligonucleotides (ASOs) increases hepcidin, leading to diminished iron uptake and recycling and improved erythropoiesis and anemia in β-thalassemic animals [14,15]. These preclinical data suggest that modulation of hepcidin expression could be a substitute for or an adjunct to iron chelation therapy in patients with thalassemia. To further investigate this possibility, we treated Hbbth3/+ animals with an LNP-formulated RNAi therapeutic targeting Tmprss6 in combination with deferiprone, an orally bioavailable iron chelator [16]. We found that siRNA combined with oral deferiprone therapy is superior to monotherapy with dietary iron deficiency, iron chelator, or Tmprss6 siRNA for the reduction of hepatic iron stores, while still maintaining some of the beneficial effects on anemia.
Methods
Other than as noted below, all mouse strains and methods are identical to those previously described [14]. Mice were born and housed in the barrier facility at Boston Children's Hospital under approved protocols, and were raised on Prolab RMH 3000 diet (Lab Diet, 380 ppm iron) until 6 weeks of age, when the diet was switched to an iron replete (50 ppm) control diet, iron deficient (3–5 ppm) diet, or an iron replete (50ppm) diet with 0.125% (1.25 g/kg) deferiprone (Harlan diet #'s, TD120277, TD120276, and TD120278, respectively). Serum hepcidin levels were measured with a novel murine-specific competitive ELISA [17].
Results
LNP-Tmprss6 siRNA plus oral deferiprone therapy diminishes secondary iron overload in murine β-thalassemia intermedia
We determined whether modulating systemic iron stores by dietary restriction or pharmacological chelation could add to the beneficial effects of Tmprss6 siRNA therapy on Hbbth3/+ anemia and iron phenotypes. To do so, we treated wild type and Hbbth3/+ animals with biweekly LNP-Tmprss6 siRNA injections in conjunction with an iron replete synthetic control diet (50 ppm iron), a low iron diet (3–5 ppm iron), or an iron replete diet containing the orally bioavailable chelator deferiprone (50 ppm iron, 0.125% wt/wt chelator). In wild-type animals, we observed qualitatively similar effects on gene expression and iron metabolism, including Tmprss6 suppression and the induction of systemic iron deficiency, as we did previously (Supporting Information Fig. 1) [14]. As expected, LNP-Tmprss6 treatment of Hbbth3/+ animals on all diets suppressed Tmprss6 mRNA expression and up-regulated serum hepcidin (Fig. 1A,B). Furthermore, treated animals had decreased transferrin saturation and serum iron (Fig. 1C,D), with the largest and most significant changes seen in animals on the diet containing deferiprone. When compared to control siRNA-treated animals on the same diet, nonheme liver iron was diminished only in animals treated with deferiprone (Fig. 1E), possibly due to the relatively low iron (50 ppm), which is only approximately two times the recommended adult murine daily requirement, in the formulated test diets compared to conventional mouse chow (380 ppm iron) that we employed previously [14]. In comparison to the control diet, LNP-Tmprss6 treated animals on either an iron deficient diet or diet with added chelator had lower liver iron levels, with the latter having a greater effect. In Hbbth3/+ animals, this change in liver iron can be visualized on DAB-enhanced Perls' stained tissue sections as decreased periportal hepatocellular iron (Fig. 1F). Total spleen iron (Fig. 1G) decreased significantly in treated Hbbth3/+ animals on the iron replete and low iron diets, but only trended downward in animals fed chlelator-enhanced chow, possibly due to effects on erythroid iron utilization or RBC turnover.
Figure 1.
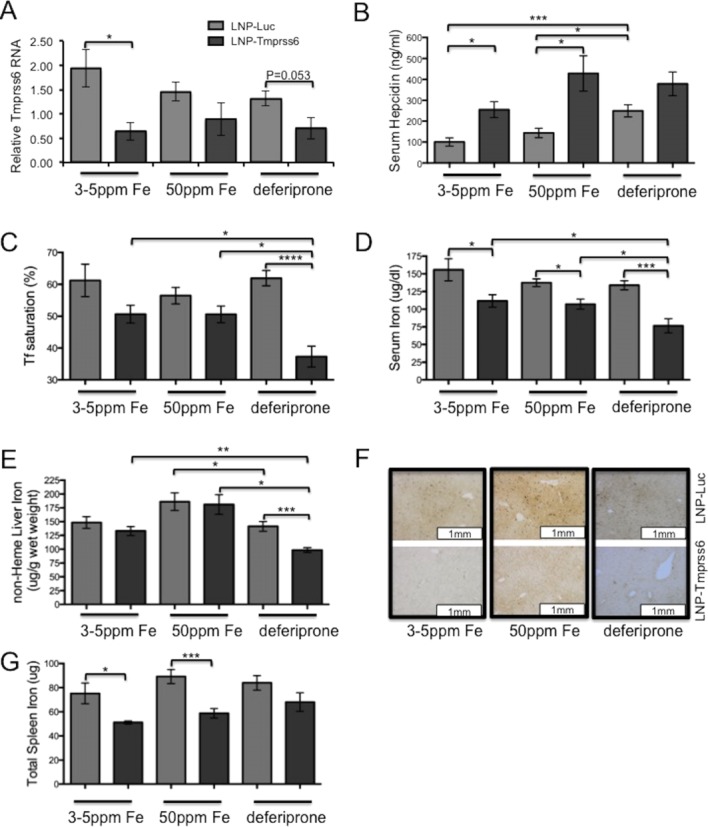
Iron parameters in Hbbth3/+ treated with LNP-Tmprss6 siRNA and dietary iron deficiency or chelation. Six-week old female animals were injected with LNP-siRNA formulations (1 mg/kg) every other week for 6 weeks and then killed 14 days after the final injection. On the day of the first injection, animals were placed on a control (50 ppm iron), iron deficient (3–5 ppm iron), or control diet supplemented with deferiprone (50 ppm iron, 0.125% deferiprone). A: Liver Tmprss6 mRNA assessed by quantitative real-time PCR and normalized to β-actin (Actb). B: ELISA serum hepcidin analysis (ng/ml). C: Serum transferrin saturation (%). D: Serum iron (μg/dl). E: Nonheme liver iron (μg/g). F: 3,3′-diaminobenzidine (DAB)-enhanced iron stain of liver sections. G: Total spleen iron (μg). Error bars are ±SEM. (n = 4−6 for each group) Student's t-test P-values: ****P < 0.001, ***P < 0.005, **P <0.01, and *P < 0.05. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
LNP-Tmprss6 siRNA plus oral deferiprone therapy improves erythropoiesis and splenomegaly in murine β-thalassemia intermedia
In earlier studies, we observed that Tmprss6 siRNA treatment induces a mild microcytic, hypochromic anemia in wild-type animals [14]. We observed the same phenotype here, regardless of the diet employed (Supporting Information Fig. 2). However, in slight contrast to published studies [11], treatment of Hbbth3/+ mice with an iron deficient diet alone did not improve their anemia. Furthermore, with 50 ppm iron in the diet, treatment of Hbbth3/+ mice with Tmprss6 siRNA improved the anemia only when combined with deferiprone therapy (Fig. 2A). Both of these results are also likely attributable to the relatively low baseline levels of iron in the control and chelator-enhanced test diets compared to historical controls. This caveat notwithstanding, other RBC and erythropoietic parameters, characteristic of a response to iron restriction, are seen in LNP-Tmprss6 treated animals on each of the test diets. All experimental siRNA-treated thalassemic groups exhibited an increase in RBC numbers (Fig. 2B) associated with a decreased mean cell volume (MCV, Fig. 2C). These changes are readily appreciated on peripheral blood smears, which demonstrate replacement of the anisopoikilocytosis of the baseline phenotype with a relatively monotonous population of evenly sized and shaped hypochromic microcytes (Fig. 2D).
Figure 2.
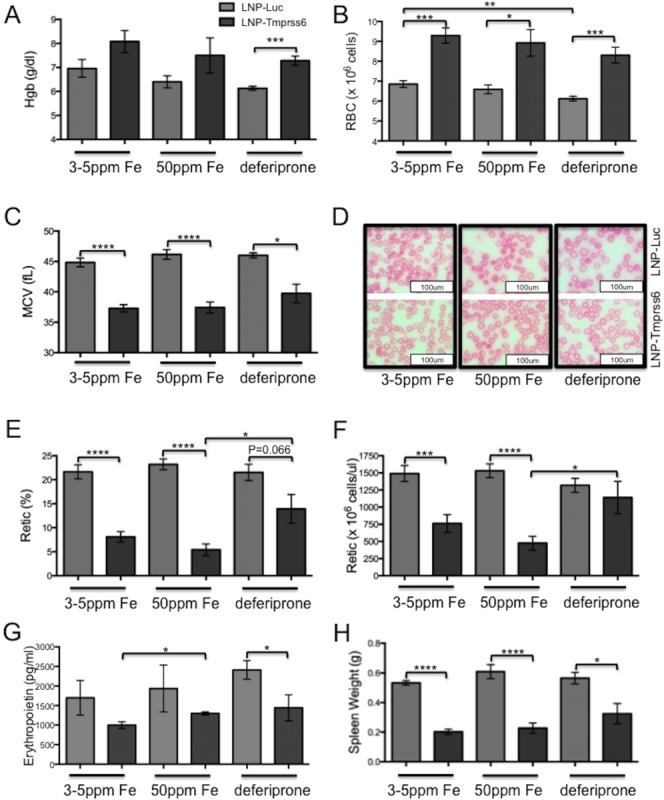
Erythropoietic parameters in Hbbth3/+ animals treated with LNP-Tmprss6 siRNA and dietary iron deficiency or chelation. Animals were treated as in Figure 1. A: Hemoglobin (g/dl). B: Red blood cell count. C: Mean red blood cell volume (fl). D: RBC morphology on Wright's stained peripheral blood smears. E: Fraction of reticulocytes (%). F: Absolute reticulocyte count. G: Serum erythropoietin levels (pg/ml). H: Spleen weight (g). Error bars are ±SEM. (n = 4−6 for each group) Student's t-test P-values: ****P < 0.001, ***P < 0.005, **P <0.01, and *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Despite the relative beneficial effect of the addition of deferiprone to LNP-Tmprss6 therapy on the Hbbth3/+ anemia, some parameters suggest that the addition of chelator at the dose employed mitigates some of the other positive effects of hepcidin-induced iron restriction. For example, while the fraction of RBCs that are reticulocytes (Fig. 2E) is decreased, there is no decrease in the absolute number of reticulocytes (Fig. 2F). Furthermore, although the decrease in serum erythropoietin is most significant in deferiprone-treated animals, changes in spleen size are not as profound as in animals on an iron deficient diet alone (Fig. 2G,H). Finally, consistent with the reduction in splenomegaly, there was partial restoration of splenic architecture in LNP-Tmprss6 treated animals also maintained on deferiprone (Supporting Information Fig. 3)
Discussion
Taken together, these data demonstrate that combined LNP-Tmprss6 siRNA and oral deferiprone chelator therapy is superior in reducing hepatic iron stores compared to dietary iron deficiency with or without LNP-Tmprss6 treatment, or chelator therapy alone. However, in this murine model and at these siRNA and chelator doses, the addition of chelation appears to partially attenuate several of the ancillary beneficial effects, including amelioration of the anemia and splenomegaly, observed in previous studies. Although this could be a direct effect of the chelator on erythropoiesis, it is likely that many of the deviations are due to the more iron-restricted baseline dietary conditions under which the current experiments were conducted. For example, it is possible that the relatively high concentrations of iron found in most mouse chows are inhibitory to thalassemic erythropoiesis, thus worsening the baseline phenotype and exaggerating any positive effects of hepcidin induction therapy. On the other hand, there is also evidence that too much hepcidin and iron restriction is equally detrimental to thalassemic hematological phenotypes [11]. For this reason, it will be prudent ultimately, if this is to be translated to the clinic, to titrate both modalities to each patient to achieve individualized chelation and hematological effects.
In addition to the beneficial effects on the anemia, combination therapy with long-acting hepcidin induction therapy, such as TMPRSS6 siRNA, has several distinct theoretical advantages over chelation alone. First, hepcidin-induced down-regulation of ferroportin sequesters iron in macrophages may limit its toxicity by reducing plasma “free” (e.g., nontransferrin bound) iron, particularly when plasma chelators are at their nadir between doses. Second, the effect of hepcidin on ferroportin expression in duodenal enterocytes responsible for iron absorption limits the ongoing absorption of iron—an effect not achieved by chelators alone. This mechanism of action will likely be critical in individuals with nontransfusional iron overload, where intestinal absorption due to hepcidin suppression is the key pathogenetic mechanism. Nevertheless, even in the transfusion-dependent patient, it has been shown that hepcidin levels, which are ordinarily increased, vary pre- and post-transfusion; hepcidin levels increase after transfusion, presumably due to the suppression of hepcidin synthesis by anemia, hypoxia, and ineffective hematopoiesis that increase as the hemoglobin drops in the interval between transfusions [2,18]. Thus, while the physiological changes induced by hepcidin induction therapy, as with TMPRSS6 siRNA, are ideally suited to the patient with transfusion-independent, iron-loading anemias, they may equally benefit patients on chronic transfusion programs.
Acknowledgments
Authors would like to thank Dean Campagna and Daniel Kierstead for excellent technical assistance and members of the Fleming laboratory for helpful discussions.
Author Contributions
PJS, JSB, KF and MDF conceived and designed the murine experiments; PJS analyzed the mouse data and wrote the manuscript; TR completed the tail injections; and JSB, KF and MDF designed research and wrote the manuscript. MW and colleagues developed and performed the serum hepcidin assays.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Papanikolaou G, Tzilianos M, Christakis JI. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Origa R, Galanello R, Ganz T. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 3.Yang B, Kirby S, Lewis J. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci USA. 1995;92:11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardenghi S, Marongiu MF, Ramos P. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Franceschi L, Daraio F, Filippini A. Liver expression of hepcidin and other iron genes in two mouse models of beta-thalassemia. Haematologica. 2006;91:1336–1342. [PubMed] [Google Scholar]
- 6.Adamsky K, Weizer O, Amariglio N. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124:123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 7.Weizer-Stern O, Adamsky K, Amariglio N. mRNA expression of iron regulatory genes in beta-thalassemia intermedia and beta-thalassemia major mouse models. Am J Hematol. 2006;81:479–483. doi: 10.1002/ajh.20549. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri L, Pagani A, Nai A. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finberg KE, Heeney MM, Campagna DR. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117:4590–4599. doi: 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardenghi S, Ramos P, Marongiu MF. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nai A, Pagani A, Mandelli G. Deletion of TMPRSS6 attenuates the phenotype in a mouse model of beta-thalassemia. Blood. 2012;119:5021–5029. doi: 10.1182/blood-2012-01-401885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Rybicki AC, Suzuka SM. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt PJ, Toudjarska I, Sendamarai AK. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood. 2013;121:1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo S, Casu C, Gardenghi S. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest. 2013;123:1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher SA, Brunskill SJ, Doree C. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database Syst Rev. 2013;8:CD004839. doi: 10.1002/14651858.CD004839.pub3. , et al. [WorldC at] [DOI] [PubMed] [Google Scholar]
- 17.Gutschow P, Schmidt PJ, Han H. A competitive enzyme-linked immunosorbent assay specific for murine hepcidin-1: Correlation with hepatic mRNA expression in established and novel models of dysregulated iron homeostasis. Haematologica. 2014 doi: 10.3324/haematol.2014.116723. , et al. Nov 25. pii: haematol.2014.116723. [Epub ahead of print], in press. [WorldCat] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with beta-thalassemia major: A longitudinal study. Blood. 2013;122:124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


