Abstract
Background & Aims
The NOD.c3c4 mouse model develops autoimmune biliary disease characterized by spontaneous granulomatous cholangitis, antimitochondrial antibodies and liver failure. This model for primary biliary cirrhosis (PBC) has evidence of biliary infection with mouse mammary tumour virus (MMTV), suggesting that the virus may have a role in cholangitis development and progression of liver disease in this mouse model. We tested the hypothesis that MMTV infection is associated with cholangitis in the NOD.c3c4 mouse model by investigating whether antiretroviral therapy impacts on viral levels and liver disease.
Methods
NOD.c3c4 mice were treated with combination antiretroviral therapy. Response to treatment was studied by measuring MMTV RNA in the liver, liver enzyme levels in serum and liver histology using a modified Ishak score.
Results
Combination therapy with the reverse transcriptase inhibitors, tenofovir and emtricitabine, resulted in a significant reduction in serum liver enzyme levels, attenuation of cholangitis and decreased MMTV levels in the livers of NOD.c3c4 mice. Furthermore, treatment with the retroviral protease inhibitors, lopinavir and ritonavir, in addition to the reverse transcriptase inhibitors, resulted in further decrease in MMTV levels and attenuation of liver disease in this model.
Conclusions
The attenuation of cholangitis with regimens containing the reverse transcriptase inhibitors, tenofovir and emtricitabine, and the protease inhibitors, lopinavir and ritonavir, suggests that retroviral infection may play a role in the development of cholangitis in this model.
Keywords: autoimmune biliary disease, combination antiretroviral therapy, mouse mammary tumour virus, NOD.c3c4, primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is a cholestatic liver disease characterized by the production of antimitochondrial antibodies (AMA) reactive to pyruvate dehydrogenase complex (PDC)-E2 1. The investigation of PBC development as well as novel therapies have been considerably enhanced by the characterization of autoimmune biliary disease in mouse models that develop cholangitis and AMA 1–6. Although these models exhibit features that are incongruous with the human disease, sufficient similarities exist to enable investigation of the complexity of genetic, environmental and immune influences that may contribute to the pathobiology of PBC 1. For example, susceptible mice develop periportal inflammation and AMA production following administration of bacteria or xenobiotics 7,8. Furthermore, several mouse models spontaneously develop AMA and hepatic inflammation, such as the NOD.c3c4 generated from the NOD mouse 2,3, the T cell TGF-β receptor II dominant-negative (dnTGFβRII) mouse 4 and the interleukin 2 receptor α deficient (IL-2Rα−/−) mouse 6. One common feature of these models is that each has markedly dysfunctional innate and adaptive immunity 1.
Our laboratory has characterized a human betaretrovirus in patients with PBC, and linked viral infection with the aberrant expression of a PDC-E2-like protein in PBC patient samples and in vitro 9–11. The association of PBC with betaretrovirus infection has been difficult to establish since both our laboratory and others have found it difficult to verify viral infection within the liver 12–14. However, more recent studies have identified evidence of viral integration in biliary epithelium of patients with PBC using linker mediated PCR and next generation sequencing technology 15. The human betaretrovirus shares approximately 95% nucleotide homology with the mouse mammary tumour virus (MMTV), which is endemic in several mouse strains 16. Therefore, we sought evidence for MMTV infection in AMA-producing mice with immune dysfunction to test whether MMTV expression might be correlated with the development of autoimmune biliary disease in vivo 16. We found that the dnTGFβRII, IL-2Rα−/− and NOD.c3c4 mice had evidence of MMTV RNA expression, and MMTV proteins were found in the same tissue distribution as aberrant PDC-E2-like protein expression 16. Further investigations in the NOD.c3c4 mouse showed a significant correlation with the production of AMA and anti-MMTV antibodies 16. Interestingly, the NOD.c3c4 mouse displayed MMTV proteins in the diseased biliary epithelium 16. Therefore, NOD.c3c4 mice were used in the present study to test whether that MMTV infection may be a factor associated with the development of intrahepatic cholangitis.
Previous studies have shown that MMTV reverse transcriptase enzyme was sensitive to zidovudine and tenofovir and the MMTV aspartyl protease could be inhibited by lopinavir in vitro 17–19. Therefore, in the present study we tested whether the treatment of NOD.c3c4 mice with several combinations of reverse transcriptase inhibitors with or without protease inhibitors may have an impact on liver disease development as assessed by intrahepatic cholangitis and serum liver enzyme levels.
Materials and methods
Animal models
NOD.c3c4 and NOD.GFP mice were purchased from Taconic Farms (Hudson, NY, USA). Mice were housed under conventional conditions, fed ad libitum with standard mouse chow, and provided with free access to drinking water. Five to eight week old female NOD.c3c4 mice were housed in groups of five per cage and treated with antiretroviral therapy or placebo using previously established dosages 17. Combination zidovudine with lamivudine in tablet form and placebo were obtained from GlaxoSmithKline (Triangle Park, NC, USA). Other antiretroviral medications were obtained in either tablet or liquid form from the University of Alberta pharmacy. Medications were added to the drinking water to achieve a daily dose of 1.5 mg lamivudine and 3 mg zidovudine, which was found to be effective in inhibiting MMTV in mice 17. The other medications were supplied at similar amounts to zidovudine/lamivudine providing a daily dose of 1.5 mg tenofovir and 1 mg emtricitabine for combination reverse transcriptase inhibitors as well as 4 mg lopinavir and 1 mg ritonavir for protease inhibitors shown to be effective in vitro 19. Groups of 10–25 mice were treated with either (i) placebo; (ii) zidovudine/lamivudine (Combivir™); (iii) tenofovir/emtricitabine (Truvada™); (iv) lopinavir/ritonavir (Kaletra™); (v) Combivir and Kaletra or (vi) Truvada and Kaletra. The consumption of drinking water containing either placebo or medication was regularly assessed to ensure that equal amounts of medication were being ingested per cage housing 5 mice. As the NOD.c3c4 model is variably penetrant, a large sample size of 20–25 per treatment was chosen for placebo, Combivir and the combination antiretroviral regimen Combivir and Kaletra. Subsequently, smaller sample sizes (n = 10) were used to demonstrate significant histological and biochemical responses with Truvada-based regimens.
Tail vein blood samples were obtained at baseline, 1 month before and at 3 months at the end of therapy to measure alkaline phosphatase (Biotron Diagnostics Inc., Hemet, CA, USA) and alanine aminotransferase (ALT, Biovision, Inc., San Francisco, CA, USA) levels, as per manufacturer's protocol. At sacrifice, liver tissues were also paraffin embedded and stained with haematoxylin and eosin or anti-MMTV p27 antibody, as previously described 16. Two pathologists assessed coded liver samples without prior knowledge of treatment, using a modified Ishak score to grade the severity of necroinflammatory activity and cholangitis, as previously described 20,21. The histological endpoints were (i) Cholangitis score, which was graded by bile duct inflammation, granulomatous cholangitis and ductular reaction; (ii) Necroinflammatory score, which included total score of the portal inflammation, interface hepatitis, parenchymal inflammation and (iii) total Ishak score derived by the combined necroinflammatory and cholangitis scores. Ductopaenia was assessed by dividing the total number of bile ducts by the number of portal triads, as previously described 20,21. The University of Alberta Health Sciences Animal Care and Use Committee approved this investigation.
Quantitative real-time polymerase chain reaction (qRT-PCR)
At sacrifice, MMTV envelope RNA and actin RNA expression were quantified in total hepatic RNA using qRT-PCR, as previously described 16. Briefly cDNA was prepared from total liver RNA and expression of the spliced MMTV envelope gene was quantified using the following primers and TAMRA TaqMan probe: env F: 5′CGGAACGGACTCACCATAGG-3′; env R: 5′-GGACCCAGATTGGTGATTCG-3′ and env Probe 5′-AGCTGCAGTCCCGCCTACGGAGA-3′. Gene expression of β-actin was also quantified using the following primers and TAMRA TaqMan probe as an endogenous control reference: β-actin F: 5′CGGTTCCGATGCCCTGA-3′; β-actin R: 5′-CGGATGTCAACGTCACACTTCA-3′; β-actin P: 5′CAGCCTTCCTTCTTGGGTATGGAATCC-3′. The thermal cycling conditions for PCR were as follows: 2 min at 50°C, 10 min at 95°C followed by 40 cycles of 15 s at 95°C, 1 min at 60°C. ΔCT was calculated by subtracting endogenous actin control CT from target CT and MMTV envelope gene expression was reported as expression levels using the formula of 2−ΔCt 16.
Sequence analysis of the MMTV pol gene
Hepatic cDNA samples from four mice receiving placebo and four mice treated with zidovudine/lamivudine were cloned to assess the variability in the MMTV pol gene in response to therapy. Nested RT-PCR was performed using the following primers: Pol-Out-F 5′ACGATATGGGAGCATTACAACCC-3′ and Pol-Out-R 5′-CGACAATGGATCTTGATGGGTG-3′ for the first amplification and Pol-In-F 5′-GTGTGCCCTCCCCTAATTTTAAG-3′ and Pol-In-R 5′ATCTTGATGGGTGTGCCAAAAG-3′ for the second. The cycling conditions for both reactions were set at 94°C for 2 min, followed by 36 cycles at 94°C for 30 s, 50°C for 30 s and 68°C for 1 min. PCR products were cloned into a pTopo 2.1 PCR cloning vector (Invitrogen, Burlington, ON, Canada) and 10 sequences were obtained for each amplicon using standard bidirectional capillary sequencing.
Statistical analysis
Changes in histology, MMTV envelope RNA levels and reduction in serum liver enzyme levels from baseline were assessed for all treatments using a non-parametric one way anova (Kruskal–Wallis). The Dunn's multiple comparison test was subsequently used to assess the difference between individual treatments and placebo. MMTV RNA levels were compared to total histological score using Spearman's ρ correlation. Significance was determined using two sided P values of less than 0.05 and these analyses were calculated using GraphPad Prism 6.0 software. The interobserver reproducibility for the histological scoring was assessed by kappa statistics using Scientific Package for Social Sciences software (SPSS 12.0, Chicago, IL, USA), as described 22. The kappa coefficients were compared for statistical significance using the Wilcoxon signed-rank test and the level of agreement for kappa values were ranked as follows: 0.0–0.2, slight; 0.21–0.4, fair; 0.41–0.6, moderate; 0.61–0.8, substantial; 0.81–1.0, almost perfect 22.
Results
Natural history of cholangitis development in the NOD.c3c4 model
Prior studies have reported that NOD.c3c4 mice develop progressive cholangitis and biliary cysts with increasing age that leads to liver failure in 50% of females within a year. However, the occurrence of liver disease was previously assessed by the detection of extrahepatic biliary dilatation and the penetrance of disease was variable 2,3. Since we were interested in determining whether antiretroviral therapy may attenuate cholangitis development in this mouse model, we assessed the serum alkaline phosphatase levels during the 12 weeks of the study in the placebo arm without any intervention. We observed that the alkaline phosphatase levels fell without any intervention, mainly between weeks 8–12 (Fig.1A) and therefore investigated whether a similar reduction was observed in the parental derived strain, NOD.GFP mice (Fig.1B). A reduction in alkaline phosphatase levels was observed in both the NOD.c3c4 and the NOD strains suggesting that the decrease was related to puberty 23. Owing to the variability in levels prior to intervention, we chose to study the overall reduction in alkaline phosphatase from baseline.
Figure 1.
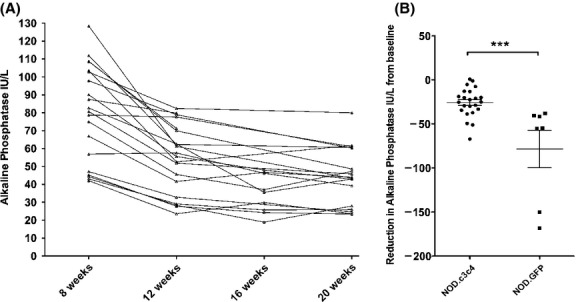
Serum alkaline phosphatase levels in NOD.c3c4 receiving no antiretroviral therapy. (A) Serial measurement of serum alkaline phosphatase in the NOD.c3c4 mice showing variance in individual levels as well as a reduction mean levels as mice aged, mainly from 8 to 12 weeks (P < 0.0001, 1 way anova). (B) Reduction in serum alkaline phosphatase levels from baseline levels between 8 and 12 weeks, showing diminished reduction in NOD.c3c4 vs. NOD.GFP (Data shown as means ± SEM, ***P < 0.001, t-test).
We also assessed the natural history of histological change without antiviral therapy (Fig.2A and B). Of note, the inter-observer reproducibility between the two pathologists was substantial for the necroinflammation score (kappa = 0.712, P < 0.001) and the cholangitis score (kappa = 0.742, P < 0.001) 22. We observed a gradual increase in necroinflammatory score; by 20 weeks, 17 of 20 (85%) mice on placebo developed hepatic inflammation and 14 of 20 (70%) cholangitis (Fig.2B). These data are consistent with previous studies that report that 44% of NOD.c3c4 mice develop lymphocytic infiltrate within the liver at 8 weeks and 90% of mice have histological disease over age 30 weeks 3. Taken together, these data suggest that bile duct damage is an early event in NOD.c3c4 mice, and inflammatory disease progresses with age. However, disease penetrance is variable in this model, mandating a large sample size for study.
Figure 2.
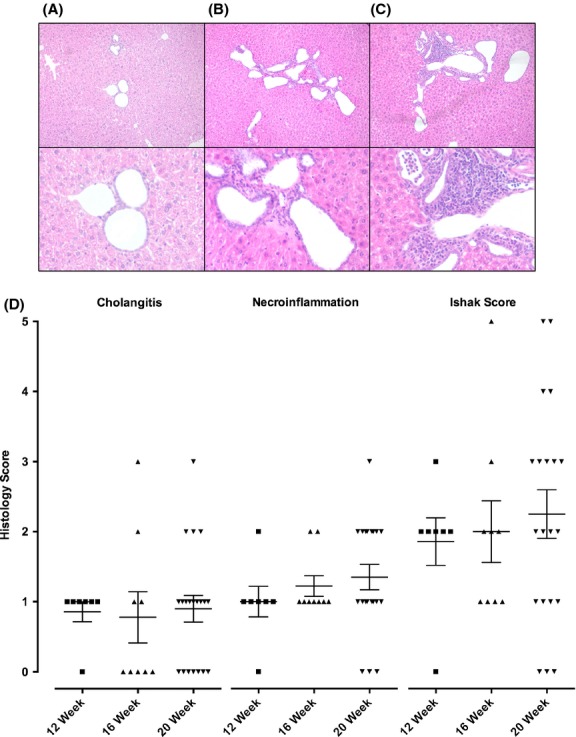
Histological features in NOD.c3c4 mice receiving no antiretroviral therapy. (A–C) Representative histological of images demonstrating total Ishak scores of (A) 0, (B) 3, and (C) 5 (Haematoxylin and Eosin, upper panel -100× magnification, lower panel - 300× magnification). (D) NOD.c3c4 mice have a variable penetrance of cholangitis and necroinflammation. As the mice age, a trend was observed for an increased variability in all histological scores as well as a gradual increase of necroinflammatory and total Ishak score in the older mice (Data shown as means ± SEM).
Histological and biochemical response to antiretroviral therapy
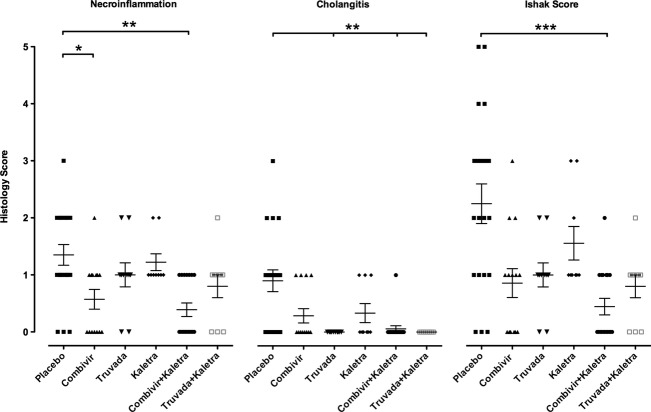
Since MMTV reverse transcriptase enzyme is sensitive to zidovudine and tenofovir and the MMTV aspartyl protease can be inhibited by lopinavir 17–19, we tested the effect of commercially available combinations of reverse transcriptase inhibitors including emtricitabine and tenofovir (Truvada™; Gilead, Foster City, CA, USA) as well as lamivudine and zidovudine (Combivir™; GlaxoSmithKline, Triangle Park, NC, USA) on disease development in NOD.c3c4 mice. To evaluate the effect of combination antiretroviral therapy, the protease inhibitors lopinavir and ritonavir (Kaletra™; AbbVie, North Chicago, IL, USA) were also used where the ritonavir acts to potentiate the activity of lopinavir 19. Following 3 months of intervention, marked differences were observed in necroinflammatory disease, in cholangitis and in total Ishak score in mice treated with antiretroviral therapy vs. placebo (Fig.3). Of note, antiretroviral regimens with Truvada had no evidence of cholangitis at the end of treatment (Fig.3). Furthermore, the inclusion of Kaletra with either combination of reverse transcriptase inhibitors was associated with incremental improvement in histological scores. Intervention with all antiretroviral regimens was associated with a significant reduction in mean alkaline phosphatase levels from baseline after 4 and 12 weeks treatment (P < 0.005 and P < 0.0001, respectively, one way anova, Fig.4A). However, when individual combinations were compared with placebo, only the combination of Truvada with Kaletra showed a significant difference after 1 month and both Truvada regimens with and without Kaletra showed significant reductions in mean alkaline phosphatase levels of more than 40 IU/L after 3 months therapy (P < 0.01 vs. placebo, Fig.4A). To further verify that Truvada and Kaletra therapy attenuated liver disease in the NOD.c3c4 mice, we determined the serum ALT levels, which were significantly reduced with this combination therapy compared to placebo (Fig.4B). Taken together, the biochemical and histological data suggest that regimens including Truvada had a significant impact on liver disease.
Figure 3.
Histological improvement in the liver following 12 weeks of antiretroviral therapy. NOD.c3c4 mice treated with antiretroviral therapy showed significant reduction in necroinflammation (P < 0.001), cholangitis (P = 0.0001) and total Ishak Score (P < 0.0005, one way anova). Combinations that included Truvada with or without Kaletra had no evidence of cholangitis and combination regimens incorporating Kaletra demonstrated improved necroinflammation, cholangitis and total Ishak score [Combivir (zidovudine and lamivudine), Truvada (tenofovir and emtricitabine), Kaletra (lopinavir and ritonavir); data shown as means ± SEM, *P < 0.05, **P < 0.01 and ***P < 0.001 vs. placebo using Dunn's multiple comparison test].
Figure 4.
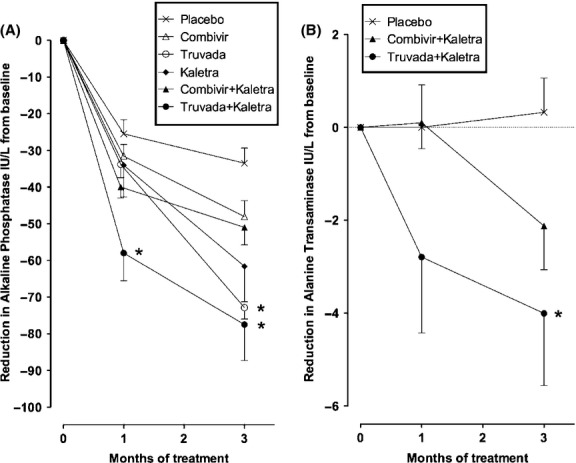
Antiretroviral regimens associated with reduction in serum liver enzyme levels from baseline. (A) After 4 weeks of treatment, only mice receiving Truvada and Kaletra experienced a significant mean reduction in alkaline phosphatase levels as compared to placebo, whereas by the end of 12 weeks therapy mice treated with regimens containing Truvada with or without Kaletra experienced significant reduction. (B) A significant reduction in serum alanine transaminase levels was observed in mice receiving Truvada and Kaletra but not Combivir and Kaletra [Combivir (zidovudine and lamivudine), Truvada (tenofovir and emtricitabine), Kaletra (lopinavir and ritonavir); data shown as means ± SEM; *P < 0.01 vs. placebo by Dunn's multiple comparison test].
Virological response to therapy
Since the combination antiretroviral therapy was found to decrease histological scores, we tested whether the antiretroviral therapy also reduced hepatic MMTV viral load through qRT-PCR 16. At the end of the study, antiretroviral therapy was found to significantly reduce hepatic MMTV RNA levels in the NOD.c3c4 mice (Fig.5A). Furthermore, treatment with Truvada led to a significant reduction in hepatic MMTV RNA compared to placebo (Fig.5A, P < 0.01), whereas, regimens based on Combivir showed no significant differences in viral burden vs. placebo. As MMTV Gag expression was previously observed in biliary epithelial cells, we tested whether this expression was affected in mice receiving combination antiretroviral therapy (Fig.5B). While Gag reactivity was readily observed in biliary epithelium of mice on placebo, mice receiving combination antiretroviral therapy were found to have reduced Gag expression in biliary epithelial cells.
Figure 5.
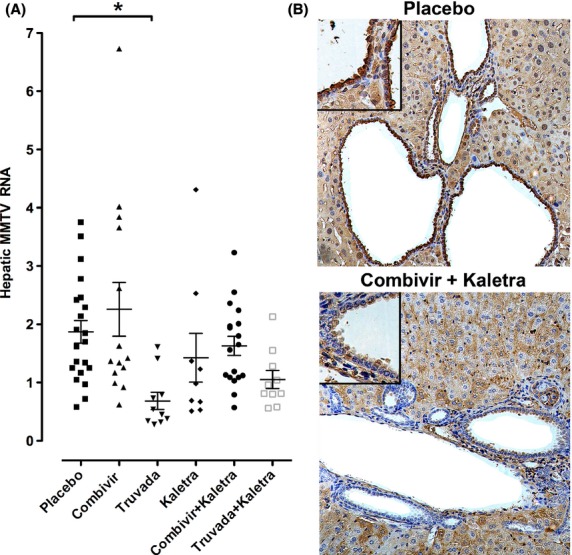
Antiretroviral therapy associated with reduced hepatic MMTV RNA and biliary epithelial cell-associated MMTV Gag expression. (A) Antiretroviral therapy modulated MMTV RNA levels in the NOD.c3c4 liver after 12 weeks therapy (P < 0.0005, 1 way anova). A significant reduction in mean hepatic MMTV RNA levels was seen with Truvada alone, whereas a trend for decreased viral load was observed with regimens containing Kaletra [Combivir (zidovudine and lamivudine), Truvada (tenofovir and emtricitabine), Kaletra (lopinavir and ritonavir); data shown as means ± SEM, *P < 0.01 vs. placebo by Dunn's multiple comparison test]. (B) Mice receiving placebo showed MMTV p27 Gag protein reactivity in biliary epithelial cells. This reactivity was attenuated in mice treated with Combivir and Kaletra. [Haematoxylin, 200× magnification with 400× in insets showing staining in biliary epithelial cells].
Since hepatic MMTV viral load was observed to be high in a proportion of mice following Combivir treatment (Fig.5A), we sought to determine whether mice in this group had escaped the effects of antiviral therapy and developed mutations within the MMTV reverse transcriptase gene. MMTV pol gene sequences were cloned from the liver of mice treated with Combivir and of the placebo group with levels of hepatic MMTV RNA greater than the mean value. Ten sequences per mouse were obtained and two common variants were observed in sequences derived from both groups (Fig.6). Additionally, five variant pol gene sequences were observed in mice treated with Combivir therapy: W150R, R176G, Y183H, M188V and L192P (Fig.6). Interestingly, M188V mutant is comparable to the M204V mutation previously observed in HBV pol gene following lamivudine therapy and the M184V mutation previously observed in HIV reverse transcriptase gene with zidovudine/lamivudine therapy 24. These results suggest that the reduced inhibitory activity of Combivir on MMTV replication might have been caused by the mutations in reverse transcriptase gene that have developed in this group.
Figure 6.
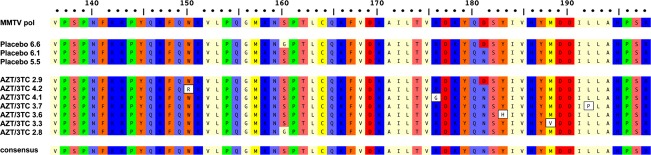
Variations in MMTV pol gene were observed after 12 weeks Combivir (zidovudine and lamivudine) therapy. Alignment of amino acid sequence 136–198 of MMTV Pol P03365.2 using ClustalW alignment (MacVector 11.1 software) showing the amino acid variations W150R, R176G, Y183H, M188V and L192P in five clones derived from two mice treated with Combivir (zidovudine and lamivudine) that were not observed in control mice on placebo. Variants G160S and D181N were observed in mice receiving placebo and antiretroviral therapy.
Discussion
Several infectious agents have been proposed as potential triggers for the development of cholangitis in mouse models 1 and of these, bacteria 7 and MMTV 16 have been linked with autoimmune biliary disease. Since MMTV replication was previously shown to be sensitive to several reverse transcriptase and protease inhibitors 17–19, we tested the effect of combination antiretroviral therapy on MMTV levels and development of cholangitis in NOD.c3c4 mice. Results from this study show that NOD.c3c4 mice treated with Truvada and Kaletra had significant reduction in intrahepatic cholangitis and necroinflammation, as well as in hepatic MMTV levels.
We chose to investigate intrahepatic cholangitis in the NOD.c3c4 mouse, since this model was previously shown to develop cholangitis progressing to liver failure 2,3, whereas other models with immune dysfunction, such as the dnTGFβRII and IL-2Rα−/−, die of extrahepatic diseases, such as inflammatory bowel disease and diffuse inflammation in other organs 25. Additionally, the NOD.c3c4 mouse model had evidence of MMTV infection in bile ducts associated with increased expression of a PDC-E2-like protein 16. While we previously have demonstrated intrahepatic replicative intermediates of MMTV as well as humoral immune responses to MMTV in NOD.c3c4 mice 16, the source of infection has yet to be resolved. This could either occur as a result of an endogenous virus encoded within the genome, an exogenous virus passaged through breast-feeding to weanling pups, or both.
In agreement with previous studies 2,3, penetrance of disease was found to be variable in this study and intrahepatic inflammation was observed in 85% of mice receiving placebo. Notably, we observed cystic dilatation of intrahepatic and extrahepatic bile ducts in all mice sacrificed at 20 weeks, irrespective of inflammation and response to therapy (data not shown). Furthermore, we observed relatively low inflammatory scores, probably because young mice were used for this study and the development of inflammatory disease increases with age. Since the disease was not completely penetrant, large numbers of mice were required to provide significant results. For example, a wide range of baseline alkaline phosphatase levels was seen prior to treatment that necessitated assessment of the reduction from baseline to correct the variability. Furthermore, we found that nearly all 8-week old mice had higher alkaline phosphatase levels that diminished with age but this observation was observed in the parental NOD strain without liver disease, suggesting that the higher alkaline phosphatase levels in 8 week old mice and younger mice may be related to puberty 23.
In the present study, combinations with Truvada had the optimal effect of abrogating cholangitis and lowering alkaline phosphatase, as well as in reducing hepatic MMTV levels. Furthermore, addition of combination protease inhibitors improved histology and biochemistry in all regimens. Interestingly, the combination of the Truvada and Kaletra therapy was reported in a newly diagnosed PBC patient with HIV and human betaretrovirus co-infection, who experienced a marked reduction in alkaline phosphatase from 700 IU/L with over a six-month period. Furthermore, alkaline phosphatase levels were then completely normalized after the institution of ursodeoxycholic acid therapy 26. Also, preliminary results from a randomized control trial of combination Truvada and Kaletra therapy for patients with PBC have reported significant improvement in reduction in alkaline phosphatase, with demonstrable incremental improvement as compared to a prior study using Combivir alone 27.
In contrast with Truvada therapy, we found Combivir therapy was not associated with detectable biochemical or virological response in NOD.c3c4 mice. Furthermore, Truvada and Kaletra had superior biochemical impact vs. Combivir and Kaletra, whereas both regimens had differential effects on histology; however, the studies were not powered to assess absolute differences between treatments. Previous head to head studies of Truvada vs. Combivir in patients with HIV infection have demonstrated the superiority of Truvada with regard to HIV suppression, lack of resistance to therapy and increased CD4 count 28.
Combivir therapy has been used in prior clinical studies to assess the effect of antiviral therapy on PBC progression; however, results from these studies were not promising 21,26,29,30. In a randomized controlled trial for 6 months using Combivir vs. placebo, no significant differences in endpoints were reached 29. However, we have gained valuable insight into the use of antiviral therapy, as significant improvements in alkaline phosphatase, cholangitis and ductopaenia were observed with Combivir treatment 21,27,29. Unfortunately, some patients demonstrated circumstantial evidence of resistance to Combivir therapy with biochemical rebound 30.
Similar findings were observed in this study, where preliminary evidence suggests that MMTV might have developed resistance to Combivir. Two of four mice treated with Combivir had unique variants of MMTV pol gene. Of particular interest, the M188V MMTV polymerase variant was detected, which is a recognized ‘YMDD’ mutant found in a similar position to the M204V mutation observed with hepatitis B virus reverse transcriptase protein associated with Lamivudine resistance and the M184V mutation seen in HIV reverse transcriptase associated with resistance to Combivir therapy 24. Further studies are required to determine whether MMTV encoding these specific reverse transcription variants are resistant to antiretroviral therapy.
In the current study, we limited our analysis to clinical parameters such as liver biochemistry and histology rather than the immune function, to test whether there was a relationship with MMTV and clinical parameters of cholangitis. Further analysis of humoral and cellular immune responses will be necessary to determine the effects of MMTV on the development of loss of tolerance to mitochondrial proteins. As AMA production occurs in less than 25% of mice by 20 weeks but increases with age 2,16, a larger sample size and a longer duration of treatment will be required to test whether antiretroviral therapy impacts on AMA production. Furthermore, another feature of autoimmune phenomena that requires investigation is that autoimmune biliary disease can be transferred by splenocytes from a syngeneic NOD.c3c4 mouse into an irradiated mice 25. Accordingly, further studies are required to investigate (i) whether MMTV may be passaged into syngeneic irradiated mice during transfer of NOD.c3c4 splenocytes and (ii) whether inhibition of MMTV replication would prevent the development of autoimmune biliary disease in irradiated NOD.c3c4 recipients following transfer.
In summary, we report that combination retroviral therapies that attenuate MMTV replication significantly impacted the development of intrahepatic cholangitis in the NOD.c3c4 mice. However, further studies are required to determine whether MMTV directly triggers cholangitis in these mice.
Acknowledgments
Financial support: This research was supported by a fellowship from the Canadian Association of Gastroenterology/Canadian Institute for Health Research (GZ), a graduate student award from the Canadian Liver Foundation (DG) and a Senior Scholar award from the Alberta Heritage Foundation for Medical Research (ALM) as well as operating funds from the Canadian Institutes of Health Research and the Canadian Liver Foundation (MOP 97798).
Conflict of interest: AbbVie and Gilead Sciences are providing medications for ongoing clinical trial using Truvada and Kaletra for patients with PBC. The authors do not have any disclosures to report.
Glossary
- AMA
antimitochondrial antibodies
- dnTGFβRII
TGF-β receptor II dominant-negative mouse
- IL-2Rα−/−
interleukin 2 receptor α deficient mouse
- MMTV
mouse mammary tumour virus
- PBC
primary biliary cirrhosis
- PDC-E2
pyruvate dehydrogenase complex-E2
References
- Hirschfield GM, Chapman RW, Karlsen TH, Lammert F. The genetics of complex cholestatic disorders. Gastroenterology. 2013;144:1357–74. doi: 10.1053/j.gastro.2013.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie J, Wu Y, Wicker LS, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–19. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarada S, Wu Y, Fertig N, et al. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–23. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- Oertelt S, Lian ZX, Cheng CM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–60. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- Salas JT, Banales JM, Sarvide S, et al. Ae2a, b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–93. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Lian Z-X, Moritoki Y, et al. IL-2 receptor α−/− mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–9. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- Mattner J, Savage PB, Leung P, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–15. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Lian ZX, Leung PS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–40. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sakalian M, Shen Z, Loss G, Neuberger J, Mason A, et al. Cloning the human betaretrovirus proviral genome from patients with primary biliary cirrhosis. Hepatology. 2004;39:151–6. doi: 10.1002/hep.20024. [DOI] [PubMed] [Google Scholar]
- Xu L, Shen Z, Guo L, et al. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci of the U S A. 2003;100:8454–9. doi: 10.1073/pnas.1433063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AL, Xu L, Guo L, et al. Detection of retroviral antibodies in primary biliary cirrhosis and other idiopathic biliary disorders. Lancet. 1998;351:1620–4. doi: 10.1016/S0140-6736(97)10290-2. [DOI] [PubMed] [Google Scholar]
- Mason AL. The evidence supports a viral aetiology for primary biliary cirrhosis. J Hepatol. 2011;54:1312–4. doi: 10.1016/j.jhep.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Johal H, Scott GM, Jones R, et al. Mouse mammary tumour virus-like virus (MMTV-LV) is present within the liver in a wide range of hepatic disorders and unrelated to nuclear p53 expression or hepatocarcinogenesis. J Hepatol. 2009;50:548–54. doi: 10.1016/j.jhep.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Selmi C, Ross SR, Ansari AA, et al. Lack of immunological or molecular evidence for a role of mouse mammary tumor retrovirus in primary biliary cirrhosis. Gastroenterology. 2004;127:493–501. doi: 10.1053/j.gastro.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Wang W, Wasilenko S, Indik S, Wong G, Mason A, et al. Isolation of the human betaretrovirus and demonstration of integration sites in patients with primary biliary cirrhosis. Can J Gastroenterol. 2012;26:84A. [Google Scholar]
- Zhang G, Chen M, Graham D, et al. Mouse mammary tumor virus in anti-mitochondrial antibody producing mouse models. J Hepatol. 2011;55:876–84. doi: 10.1016/j.jhep.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Held W, Waanders GA, Acha-Orbea H, MacDonald HR, et al. Reverse transcriptase-dependent and -independent phases of infection with mouse mammary tumor virus: implications for superantigen function. J Exp Med. 1994;180:2347–51. doi: 10.1084/jem.180.6.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihlar T, Douglas JL, Gibbs CS, et al. Methods of Inhibition of MMTV-Like Viruses. USA: Gilead Sciences Inc; 2005. U.P.a.T. Office, Editor. [Google Scholar]
- Montano-Loza AJ, Wasilenko S, Bintner J, Mason AL. Cyclosporine A inhibits in vitro replication of betaretrovirus associated with primary biliary cirrhosis. Liver Int. 2009;30:871–7. doi: 10.1111/j.1478-3231.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Mason AL, et al. Pilot studies of single and combination antiretroviral therapy in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2348–55. doi: 10.1111/j.1572-0241.2004.40741.x. [DOI] [PubMed] [Google Scholar]
- Sis B, Dadras F, Khoshjou F. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006;6:1444–50. doi: 10.1111/j.1600-6143.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- Dimai HP, Linkhart TA, Linkhart SG. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone. 1998;22:211–6. doi: 10.1016/s8756-3282(97)00268-8. [DOI] [PubMed] [Google Scholar]
- Locarnini S, Warner N. Major causes of antiviral drug resistance and implications for treatment of hepatitis B virus monoinfection and coinfection with HIV. Antivir Ther. 2007;12(Suppl. 3):H15–23. [PubMed] [Google Scholar]
- Mason AL. An autoimmune biliary disease mouse model for primary biliary cirrhosis: something for everyone. Hepatology. 2006;44:1047–50. doi: 10.1002/hep.21390. [DOI] [PubMed] [Google Scholar]
- Schembri G, Schober P. Killing two birds with one stone. The Lancet. 2011;377:96. doi: 10.1016/S0140-6736(10)61343-8. [DOI] [PubMed] [Google Scholar]
- Mason AL, Montano-Loza AJ, Saxinger L. Letter: biochemical response to combination anti-retroviral therapy in patients with primary biliary cirrhosis. Aliment Pharmacol Ther. 2014;39:236–7. doi: 10.1111/apt.12575. [DOI] [PubMed] [Google Scholar]
- Gallant JE, Dejesus E, Arribas JR. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- Mason AL, Lindor KD, Bacon BR, Vincent C, Neuberger JM. Clinical trial: randomized controlled study of zidovudine and lamivudine for patients with primary biliary cirrhosis stabilized on ursodiol. Aliment Pharmacol Ther. 2008;28:886–94. doi: 10.1111/j.1365-2036.2008.03799.x. [DOI] [PubMed] [Google Scholar]
- Mason AL, Wasilenko ST. Other potential medical therapies: the use of antiviral agents to investigate and treat primary ciliary cirrhosis. Clin Liver Dis. 2008;12:445–60. doi: 10.1016/j.cld.2008.02.006. ; xi. [DOI] [PubMed] [Google Scholar]