Abstract
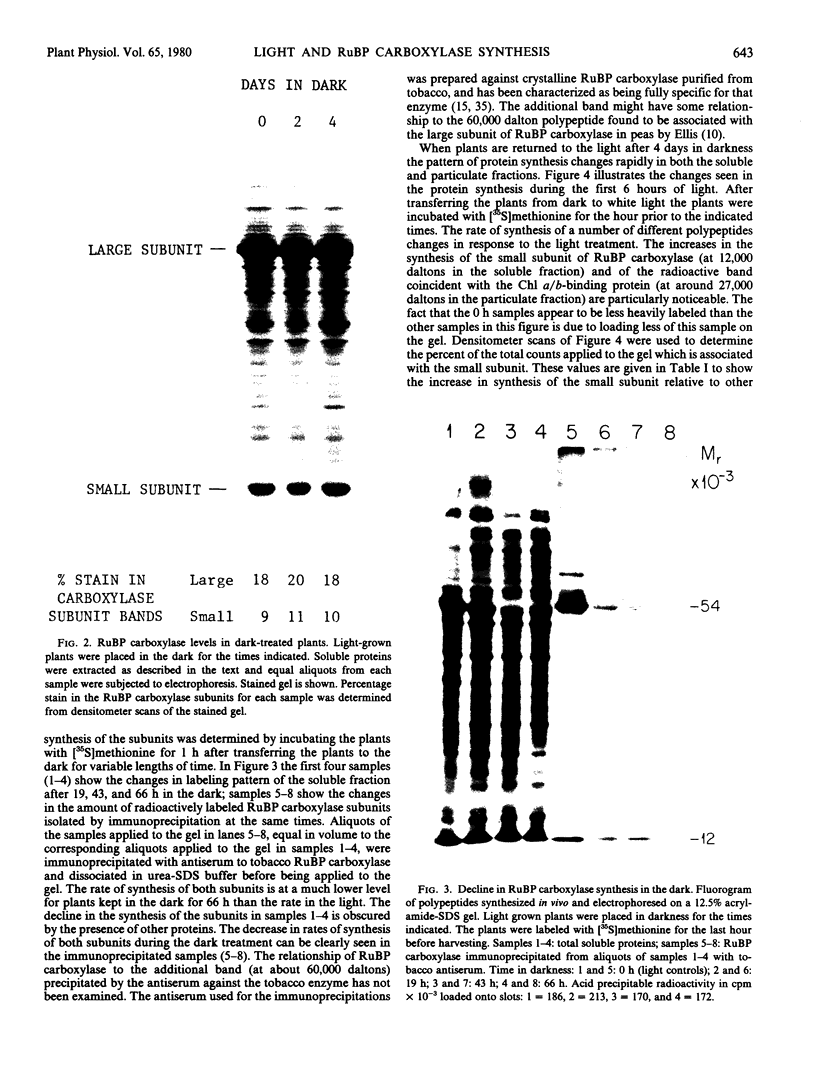
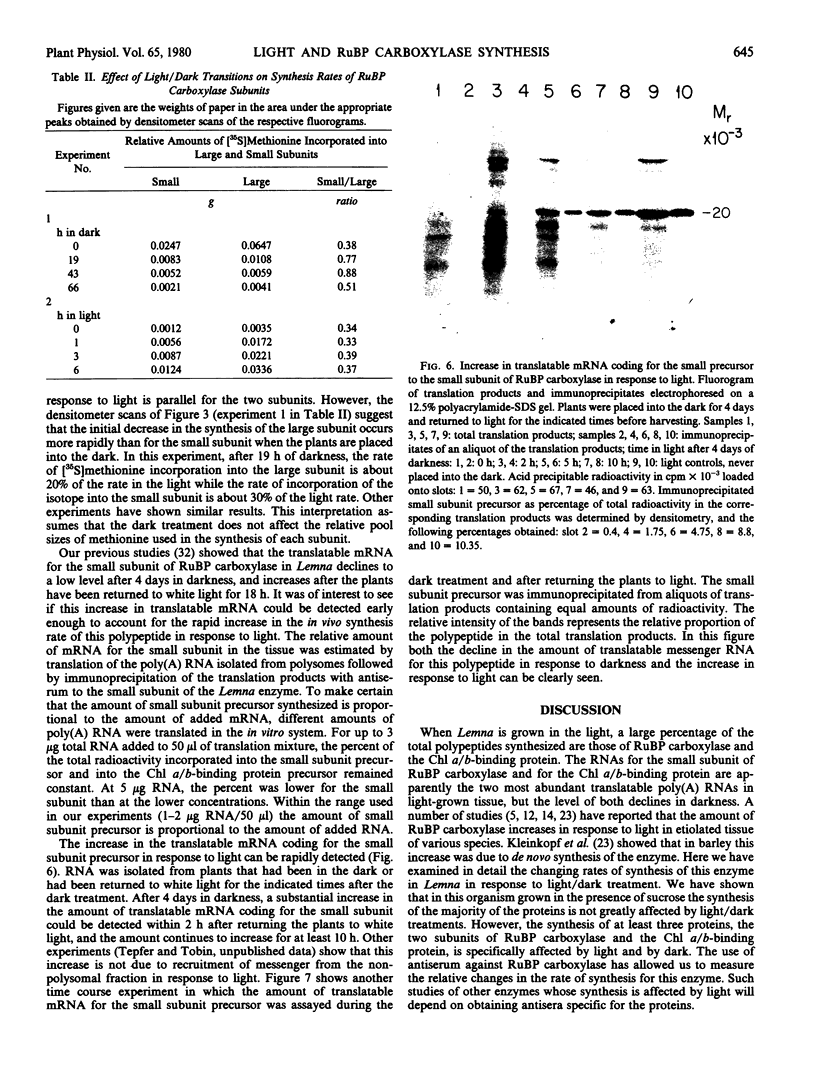
Placing light-grown Lemna gibba L. G-3 into the dark results in a changed pattern of protein synthesis. Although the amount of protein in the tissue and the over-all rate of incorporation of [35S]methionine into protein does not significantly decline during four days of darkness, the rate of synthesis of three polypeptides declines dramatically. One of these polypeptides is the chlorophyll a/b-binding protein and the two others are the large and small subunits of ribulose-1,5-bisphosphate carboxylase. The changed rates of synthesis of the two subunits were examined after transitions of plants from light to dark and dark to light. The in vivo synthesis of both subunits, while declining to a low level during four days of darkness, increases rapidly upon returning the plants to white light. In addition, the level of poly(A) mRNA coding for the precursor polypeptide of the small subunit of the enzyme falls to a low level in the dark and increases rapidly in response to white light. The increase in translatable mRNA for the small subunit is rapid enough to account for a major part of the increased synthesis of this subunit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Barraclough R., Ellis R. J. The biosynthesis of ribulose bisphosphate carboxylase. Uncoupling of the synthesis of the large and small subunits in isolated soybean leaf cells. Eur J Biochem. 1979 Feb 15;94(1):165–177. doi: 10.1111/j.1432-1033.1979.tb12883.x. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Protein synthesis in plant leaf tissue. The sites of synthesis of the major proteins. J Biol Chem. 1976 May 10;251(9):2848–2853. [PubMed] [Google Scholar]
- Cleland C. F., Briggs W. S. Gibberellin and CCC Effects on Flowering and Growth in the Long-day Plant Lemna gibba G3. Plant Physiol. 1969 Apr;44(4):503–507. doi: 10.1104/pp.44.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle R. S., Dau B., Kleinkopf G. E., Huffaker R. C. Differential synthesis of ribulosediphosphate carboxylase subunits. Biochem Biophys Res Commun. 1970 Nov 9;41(3):621–627. doi: 10.1016/0006-291x(70)90058-6. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Wildner G. Formation of the small subunit in the absence of the large subunit of ribulose 1,5-bisphosphate carboxylase in 70 S ribosome-deficient rye leaves. Arch Biochem Biophys. 1978 Mar;186(2):283–291. doi: 10.1016/0003-9861(78)90437-x. [DOI] [PubMed] [Google Scholar]
- Filner B., Klein A. O. Changes in enzymatic activities in etiolated bean seedling leaves after a brief illumination. Plant Physiol. 1968 Oct;43(10):1587–1596. doi: 10.1104/pp.43.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai A., Wildman S. G. Kinetic analysis of fraction I protein biosynthesis in young protoplasts of tobacco leaves. Biochim Biophys Acta. 1977 Nov 2;479(1):39–52. doi: 10.1016/0005-2787(77)90124-1. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Stegeman W. J. Kinetics and regulation of synthesis of the major polypeptides of thylakoid membranes in Chlamydomonas reinhardtii y-1 at elevated temperatures. J Cell Biol. 1976 Aug;70(2 Pt 1):326–337. doi: 10.1083/jcb.70.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanij V., Chua N. H., Siekevitz P. Synthesis and turnover of ribulose biphosphate carboxylase and of its subunits during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1975 Mar;64(3):572–585. doi: 10.1083/jcb.64.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N. Non-synchronous incorporation of C14O2 into amino acids of the two subunits of fraction I protein. Biochem Biophys Res Commun. 1970 Jan 6;38(1):119–124. doi: 10.1016/0006-291x(70)91092-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner A., Jakob K. M., Gressel J., Sagher D. The early synthesis and possible function of a 0.5 X 10(6) Mr RNA after transfer of dark-grown Spirodela plants to light. Biochem Biophys Res Commun. 1975 Nov 3;67(1):383–391. doi: 10.1016/0006-291x(75)90327-7. [DOI] [PubMed] [Google Scholar]
- Schantz R., Bar-Nun S., Ohad I. Preparation of Antibodies against Specific Chloroplast Membrane Polypeptides Associated with the Formation of Photosystems I and II in Chlamydomonas reinhardi y-1. Plant Physiol. 1977 Feb;59(2):167–172. doi: 10.1104/pp.59.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Tobin E. M., Klein A. O. Isolation and translation of plant messenger RNA. Plant Physiol. 1975 Jul;56(1):88–92. doi: 10.1104/pp.56.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M. Light regulation of specific mRNA species in Lemna gibba L. G-3. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4749–4753. doi: 10.1073/pnas.75.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Key J. L., Ross C. W. Activation of 80S Maize Ribosomes by Red Light Treatment of Dark-grown Seedlings. Plant Physiol. 1974 Jan;53(1):28–31. doi: 10.1104/pp.53.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]








