Abstract
Objective
The long-term effects of traumatic brain injury (TBI) can resemble observed in normal ageing, suggesting that TBI may accelerate the ageing process. We investigate this using a neuroimaging model that predicts brain age in healthy individuals and then apply it to TBI patients. We define individuals' differences in chronological and predicted structural "brain age," and test whether TBI produces progressive atrophy and how this relates to cognitive function.
Methods
A predictive model of normal ageing was defined using machine learning in 1,537 healthy individuals, based on magnetic resonance imaging–derived estimates of gray matter (GM) and white matter (WM). This ageing model was then applied to test 99 TBI patients and 113 healthy controls to estimate brain age.
Results
The initial model accurately predicted age in healthy individuals (r * 0.92). TBI brains were estimated to be "older," with a mean predicted age difference (PAD) between chronological and estimated brain age of 4.66 years (±10.8) for GM and 5.97 years (±11.22) for WM. This PAD predicted cognitive impairment and correlated strongly with the time since TBI, indicating that brain tissue loss increases throughout the chronic postinjury phase.
Interpretation
TBI patients' brains were estimated to be older than their chronological age. This discrepancy increases with time since injury, suggesting that TBI accelerates the rate of brain atrophy. This may be an important factor in the increased susceptibility in TBI patients for dementia and other age-associated conditions, motivating further research into the age-like effects of brain injury and other neurological diseases.
Traumatic brain injury (TBI) causes long-term structural and functional alterations to the brain. Some of these changes are thought to be progressive in nature,1,2 and potentially underlie the increased risk for early cognitive decline3 and dementia4 observed in TBI patients. Similar behavioral and anatomical changes are also associated with normal ageing,5,6 raising the possibility that the chronic consequences of TBI may contribute to the premature development of age-associated changes to the brain.2
Normal ageing can be considered as the progression along a temporal trajectory, where individuals gradually accumulate pathologies associated with physical decline, cognitive impairment, and brain volume loss.7,8 Insults, such as TBI, may trigger a sequence of neurobiological events that alter that trajectory, prematurely causing brain atrophy, and potentially manifesting as an early onset of neurodegeneration.9 As illustrated in Figure 1, an environmental insult like TBI might cause a one-off increase in apparent "brain age," or could result in an ongoing interaction between injury and ageing-related or other neurodegenerative processes that cause progressive brain atrophy.2 In the latter case, as more time passes since the TBI occurred, the greater the discrepancy between chronological age and estimated brain age will be. This possibility is consistent with the progressive decline associated with TBI, even years after injury, as demonstrated by neuropsychological,10 neuroimaging,11–14 and animal1 research.
Figure 1.
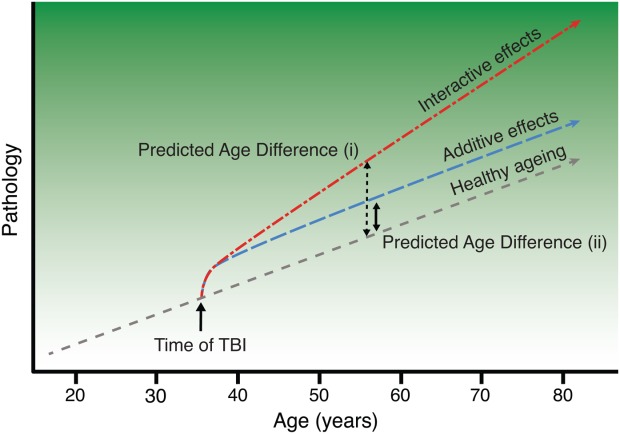
Model of premature brain ageing in traumatic brain injury. Illustration of the conceptual framework for the investigation of brain age in traumatic brain injury (TBI). The short-dashed line represents the trajectory of healthy ageing as age (x-axis) increases, against a background gradient of increasing susceptibility to age-related pathology (y-axis), such as cognitive decline and dementia. Occurrence of TBI is indicated (black arrow), with acute pathology causing an immediate departure from a healthy brain state. Two alternative brain ageing trajectories post-TBI are shown. The long-dashed "additive effects" line depicts a trajectory assuming a one-off hit, with damage leading to the patient's brain structure resembling an older individual, followed by a normal rate of subsequent ageing. The dash–dot "interactive effects" line represents an accelerated rate of brain atrophy caused by TBI and an interaction with normal ageing processes, with the discrepancy between normal ageing and pathological changes increasing the greater the time since injury (TSI). Comparing predicted age difference (PAD) scores (i; dashed black line) and (ii; solid black line) illustrates how a greater PAD score would be expected under the interactive effects model with accelerating atrophy (i), compared to the added effects model (ii), at equivalent TSI (figure adapted from Smith and colleagues2). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Using neuroimaging, it is possible to predict age in healthy individuals,15 allowing the discrepancy between chronological age and predicted brain age to be calculated. In clinical samples, this discrepancy can be considered as an index of the deviation from a normal ageing trajectory. Estimates of brain age, derived using machine learning, have previously been used in a number of contexts. These include measuring normal brain maturation during development,16,17 predicting conversion from mild cognitive impairment to Alzheimer disease,18 and in neurodevelopmental disorders such as schizophrenia and borderline personality disorder, where patients were shown to have apparently "older" brains.19 The deviation from normal ageing may reflect important neurological changes relating to clinical features such as cognitive impairment. Characteristic changes to cognition seen during normal ageing affect the domains of executive function, memory, and information processing speed.20 TBI patients show a similar pattern of cognitive deficits,10 further suggesting links between TBI and ageing of the brain.
Here we developed and tested a predictive model of brain age. Machine learning techniques were used to define a model that accurately predicted chronological age in healthy individuals. The model was then applied to brain images from TBI patients, allowing a prediction of their brains' ages to be made. It was expected that TBI patients' brains would be older than their chronological age and that this discrepancy would increase with longer time since injury (TSI), reflecting a progressive atrophy of brain tissue. Furthermore, we hypothesized that the discrepancy between chronological age and predicted brain age would be reflected in cognitive changes that would be consistent with age-related cognitive impairment normally seen in older individuals.
Subjects and Methods
Participants
Training Set
T1-weighted magnetic resonance imaging (MRI) data from 1,537 healthy controls were obtained from 8 publically accessible neuroimaging initiatives (Supplementary Table). This provided an unbiased source of data with which to train the age prediction model that was entirely independent from the TBI and control test data sets. Controls had no history of significant neurological or psychiatric problems, with further specific recruitment criteria made by each independent study. Exclusions were made due to poor data quality leading to image processing failure, identified during imaging quality assessment. All training set data had been previously anonymized, and ethical approval and informed consent were obtained by each specific study.
Test Set
Ninety-nine patients with persistent neurological problems after TBI (72 males, mean age ± standard deviation [SD]: 37.98 ± 12.43 years) were recruited (Table1). A comparison group of 113 healthy controls assessed on the same scanner was used to validate the accuracy of the age prediction model (49 males, 43.3 ± 20.24 years). All patients were scanned at least 1 month post-injury (mean * 28.4 months), with a range of 1 to 563 months. This variation allowed us to examine the influence of TSI on brain changes. The severity of TBI was classified using the Mayo Classification System criteria, with 17% being classified as mild (probable) and 83% being moderate/severe. Cause of injury was reported as follows: road–traffic accident (RTA; 39%), fall (24%), assault (23%), sport-related injury (6%), other (7%). Cerebral contusions were identified in 52 patients (53%) by an experienced neuroradiologist. Exclusion criteria were as follows: psychiatric or neurological illness, previous traumatic brain injury, antiepileptic medication, current or previous drug or alcohol abuse, MRI contraindication. All participants gave written informed consent, and the local ethics committee approved the study.
Table 1.
Details of TBI Patients
| Characteristic | TBI Patients | Controls |
|---|---|---|
| No. | 99 | 113 |
| Age, yr | 37.98 ± 12.43 | 43.3 ± 20.24 |
| Sex, M/F | 72/27 | 49/64 |
| Time since TBI, mo | 28.4 ± 63.57 | — |
| Mechanism of injury, RTA/fall/assault/sports/other | 39/24/23/6/7 | — |
| Mayo Clinic criteria, probable–mild/moderate–severe | 17/82 | — |
| Patient has contusions on MRI, present/absent | 52/47 | — |
| GCS | 9.71 ± 4.75 | — |
| Patient medicated at first visit, medicated/not medicated | 55/44 | — |
Values are reported in mean ± standard deviation or absolute numbers.
F * female; GCS * Glasgow Coma Scale; M * male; MRI * magnetic resonance imaging; RTA * road–traffic accident; TBI * traumatic brain injury.
Neuropsychological testing was carried out using a standardized battery of tests previously shown to be sensitive to cognitive abnormalities in TBI.11 These were the Trail Making Task, Stroop color naming and word reading, Wechsler Abbreviated Scale for Intelligence (WASI) matrix reasoning and similarities subscales, Choice Reaction task, People and Doors immediate recall test, letter fluency, and task inhibition/switching.
Procedures
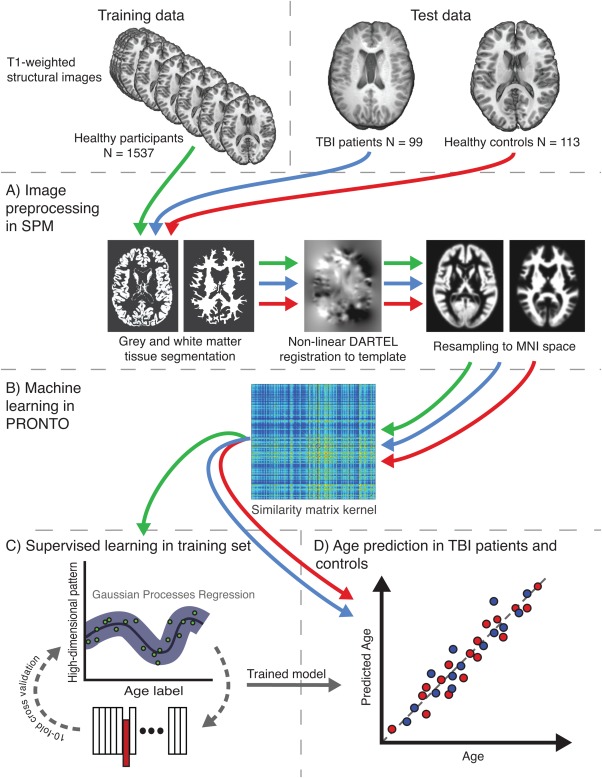
A high-level overview of the methods is provided in Figure 2. Methods are summarized below.
Figure 2.
Overview of the study methods. Study data comprised 2 sets, training and test. The training set used structural magnetic resonance imaging from 1,537 healthy individuals from multiple cohorts, whereas the test set included 2 groups, 99 traumatic brain injury (TBI) patients and 113 healthy controls, all scanned on the same scanner. (A) Conventional Statistical Parametric Mapping (SPM) structural preprocessing pipeline was used to generate gray and white matter maps, normalized to Montreal Neurological Institute (MNI) space and modulated to retain data relating to brain size. (B) Separately for gray and white matter, all 1,749 data sets were converted to a kernel matrix based on voxelwise similarity using Pronto. (C) The training data only were run through a supervised learning stage where a Gaussian Processes Regression (GPR) machine was trained to recognize patterns of imaging data that matched a given age label. To assess model accuracy, 10-fold cross-validation was conducted where 10% of samples were excluded from the training step and the ages of these samples were estimated. This was iterated 9 further times to generate age predictions on all samples. (D) The trained GPR model was then applied to the 2 test data sets, to assess accuracy of the model on healthy controls and then predict brain age of TBI patients. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MRI Acquisition Parameters
For the training set of high-resolution T1-weighted magnetic resonance images, multiple scanners were used, including different vendors, field strengths, and acquisition protocols (see Supplementary Table). For the test set, T1 images were acquired using a Philips 3T Intera scanner (Philips Medical Systems, Best, the Netherlands) with the following parameters: matrix size * 208 × 208, slice thickness * 1.2mm, 0.94 x 0.94mm in-plane resolution, 150 slices, repetition time * 9.6 milliseconds, echo time * 4.5 milliseconds, flip angle * 8°.
Image Preprocessing
All MRI data were preprocessed (see Fig 2A) using the Statistical Parametric Mapping (SPM8) software package (University College London, London, UK). This included tissue segmentation into gray matter (GM) and white matter (WM) maps, then registration using the nonlinear DARTEL algorithm21 to Montreal Neurological Institute space and resampling with a 4mm smoothing kernel. Each tissue class (ie, GM and WM) was processed independently after segmentation. The preprocessing procedure ensured that all images were well aligned and appropriate for voxelwise analysis at the machine learning stage.
Machine Learning Prediction of Age
Machine learning analysis (see Fig 2B) was conducted using the Pattern Recognition for Neuroimaging Toolbox (Pronto22) and run on GM and WM separately. Data from all subjects were converted to a similarity matrix kernel to improve computational efficiency in Pronto by generating a vector representation of voxelwise intensity levels22 for all data and calculating the dot product between each image. Next, the data were mean-centered and a Gaussian Processes Regression (GPR) model was defined using age as the dependent variable and the similarity matrix of imaging data as the independent variables. GPR is a machine learning extension of the classical regression model, which incorporates nonlinear and Gaussian probabilistic elements to allow quantitative predictions to be made using continuous variables.23
Model Validation
Model validation (see Fig 2C) proceeded in 3 stages to ensure independence between training and test sets and to enable an unbiased demonstration of model generalizability. The first stage involved running 10-fold cross-validation on the training set, to determine the accuracy of the model. This entailed randomly selecting one-tenth of the training data to be a temporary test set, with the remainder used for model definition. Using the learned pattern from this training set, age was predicted on the temporary test set. This process was iterated until all images had been included in the test set and had an age value predicted. This was followed by permutation testing with 1,000 randomizations to derive a p-value for each model. The second stage involved validating the model on the independent controls test data set. Here, the model was defined using the entire training set (N * 1,537) and then used to predict the brain ages for the control test data set. Finally, the trained model was applied to estimate predicted brain ages for the TBI patients (see Fig 2D).
Statistical Analysis
Model accuracy was assessed using Pearson correlation coefficients between predicted age and chronological age of the training subjects. The proportion of the variation explained by the trained model (R2) was examined, as was the mean absolute error (MAE) between predicted and chronological age. Accuracy was also evaluated for the control test data set, using the above parameters. Predicted age was subtracted from chronological age, generating a predicted age difference (PAD) score per participant. PAD scores were statistically analyzed in R (http://www.R-project.org/) using an analysis of covariance (ANCOVA) to test for group differences between TBI patients and controls, while covarying for age and sex. Within the TBI group, PAD was also assessed for Spearman nonparametric correlations with age, TSI, neuropsychological measures, and differences between subgroups based on TBI severity, mechanism of injury, and the presence of contusions. Analysis of PAD score was repeated for both GM and WM, and an exploratory analysis of the relationship between the 2 brain tissue classes was also carried out.
Results
Chronological Age Can Be Predicted from Structural Neuroimaging
The model was able to accurately predict chronological age for both training and control test data sets. For the training set (Fig 3), age was accurately predicted based on both GM (predicted–chronological age correlation r * 0.921, R2 * 0.848, MAE * 6.2 years) and WM (r * 0.922, R2 * 0.851, MAE * 6.16 years). Permutation testing resulted in a corrected p-value of 0.001 for both GM and WM models. The mean PAD for the training group was −0.003 years (±7.82) for GM and 0.037 years (±7.74) for WM. For the healthy control test data set, age was accurately predicted for both GM (r * 0.931, R2 * 0.867, MAE * 5.80) and WM (r * 0.931, R2 * 0.867, MAE * 6.35). These results validated the generalizability of the GPR modeling approach, as high accuracy was achieved when training the model on one data set and testing on an entirely independent data set.
Figure 3.
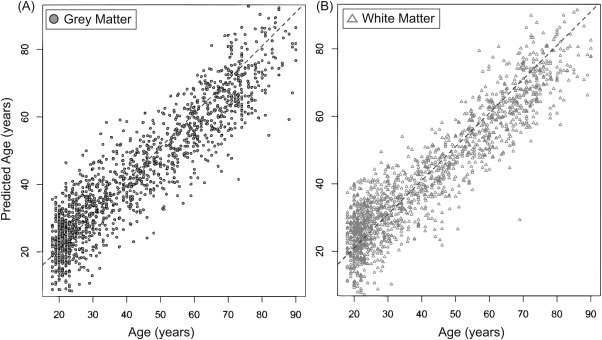
Machine learning model provides accurate age prediction in healthy training set. Predicted age for each healthy individual in the training set (n * 1,537) is shown, derived by running 10-fold cross-validation on the Gaussian Processes Regression model. (A) Chronological age (x-axis) is plotted against predicted age (y-axis), for gray matter (dark gray circles). (B) Chronological age and predicted age for white matter (light gray triangles). Diagonal dashed line represents the line of identity (x * y).
TBI Patients Have Increased Brain Age
TBI patients (GM mean PAD * 4.66 ± 10.8; WM mean PAD * 5.97 ± 11.22) showed increased PAD compared to controls (GM mean PAD * 0.07 ± 7.41; WM mean PAD * 2.06 ± 7.41; Fig 4). Comparing these PAD scores between TBI and control groups showed significant differences for both GM (F * 14.8, p < 0.001) and WM (F * 9.7, p * 0.002).
Figure 4.
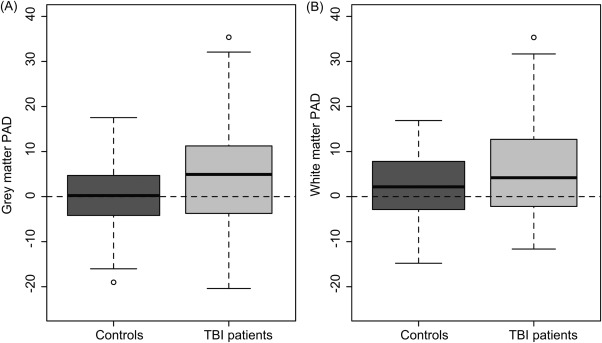
Traumatic brain injury (TBI) patients show increased predicted age difference (PAD) score for gray matter and white matter. Boxplots of PAD score are shown, calculated by subtracting chronological age from predicted age for the test data sets of 99 TBI patients and 113 healthy controls. (A) PAD scores derived from the gray matter model showing a significant increase in TBI patients. (B) PAD scores from white matter also show an increase in TBI patients.
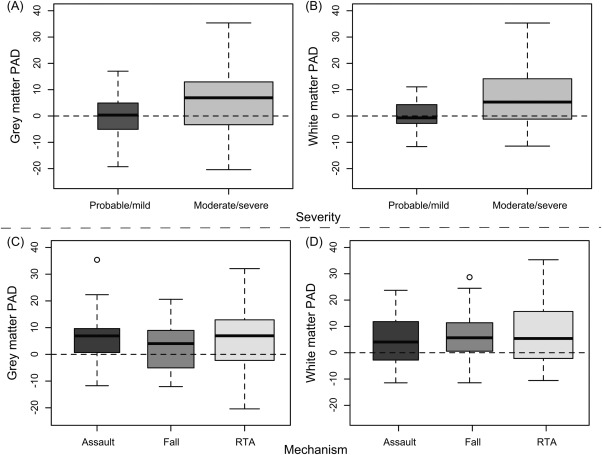
The discrepancy between predicted and chronological age was related to the severity of injury (Fig 5A, B). Patients with a moderate/severe classification showed increased mean PAD score (GM mean * 5.72 ± 10.97; WM mean * 7.24 ± 11.64), whereas mild (probable) patients were not significantly different from controls (GM mean * −0.42 ± 8.48; WM mean * −0.14 ± 6.1). The presence of focal lesions had no influence on PAD score, as there were no significant differences between those with and without contusions for either GM (mean: 5.99 vs 5.55, p * 0.85) or WM (mean: 6.89 vs. 7.44, p * 0.83). Furthermore, when limiting the TBI patient group to only moderate/severe lesion-free patients (n * 31), ANCOVA for GM and WM still showed significantly greater PAD scores compared to controls (p < 0.001). Although mean PAD score for TBI patients and controls was greater for WM than GM, the relatively high variability meant that this difference was not significant (p > 0.1).
Figure 5.
Gray matter (GM) and white matter (WM) predicted age difference (PAD) score, stratified by injury severity and injury mechanism. Boxplots of PAD score in the traumatic brain injury (TBI) patient group are shown, stratified by clinical characteristics. (A) GM PAD score distributions for each Mayo classification: probable/mild, moderate/severe, indicating that brain age is only increased in moderate/severe patients, not in mild TBI. (B) Mayo classification for WM. (C) GM PAD score by mechanism of injury (assault, fall, road–traffic accident [RTA]), indicating that similar levels of increased brain ageing occur independent of mechanism of injury. (D) Mechanism of injury for WM.
Brain Atrophy Correlates with Cognitive Impairment
Performance on a number of cognitive measures correlated strongly with PAD scores (Table2). After correction for the multiple tests conducted (using the false discovery rate24), measures relating to information processing speed and memory were significantly correlated with GM and WM PAD, with increased reaction time being related to increases in PAD score. Executive function measures were significantly correlated for GM PAD scores, but not for WM. Scores on the WASI subscales were not correlated with PAD scores.
Table 2.
Relationship between Neuropsychological Measures and PAD in TBI Patients
| Cognitive Domain | Neuropsychological Test | No. | Mean | GM PAD, rho | p | WM PAD, rho | p |
|---|---|---|---|---|---|---|---|
| Processing speed | Trail Making Test A, s | 90 | 29.59 (12.15) | 0.338 | 0.001a | 0.379 | <0.001a |
| Trail Making Test B, s | 90 | 68.79 (39.79) | 0.343 | 0.001a | 0.271 | 0.009a | |
| Stroop color naming, s | 90 | 34.47 (8.83) | 0.279 | 0.007a | 0.281 | 0.006a | |
| Stroop word reading, s | 90 | 23.93 (5.49) | 0.269 | 0.009a | 0.306 | 0.003a | |
| Choice reaction task median reaction time, ms | 66 | 478 (124) | 0.331 | 0.005a | 0.243 | 0.047 | |
| Executive function | Trail Making Test B minus A, s | 90 | 39.15 (32.51) | 0.262 | 0.011a | 0.154 | 0.146 |
| Inhibition/switching, s | 89 | 69.54 (22.18) | 0.247 | 0.018a | 0.205 | 0.053 | |
| Inhibition/switching minus baseline Stroop performance, s | 89 | 37.24 (18.41) | 0.213 | 0.043 | 0.168 | 0.115 | |
| Letter fluency total | 89 | 39.01 (12.73) | −0.271 | 0.009a | −0.133 | 0.214 | |
| Intellectual ability | WASI similarities | 90 | 37.04 (5.22) | −0.160 | 0.132 | −0.136 | 0.200 |
| WASI matrix reasoning | 88 | 26.34 (5.53) | −0.104 | 0.336 | 0.013 | 0.908 | |
| Memory | People Test immediate recall | 90 | 22.94 (7.33) | −0.253 | 0.015a | −0.254 | 0.014a |
Correlations with PAD score were conducted with variance accounting for chronological age partialed out, using the Spearman rank-order approach.
Denotes statistical significance after false discovery rate correction for multiple comparisons.
GM* gray matter; PAD * predicted age difference score; TBI * traumatic brain injury; WASI * Wechsler Abbreviated Scale for Intelligence; WM * white matter.
Evidence for Accelerated Atrophy following TBI
There was a strong correlation between PAD score and TSI for both GM (r * 0.535, p < 0.001) and WM (r * 0.496, p < 0.001), controlling for age. This reflected an increasing discrepancy between predicted and chronological age with a longer TSI (Fig 6). As there was also a correlation between TSI and age (r * 0.28, p * 0.0047) and between PAD score and age (GM: r * 0.18, p * 0.068; WM: r * 0.21, p * 0.037), a partial correlation approach was used to examine directly the relationship between PAD score and time since TBI, to remove variance associated with age. Three outliers in terms of TSI were present in the TBI group (168, 206, and 563 months), defined as being ±2 SD away from the mean (mean * 28.38 ± 63.58, 2-SD range * 0–155.54 months). These patients were not driving the association, as the results were still highly significant after these patients were removed (GM: r * 0.506, p < 0.001; WM: r * 0.467, p < 0.001). Removing patients with focal lesions also did not alter the association between TSI and PAD score, as analysis in contusion-free patients (n * 47) was still significant (GM: r * 0.353, p * 0.01; WM: r * 0.342, p * 0.016).
Figure 6.
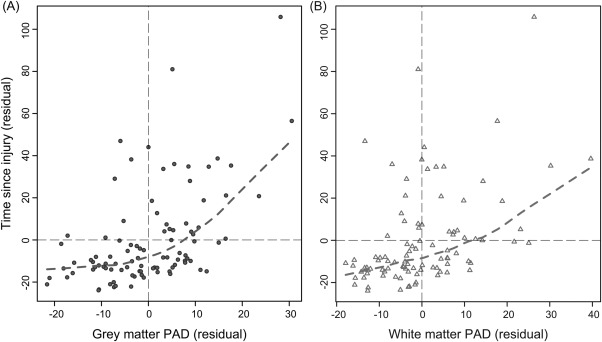
Gray matter and white matter predicted age difference (PAD) score increases with greater time since injury (TSI). Scatterplots depicting the relationship between PAD score (x-axis) and TSI (y-axis) are shown. Plotted values are the residuals derived from a linear regression with PAD score or TSI, regressing out chronological age. (A) Gray matter (dark gray circles) PAD scores, with dashed lines representing the locally weighted scatterplot smoothing (lowess) line calculated (dashed gray line). B) White matter (light gray triangles) PAD scores with lowess line (dashed light gray line). Both analyses were conducted after the removal of 3 outliers, identified based on having a TSI of ±2 standard deviations from the mean.
Brain "Ageing" Does Not Vary in Patients with Different Mechanism of Injury
We assessed whether the mechanism of injury affected brain ageing. Patients who suffered TBI due to RTA, assault, or fall all showed increased mean PAD scores (5.93, 6.15, and 4.11 for GM; 7.52, 4.42, and 6.51 for WM), indicating that TBI patients' brains appeared to be older, irrespective of the cause of injury (see Fig 5C, D). There were no significant differences in PAD between mechanism subgroups for either GM or WM. The correlation between TSI and PAD was still present for patients injured during RTAs and assaults, although not for sufferers of falls. Mean TSI associated with falls was only 19.8 (±35.2) months, considerably less than for RTA (33.2 ± 43.1 months) and assaults (41.5 ± 120.7 months). This decreased duration and narrower distribution may have led to lower sensitivity to the effects of TSI on PAD in sufferers of falls.
Discussion
Using a multivariate method to investigate the spatial patterns of age-associated brain atrophy, we show that TBI produces a pattern of structural brain changes that affects the apparent brain age of both GM and WM. The predictive model we generated was highly accurate at estimating chronological age in healthy participants, based only on the appearance of T1 MRI scans. In contrast, following TBI, the model estimated brain age to be on average >4 years older than the patient's chronological age. This discrepancy was only seen in patients with more severe injuries, was independent of the mechanism of injury, and was predictive of cognitive impairment. These results support the theory proposed by Moretti and colleagues3 that TBI may hasten the ageing process and is in keeping with long-term structural and functional brain abnormalities reported in neuroimaging and neuropathology studies of TBI patients.11–13,25,26
Animal work demonstrates a progressive loss of brain tissue after experimental TBI.1 A number of mechanisms for this have been proposed, including chronic neuroinflammation,12,26 Wallerian degeneration of WM,27 and the deposition of abnormal tau and amyloid-β proteins.28 These processes lead to alterations in cellular morphology, loss of trophic support, and eventual cell death and gross atrophy.2 It is also possible that a reduction in brain volume might be due to the clearance of tissue damaged at the time of injury. Previous neuroimaging studies have typically shown atrophy when comparing acute and chronic scans, making it difficult to disentangle these possibilities.14 One longitudinal study has shown that GM volume decreases continue between 1 and 4 years after TBI, demonstrating that tissue loss continues well beyond the stage when clearance of acutely damaged tissue is likely to have finished.29 This suggests a progressive process, which is supported by our observation that patients who were assessed further from their injury (high TSI) showed more atrophy. This supports the idea that tissue loss accelerates over time, in keeping with a progressive neurodegenerative process triggered by the injury (see Figs 1 and 4 and reviews by Smith and colleagues2 and Bigler9).
Our model explained much of the variation in chronological age, in line with previous research showing that multivariate brain imaging analysis can accurately predict chronological age.15,30 Much like outwardly visible signs of age such as the presence of wrinkles or gray hair, this shows that the brain also varies in its apparent age. Discrepancies between brain and chronological age appear to be biologically informative, perhaps reflecting important differences in susceptibility or resistance to age-related pathology. This possibility is supported by the relationship we observed between PAD score and cognitive function. Our structural imaging measures correlated with cognitive impairment in domains typically affected by TBI,10,11 namely information processing speed, memory, and executive function. As the vast majority of these patients were at least 3 months postinjury, it is likely that such impairments are persistent, rather than acute and transient, implying that there is a relationship between the observed brain changes and the chronic post-TBI cognitive profile. Intriguingly, these are the same cognitive domains often affected by age-related cognitive decline, which leads us to speculate that PAD score may be relevant to the effects of ageing in a broader sense. In particular, the strongest associations with our measure of brain ageing were found with information processing speed measures, and impairments in this domain have been posited as central to the cognitive decline associated with typical ageing.20 The nature of our sample did not permit an exhaustive examination of TBI-related and age-related cognitive dysfunction; however, our interpretation of results supports the idea that the pattern of brain changes detected in TBI patients has similar functional consequences to normal ageing, albeit occurring in an accelerated form. Measures of age discrepancy might be useful for screening clinical and population samples to identify those at increased risk of age-associated pathologies, for quantifying the effects of vascular risk factors or neuropsychiatric diseases on general brain health, and for stratifying patients for targeted treatments or clinical trial enrollment.
As predicted, the degree of apparent ageing reflects the severity of initial injury. In contrast to moderate/severe TBI, patients with minor TBI showed no brain atrophy. This suggests that a significant biomechanical force is necessary to trigger ongoing neurodegenerative processes that lead to progressive atrophy. In addition, the mechanism of injury did not influence PAD score, with those suffering falls, assaults, and RTAs showing similar levels of atrophy. That widely varying mechanisms of TBI result in similar patterns of premature atrophy is important, as it suggests that the underlying differences in brain structure are unlikely to be secondary to nonspecific neurological or demographic factors that might be expected to vary across individuals with TBI from very different causes. For example, a possible confound is that patients predisposed to TBI might have smaller brains prior to injury. However, there is no evidence from other studies that this is the case, and it is highly unlikely that patients with such contrasting mechanisms of injury would also show the same systematic bias in brain size.
It is unclear whether the brain atrophy we observe after TBI reflects ongoing neurodegeneration triggered by the injury or an interaction with normal ageing. In part, this reflects the complexity of defining ageing. Rates of ageing vary between individuals, but also can affect different tissues in the same person at different rates.31 This makes comprehensively modeling age challenging. One limit of the work is that we focus only on a single facet of ageing, demonstrating that individuals have more brain atrophy after TBI. We are unable to disentangle the degree to which this atrophy results from an acceleration of processes seen in healthy ageing, whether the injury triggers new neurodegenerative processes, or the extent to which these two possibilities are inter-related. Processes involved in neurodegeneration such as inflammation and the accumulation of misfolded proteins are also seen to varying degrees in healthy ageing, and comparative studies focusing on these factors will be necessary to define to what extent TBI patients resemble healthy older individuals or should be viewed as having distinct neuropathology.
A number of other potential limitations of the study should also be noted. First, some TBI patients have small focal lesions that could potentially cause problems with the image registration techniques that our age prediction requires. However, we use advanced registration algorithms that perform well in our patient group, and our results are similar when the analysis is restricted to patients without focal lesions. Second, the estimates of age were variable, indicating a degree of residual measurement error, which is to be expected as we were using only brain structure to predict chronological age. Nevertheless, the large sample size meant we were sufficiently powered to detect statistically significant group differences in PAD score.
We exploited the wide range of TSI to explore the chronic effects of TBI. Our analysis indicated both that increased atrophy is present after TBI, and also that the amount of atrophy increases over time. Removing the patients with the greatest TSI did not alter the results, implying that this apparent acceleration is a robust effect even over a relatively short time period. As there are a number of potential confounds inherent with cross-sectional analyses, our evidence for accelerating atrophy should be interpreted with caution. For example, brain ageing is likely to be in part genetically mediated,32 so information about genotype should be added in future studies to potentially explain even greater amounts of variance in age. In addition, the severity of the initial injury will influence the extent of brain atrophy. We only observed increased atrophy in patients with moderate/severe injuries, which shows the expected impact of injury on apparent brain age. However, within the moderate/severe group there will be variations in injury severity, and these could potentially confound the relationship between TSI and brain atrophy. Individual indices of injury severity such as Glasgow Coma Scale score and duration of post-traumatic amnesia are known to be crude measures of injury severity.33 Therefore, large longitudinal studies will be necessary to completely resolve these issues, ideally with individual follow-up at multiple time points to allow longitudinal within-subject analysis that incorporates other genetic and biomarker information about the ageing process.
In summary, our study is the first empirical in vivo demonstration that TBI causes structural brain changes that resemble the atrophy seen during ageing. The accelerated trajectory of brain atrophy we observed is in keeping with a progressive neurodegenerative process triggered by the injury. The effects of age and injury are likely to act synergistically, leading to deficits in information processing speed and other neuropsychological measures known to be impaired in normal ageing. Future studies could use PAD score as a predictor of clinical outcome or as a surrogate marker of treatment efficacy. The work gives insight into the etiology of the long-term chronic effects of TBI and has implications for the long-term care and potential future treatments for TBI patients, as methods that attenuate the negative effects of ageing may also be effective treatments for patients who have suffered a TBI.
Acknowledgments
This research was supported by a European Union Seventh Framework Programme grant to the Comorbidity in Relation to AIDS (COBRA) project (FP-7-HEALTH 305522; J.H.C.), an National Institute for Health Research (NIHR) Professorship (NIHR-RP-011-048; D.J.S.), and the NIHR Imperial Biomedical Research Centre.
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/). As such, the investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. The draft manuscript was certified by the ADNI Data and Publications Committee as meeting the ADNI data sharing requirements. Full details of the ADNI data sharing policy and further information fully acknowledging the relevant funding sources can be found here: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_DSP_Policy.pdf
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
Potential Conflicts of Interest
D.J.S.: investigator led grant, Pfizer.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Smith DH, Chen XH, Pierce JES. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- 2.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretti L, Cristofori I, Weaver SM. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11:1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 4.Bazarian JJ, Cernak I, Noble-Haeusslein L. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24:439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- 5.Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira D, Molina Y, Machado A. Cognitive decline is mediated by gray matter changes during middle age. Neurobiol Aging. 2014;35:1086–1094. doi: 10.1016/j.neurobiolaging.2013.10.095. [DOI] [PubMed] [Google Scholar]
- 7.Sowell ER, Peterson BS, Thompson PM. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 8.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. 2013;7:395. doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- 11.Kinnunen KM, Greenwood R, Powell JH. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramlackhansingh AF, Brooks DJ, Greenwood RJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 13.Sharp DJ, Beckmann CF, Greenwood R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 14.Ross DE. Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Inj. 2011;25:1271–1278. doi: 10.3109/02699052.2011.624568. [DOI] [PubMed] [Google Scholar]
- 15.Franke K, Ziegler G, Klöppel S, Gaser C. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50:883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown T, Kuperman JM, Chung Y. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosenbach NUF, Nardos B, Cohen AL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaser C, Franke K, Klöppel S. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer's disease. PLoS One. 2013;8:e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsouleris N, Davatzikos C, Borgwardt S. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40:1140–1153. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Schrouff J, Rosa MJ, Rondina JM. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11:319–337. doi: 10.1007/s12021-013-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle OM, Ashburner J, Zelaya FO. Multivariate decoding of brain images using ordinal regression. Neuroimage. 2013;81:347–357. doi: 10.1016/j.neuroimage.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 25.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Johnson VE, Stewart JE, Begbie FD. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 28.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farbota KD, Sodhi A, Bendlin BB. Longitudinal volumetric changes following traumatic brain injury: a tensor-based morphometry study. J Int Neuropsychol Soc. 2012;18:1006–1018. doi: 10.1017/S1355617712000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groves AR, Smith SM, Fjell AM. Benefits of multi-modal fusion analysis on a large-scale dataset: life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. Neuroimage. 2012;63:365–380. doi: 10.1016/j.neuroimage.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 31.Simm A, Johnson TE. Biomarkers of ageing: a challenge for the future. Exp Gerontol. 2010;45:731–732. doi: 10.1016/j.exger.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Filippini N, Ebmeier KP, MacIntosh BJ. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54:602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Malec JF, Brown AW, Leibson CL. The mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.