Abstract
Pea cotyledons were injected with d-[14C]mannose or d-[14C]-glucosamine and incubated for 1 to 1.5 hours. Cotyledons were homogenized and subcellular fractions were isolated by differential centrifugation followed by linear sucrose density gradient centrifugation.
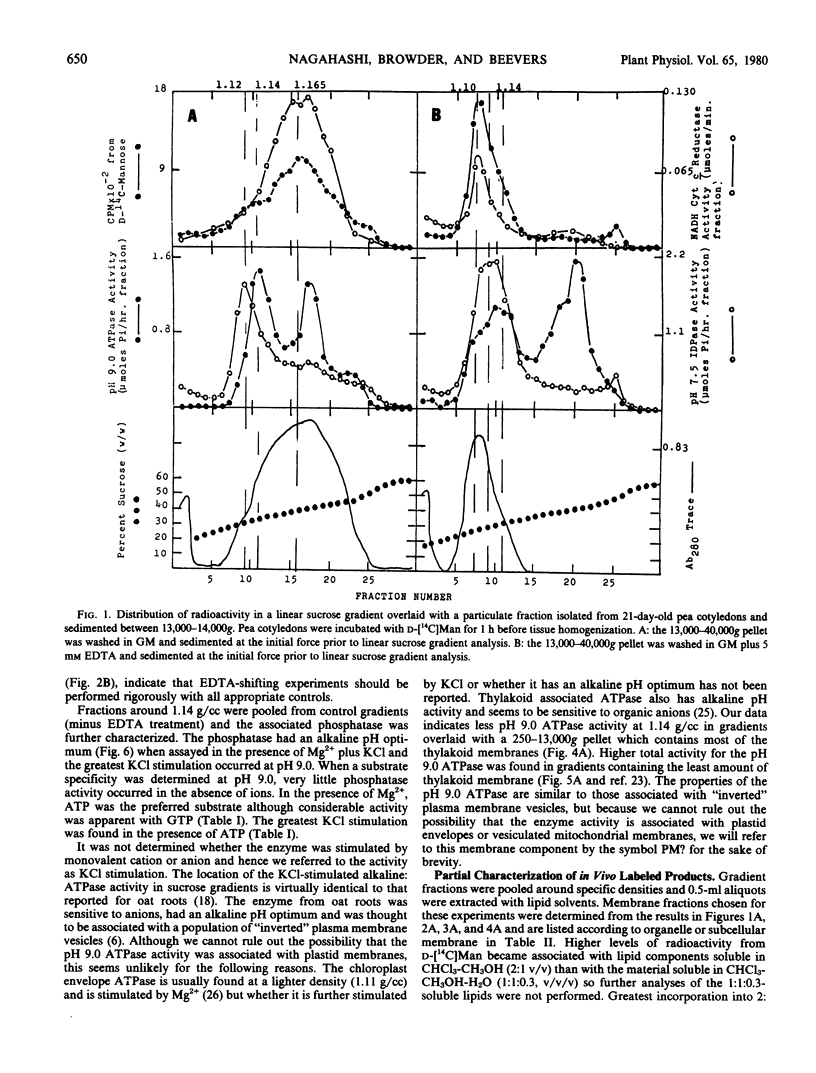
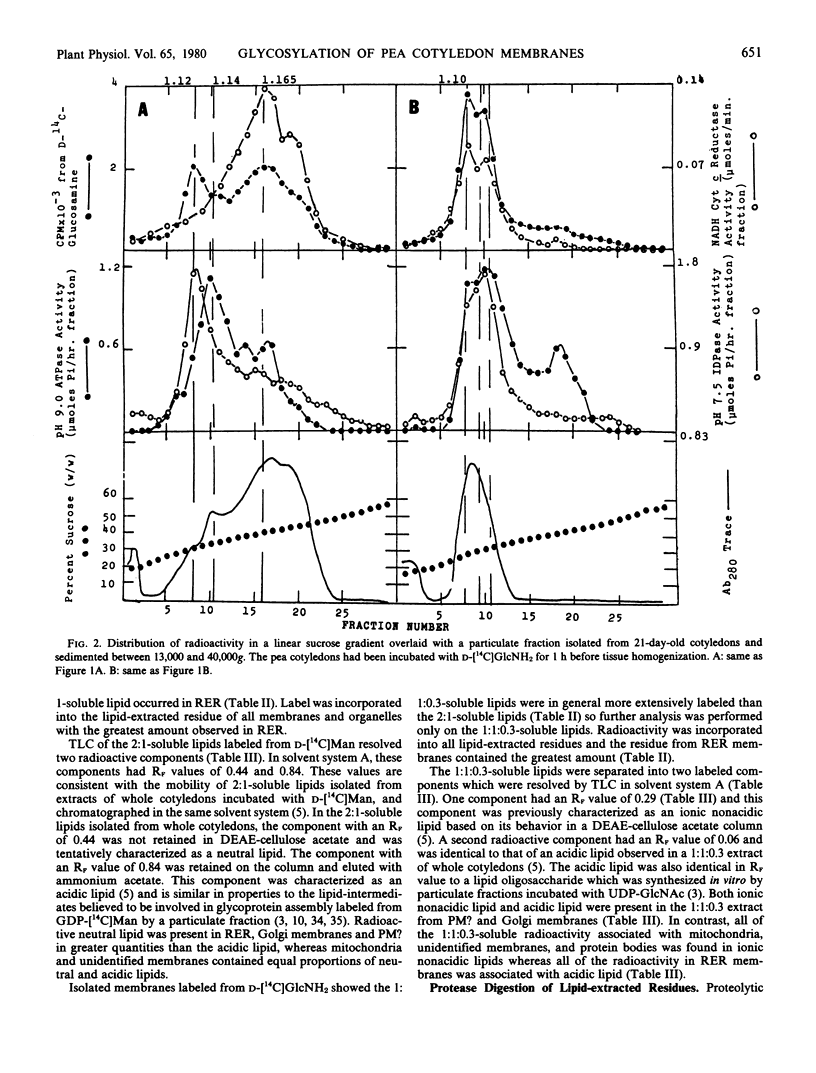
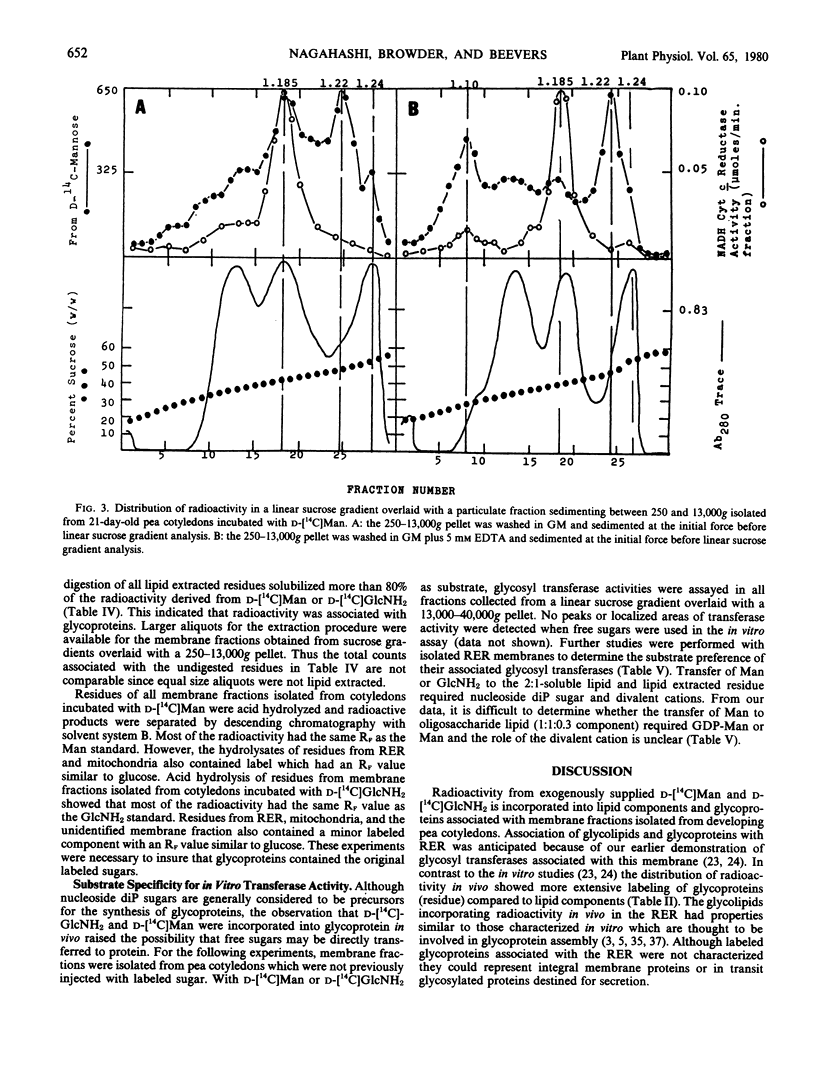
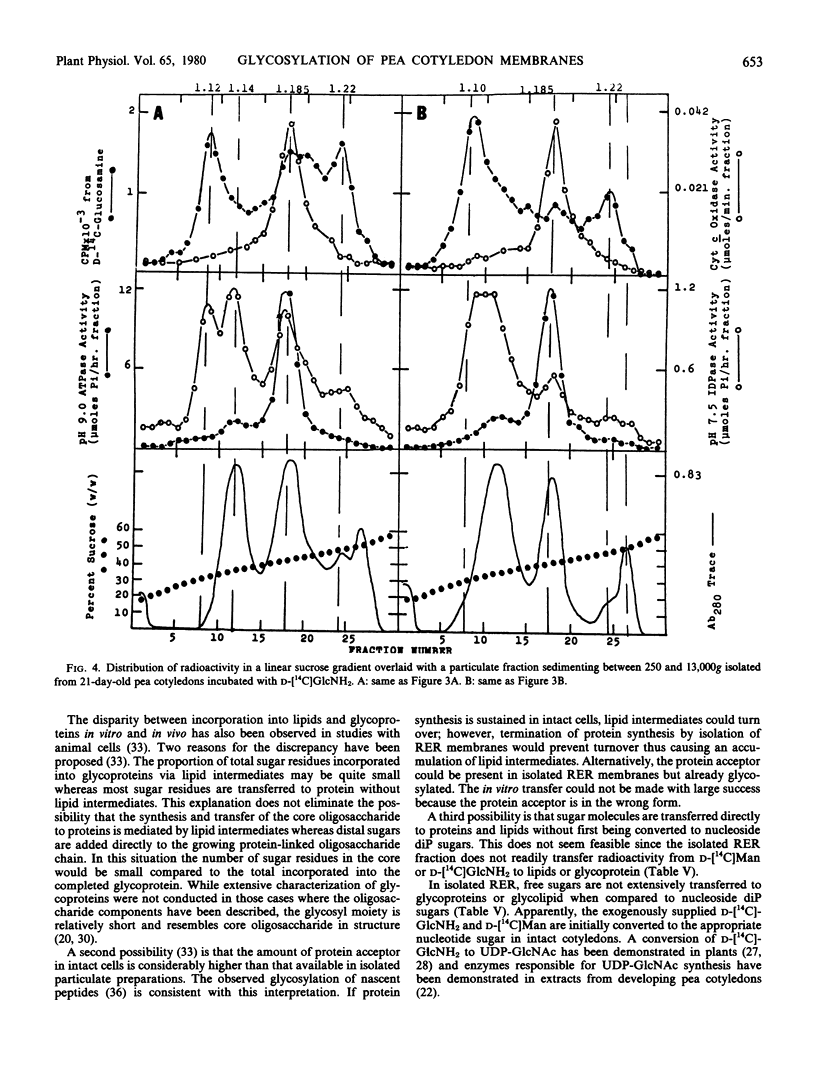
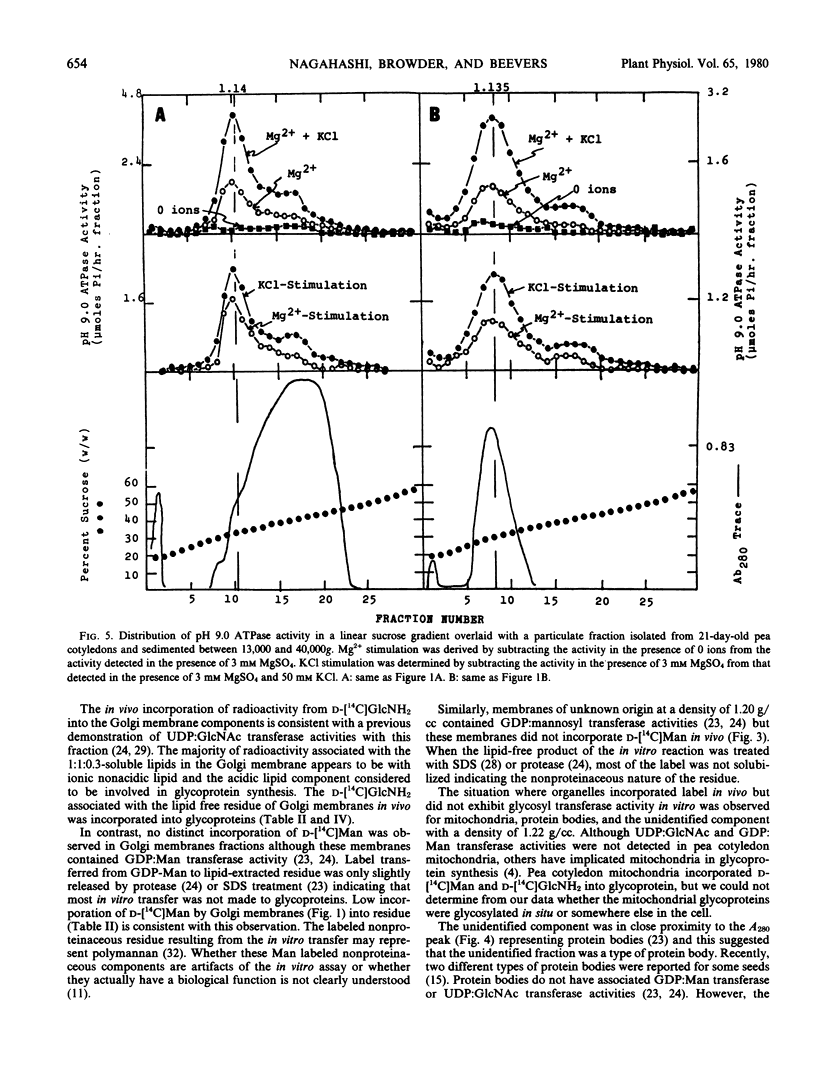
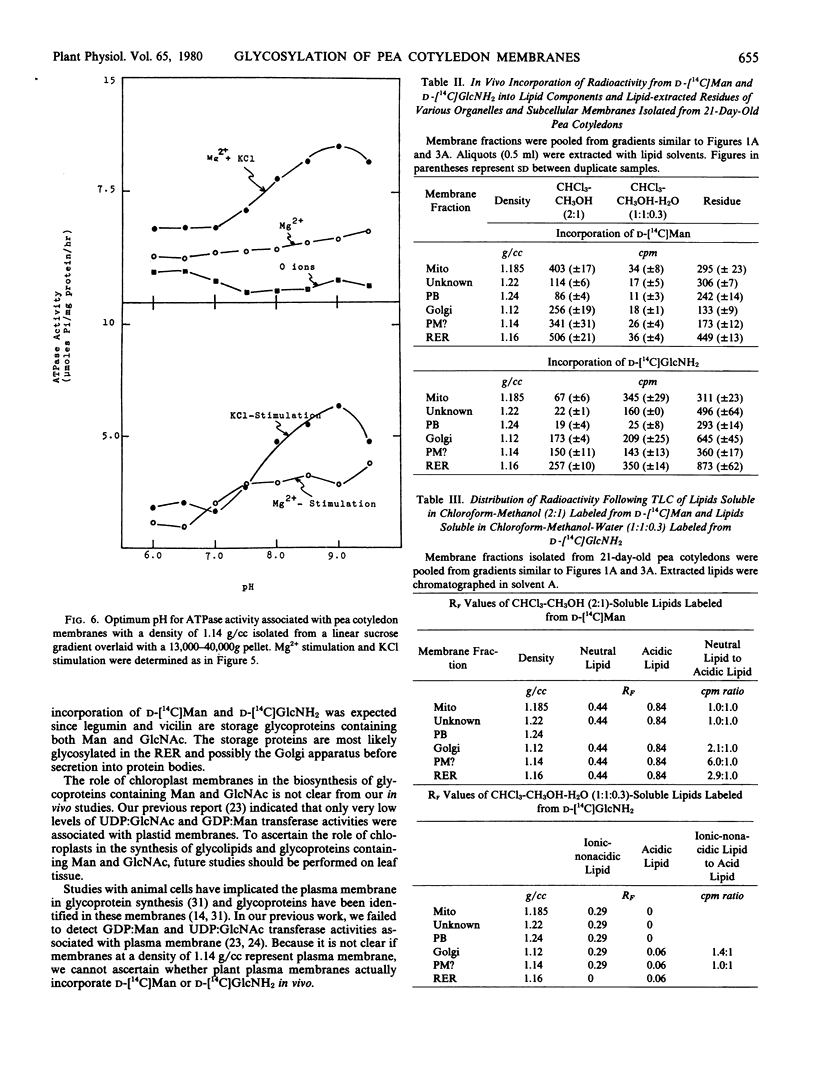
Radioactivity that was precipitated by trichloroacetic acid was associated most extensively with rough endoplasmic reticulum, Golgi membranes, a membrane with a density of 1.14 grams per cubic centimeter (possibly plasma membrane) and an unidentified subcellular component with a density of 1.22 grams per cubic centimeter. Lower levels of incorporation were observed in protein bodies and mitochondria.
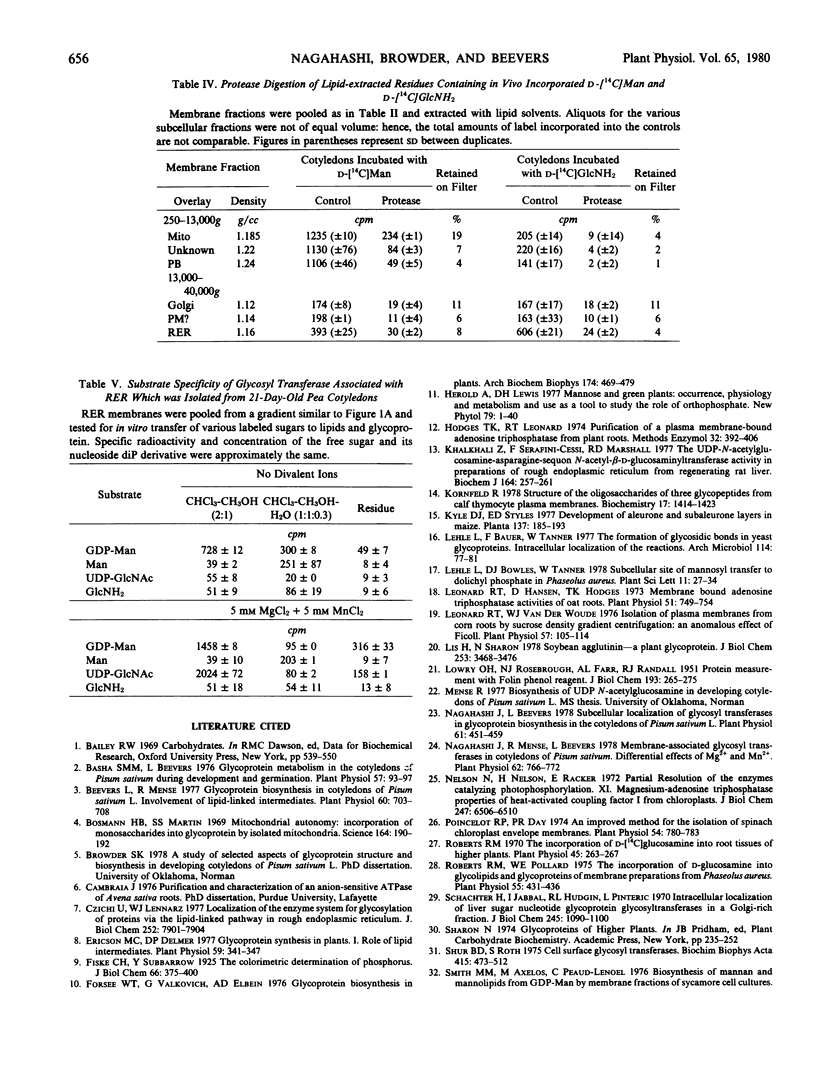
Isolated membrane fractions were lipid-extracted to determine which components of the membrane contained the label. Rough endoplasmic reticulum contained the most extensively labeled lipids which had similar properties to the lipid intermediates thought to be involved in glycoprotein assembly. The lipid free residues of the various membrane fractions contained radioactivity that was released by protease treatment. Acid hydrolysis of the residues indicated that most of the radioactivity was associated with mannose or glucosamine. It appears that various subcellular components of the pea cotyledon possess glycoproteins that contain mannose and glucosamine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Beevers L. Glycoprotein Metabolism in the Cotyledons of Pisum sativum during Development and Germination. Plant Physiol. 1976 Jan;57(1):93–97. doi: 10.1104/pp.57.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Mense R. M. Glycoprotein Biosynthesis in Cotyledons of Pisum sativum L: Involvement of Lipid-linked Intermediates. Plant Physiol. 1977 Nov;60(5):703–708. doi: 10.1104/pp.60.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Martin S. S. Mitochondrial autonomy: incorporation of monosaccharides into glycoprotein by isolated mitochondria. Science. 1969 Apr 11;164(3876):190–192. doi: 10.1126/science.164.3876.190. [DOI] [PubMed] [Google Scholar]
- Czichi U., Lennarz W. J. Localization of the enzyme system for glycosylation of proteins via the lipid-linked pathway in rough endoplasmic reticulum. J Biol Chem. 1977 Nov 25;252(22):7901–7904. [PubMed] [Google Scholar]
- Ericson M. C., Delmer D. P. Glycoprotein synthesis in plants: I. Role of lipid intermediates. Plant Physiol. 1977 Mar;59(3):341–347. doi: 10.1104/pp.59.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsee W. T., Valkovich G., Elbein A. D. Glycoprotein biosynthesis in plants. Formation of lipid-linked oligosaccharides of mannose and N-acetylglucosamine by mung bean seedlings. Arch Biochem Biophys. 1976 Jun;174(2):469–479. doi: 10.1016/0003-9861(76)90375-1. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Khalkhali Z., Serafini-Cessi F., Marshall R. D. The UDP-N-acetylglucosamine-asparagine-sequon N-acetyl-beta-D-glucosaminyltransferase activity in preparations of rough endoplasmic reticulum from regenerating rat liver. Biochem J. 1977 Apr 15;164(1):257–261. doi: 10.1042/bj1640257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. Structure of the oligosaccharides of three glycopeptides from calf thymocyte plasma membranes. Biochemistry. 1978 Apr 18;17(8):1415–1423. doi: 10.1021/bi00601a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehle L., Bauer F., Tanner W. The formation of glycosidic bonds in yeast glycoproteins. Intracellular localisation of the reactions. Arch Microbiol. 1977 Jul 26;114(1):77–81. doi: 10.1007/BF00429634. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Mense R. M., Beevers L. Membrane-associated Glycosyl Transferases in Cotyledons of Pisum sativum: Differential Effects of Magnesium and Manganese Ions. Plant Physiol. 1978 Nov;62(5):766–772. doi: 10.1104/pp.62.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling factor I from chloroplasts. J Biol Chem. 1972 Oct 25;247(20):6506–6510. [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. An improved method for the isolation of spinach chloroplast envelope membranes. Plant Physiol. 1974 Nov;54(5):780–783. doi: 10.1104/pp.54.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M., Pollard W. E. The Incorporation of d-Glucosamine into Glycolipids and Glycoproteins of Membrane Preparations from Phaseolus aureus Hypocotyls. Plant Physiol. 1975 Mar;55(3):431–436. doi: 10.1104/pp.55.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M. The incorporation of D-glucosamine-14C into root tissues of higher plants. Plant Physiol. 1970 Mar;45(3):263–267. doi: 10.1104/pp.45.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Speake B. K., White D. A. The formation of lipid-linked sugars as intermediates in glycoprotein synthesis in rabbit mammary gland. Biochem J. 1978 Feb 15;170(2):273–283. doi: 10.1042/bj1700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Tucker P., Pestka S. De novo synthesis and glycosylation of the MOPC-46B mouse immunoglobulin light chain in cell-free extracts. J Biol Chem. 1977 Jul 10;252(13):4474–4486. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]



