Abstract
Rationale
Nicotine and ethanol are commonly coabused drugs, and nicotine-laced ethanol products are growing in popularity. However, little is known about time-course changes in extracellular nicotine and cotinine levels in rat models of ethanol and nicotine coabuse.
Objectives
The objective of the present study was to determine the time-course changes in brain levels of nicotine and cotinine following subcutaneous (SC) and intragastric (IG) nicotine administration in alcohol-preferring (P) and Wistar rats.
Methods
In vivo microdialysis was used to collect dialysate samples from the nucleus accumbens shell (NACsh) for nicotine and cotinine determinations, following SC administration of (−)-nicotine (0.18, 0.35, and 0.70 mg/kg) in female P and Wistar rats or IG administration of (−)-nicotine (0.35 and 0.70 mg/kg) in 15 % (v/v) ethanol or water in female P rats.
Results
SC nicotine produced nicotine and cotinine dialysate levels as high as 51 and 14 ng/ml, respectively. IG administration of 15 % EtOH + 0.70 mg/kg nicotine in P rats resulted in maximal nicotine and cotinine dialysate levels of 19 and 14 ng/ml, respectively, whereas administration of 0.70 mg/kg nicotine in water resulted in maximal nicotine and cotinine levels of 21 and 25 ng/ml, respectively. Nicotine and cotinine levels were detectable within the first 15 and 45 min, respectively, after IG administration.
Conclusions
Overall, the results of this study suggest that nicotine is rapidly adsorbed and produces relevant extracellular brain concentrations of nicotine and its pharmacologically active metabolite, cotinine. The persisting high brain concentrations of cotinine may contribute to nicotine addiction.
Keywords: Nicotine, Metabolism, Microdialysis, Nucleus accumbens, Alcohol, Rat, Brain
Introduction
Although many studies have examined the metabolism of nicotine in the periphery (Kyerematen and Vesell 1991; Gorrod and Wahren 1993; Gorrod and Jacobs 1999), few studies have examined the levels of nicotine and its metabolites in the brain following peripheral nicotine administration. Examining the profile of nicotine metabolites in the brain is important because of their potential to produce neuropharmacological effects. In addition, blood levels of nicotine and cotinine may not accurately reflect brain levels, especially after repeated nicotine administration regimens (Ghosheh et al. 1999).
The major nicotine metabolite, cotinine, is found in the brain of mice and cats after intravenous nicotine administration (Applegren et al. 1962; Deutsch et al. 1992). It has also been demonstrated that cotinine resides in the brain six times longer than nicotine after peripheral administration (Ghosheh et al. 1999). In addition to cotinine, other nicotine metabolites are found in the brain after peripheral nicotine administration (Schmiterlöw et al. 1967; Peterson et al. 1984; Crooks et al. 1995). Cotinine has been shown to be pharmacologically active. For example, cotinine increases urinary 5-hydroxyindole acetic acid in smokers and increases serotonin turnover in the rat brain (Essman 1973). Furthermore, cotinine administration to abstinent smokers attenuated the desire to smoke and decreased irritability, restlessness, anxiety, tension, and insomnia associated with nicotine abstinence (Benowitz and Jacob 1993; Keenan et al. 1994).
Innate differences in nicotine metabolism may be responsible for differential behavioral and/or neurochemical responses to nicotine. However, the Alko alcohol (AA) and Alko non-alcohol (ANA) rat lines, selectively bred for differences in voluntary ethanol consumption (Eriksson 1968, 1969), displayed identical plasma nicotine concentrations after subcutaneous (SC) nicotine administration (Kiianmaa et al. 2000). In contrast, nicotine and cotinine levels were higher in the blood of Lewis compared to Fisher rats at 15 min post-intravenous injection. However, brain nicotine levels were statistically similar between the Lewis and Fisher rat strains (Sziraki et al. 2001).
A recent binge ethanol-nicotine coabuse model produced pharmacologically relevant blood levels of both drugs following oral consumption (Hauser et al. 2012). Oral intake of nicotine is on the increase in the USA with the introduction of nicotine-containing products specifically designed for oral intake. Some notable examples of these products include nicotine-laced water, nicotine-infused fruit drinks (Platinum Products), beer brewed with tobacco, nicotine energy drinks, as well as candy-like oral nicotine products. Therefore, it would be important to have a better understanding of the time-course changes of nicotine levels in the brain following the oral route of administration. Nicotine is subject to first-pass metabolism resulting in removal of approximately 70 % of the drug from the blood as it passes through the liver (Matta et al. 2007). Therefore, only 30 % of orally or systemically administered nicotine can be expected to reach the circulation, with the other 70 % being metabolized primarily to cotinine. Thus, when conducting researchwhere nicotine is given orally or systemically, it is important to consider first-pass metabolism and its effect on the relative exposure to nicotine, as well as the resultant effects of accumulating cotinine levels (Matta et al. 2007; Benowitz et al. 1990). Few, if any, studies have examined the time-course of in vivo concentrations of nicotine and cotinine in the brain after oral nicotine administration.
The objectives of the present study were to determine the time-course changes in the brain levels of nicotine and cotinine following (1) SC nicotine administration in P and Wistar rats and (2) intragastric (IG) administration of nicotine, with or without ethanol in P rats.
Methods
Animals
Adult, drug-naïve, female alcohol-preferring P rats, from the 69th generation, and Wistar rats (Harlan, Indianapolis, IN) weighing 270–370 g at time of surgery were used for the SC nicotine experiment. For the IG experiment, adult, drug naïve, female P rats, from the 69th, 70th, and 71st generations, weighing 280–370 g at time of surgery were used. Rats were housed in temperature- and humidity-controlled rooms maintained on a reversed 12-h light cycle (lights on at 9:00 p.m.). Food and water were available ad libitum. Female rats were used because these rats maintain their head size better than male rats for more accurate stereotaxic placements. Wistar rats were used as the outbred progenitor strain to compare with the selected P line, to establish the dose effects in the SC experiments, and to include a strain used in previous nicotine work. Since P rats drink ethanol and nicotine solutions and are currently being used to study oral ethanol/nicotine coabuse (Hauser et al. 2012), they were used in the IG nicotine/nicotine+ethanol experiments. All protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were conducted in accordance with the principles of laboratory animal care as outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Nicotine administration
Separate groups of female P and Wistar rats were used for each dose of (−)-nicotine (0.18, 0.35, and 0.70 mg/kg; free base) administered subcutaneously. Nicotine was dissolved in sterile saline (0.9%), and the pH was adjusted to 7.2–7.4 prior to injection. The volume per unit body weight of the nicotine solution administered subcutaneously was 1.0 ml/kg. The SC administration is a typical route of administration for systemic nicotine treatment in animal models, so it was important to determine if there were any differences in time-course nicotine levels in the brain between strains using this route of administration.
Separate groups of female P rats were used for each IG administered dose of (−)-nicotine: (0.35 or 0.70 mg/kg) in 15 % (v/v) ethanol or 0.70 mg/kg nicotine in water. The concentration of the nicotine in all IG solutions administered was 0.05 mg/ml. The volume per unit body weight administered IG was 7.0 ml/kg for the 0.35 mg/kg and 14.0 ml/kg for the 0.70 mg/kg nicotine treatments. A curved stainless steel gavage needle was used to administer all IG solutions.
In vitro probe recovery
An in vitro probe recovery experiment was performed to determine the percent relative recoveries of nicotine and cotinine. Two probes with an active membrane length of 2 mm were immersed in a beaker containing three different concentrations of nicotine and cotinine (0, 50, 150, and 200 ng/ml; free base) in artificial cerebrospinal fluid (aCSF) at 20 to 21 °C, while solutions were constantly stirred. Four dialysate samples were collected every 15 min at a flow rate of 2.0 µl/ min for each of the three nicotine and cotinine concentrations given in ascending order.
In vivo microdialysis
Stereotaxic surgery for insertion of guide cannulae and probes followed the procedure described previously (Ding et al. 2009). Briefly, guide cannulae were stereotaxically implanted 3.0 mm above the nucleus accumbens shell subregion (NACsh) [AP + 1.2 mm, ML +2.3 mm, DV −5.4 mm], according to the brain atlas of Paxinos and Watson (1998). The NACsh was targeted as it is a component of the mesolimbic dopamine system, is thought to be involved in both ethanol and nicotine reward, and may be a key site in mediating their coabuse (Lee and Messing 2011). Stylets were inserted into the cannula when no experiments were being conducted. Rats were allowed to recover from surgery for at least 5 days before testing.
Loop-style dialysis probes with active membrane length 2.0 mm were inserted into the NACsh (Engleman et al. 2004; Perry and Fuller 1992). Perfusion of probes started on the microdialysis day approximately 16–18 h after probe insertion, as previously described (Ding et al. 2009). On microdialysis day, rats were placed into Plexiglas chambers and connected to a Harvard pump with PE20 tubing; aCSF (140.0 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2, 2.0 mM Na2HPO4, 1.0 mMMgCl2, pH7.2–7.4) was perfused through the probes at a rate of 2.0 µl/min. Baseline samples were collected following a 90-min washout period. Samples were collected at 15-min intervals and were frozen immediately on dry ice before being stored at −70 °C. For SC and IG experiments, samples were collected 5 to 125 min and 15 to 195 min, respectively, postinjection.
At the end of each experiment, rats were euthanized and 1 % bromophenol blue was perfused through probes in the NACsh. Brains were removed quickly and frozen immediately on dry ice and stored at −20 °C. Sections (40 µm) were sliced on a cryostat microtome and stained with cresyl violet for verification of probe placement with reference to the rat brain atlas of Paxinos and Watson (1998). Probes were mainly located in the NACsh (at least 75 % of the active membrane). Approximately 85 % of rats had correct placements and were included in the analysis.
Nicotine and cotinine analysis
All chemicals and reagents were purchased from Sigma- Aldrich, St. Louis, MO, USA, except acetonitrile, which was purchased from Fisher Scientific, Fairlawn, NJ, USA. Triethylamine and acetonitrile were HPLC grade.
Dialysates (15 µl/sample) were analyzed to determine nicotine and cotinine concentrations using high-performance liquid chromatographywith ultraviolet detection. Separation was performed on a Zorbax SB-C8 column (2.1 × 100 mm, 3.5 um; Agilent Technology). The mobile phase contained 15 % (v/v) acetonitrile in phosphate-citrate buffer (30 mM K2HPO4, 30 mM citric acid, 0.5 % (v/v) triethylamine-adjusted to pH 6.7 with 10 M NaOH). The mobile phase was maintained at a flow rate of 0.2 ml/min, and analytes were detected by their UV absorbance at 260 nm using an ESA Model 520 UV/VIS detector (Thermo Scientific, Sunnyvale, CA, USA) or a Shimadzu model SPD-20A UV/VIS detector (Shimadzu Scientific Instruments, Columbia, MD, USA).
A ten-port HPLC valve was used in a configuration with a small sample clean-up column (Thermo Scientific BDShypersil-C18, 5 µm, 10 × 1 mm Javelin), which trapped a late-eluting peak contained in the samples (see Perry and Fuller (1992) for details). When the valve was in the inject position, samples from the sample loop were injected onto the sample clean-up column and then onto the analytical column. When the valve was in the load position, the sample clean-up column was back flushed with mobile phase. When a sample was injected, the valve was in the inject position for 90 s after injection and in the load position for the remainder of the 12-min run time per sample. The same mobile phase was used for both the analytical and clean-up columns. The flowrate for the clean-up column was 0.9 ml/min.
Nicotine and cotinine concentrations were quantified by relating peak areas to those of calibrating nicotine and cotinine standard solutions. Analysis and quantification of chromatograms (peak area) were undertaken using EZChrom Software (Agilent Technologies, Santa Clara, CA, USA).
Statistical analyses
All analyses were performed in the IBM SPSS Statistics package (v. 21), and the level of significance was 0.05. SC nicotine and cotinine data were analyzed by a three-way repeated measures ANOVA (line × dose × time). Subsequently, nicotine and cotinine levels were analyzed by a two-way repeated measures ANOVA (dose × time). One-way repeated measures ANOVAs were used to examine the effect of dose at each time point and the effect of time at each dose. Post hoc tests were used to examine differences between doses on nicotine and cotinine levels and the effects of dose at each time point. A Tukey’s post hoc test was used to determine if there were significant changes in nicotine and cotinine levels between time points at a given dose.
Nicotine elimination rates following SC administration were calculated from the linear (pseudo-zero order) portions of the nicotine curves. Nicotine elimination rates, area under the curve (AUC), maximum concentration (Cmax), and time to reach Cmax (Tmax) for the 0.70 mg/kg SC nicotine dose were analyzed by independent t tests.
A two-way repeated measures ANOVA (treatment × time) was used to compare differences in nicotine and cotinine levels for the 15 % EtOH+0.35 mg/kg nicotine (15E+ 0.35NIC) and 15 % EtOH+0.70 mg/kg nicotine (15E+ 0.70NIC) IG treatments. After observing a significant effect of time, a Tukey’s post hoc test was used to examine differences in nicotine and cotinine levels between different time points. Independent t tests were used to examine differences between treatment conditions on AUC, Cmax, and Tmax for nicotine and cotinine levels. AUC was derived by summing the values over the indicated time-course for each animal.
A two-way repeated measures ANOVA (treatment × time) was used to examine differences between the 15E+0.70NIC and water+0.70 mg/kg nicotine (water+0.70NIC) IG treatments on nicotine and cotinine levels. After observing a significant effect of time, a Tukey’s post hoc test was used to examine differences between time points on nicotine and cotinine levels. In addition, after observing a significant treatment × time interaction, independent t tests were employed to examine the effect of treatment at each time point to determine if there were significant differences between 15E+0.70NIC and water+0.70NIC treatments on cotinine levels. After observing a significant effect of time, a Tukey’s post hoc test was used to examine the effect of time for each treatment condition on cotinine levels. Independent t tests were used to examine differences between treatments on AUC, Cmax, and Tmax on nicotine and cotinine levels.
Results
SC nicotine administration
The in vitro relative recoveries (probe efficiency) of nicotine and cotinine were 15.1±3.1 and 13.1±1.8 %, respectively. Thus, the levels reported represent a fraction of the extracellular levels of nicotine and cotinine sampled at the time points indicated in these experiments. The reported levels are not corrected for recovery and do not necessarily reflect the intracellular concentrations of these compounds.
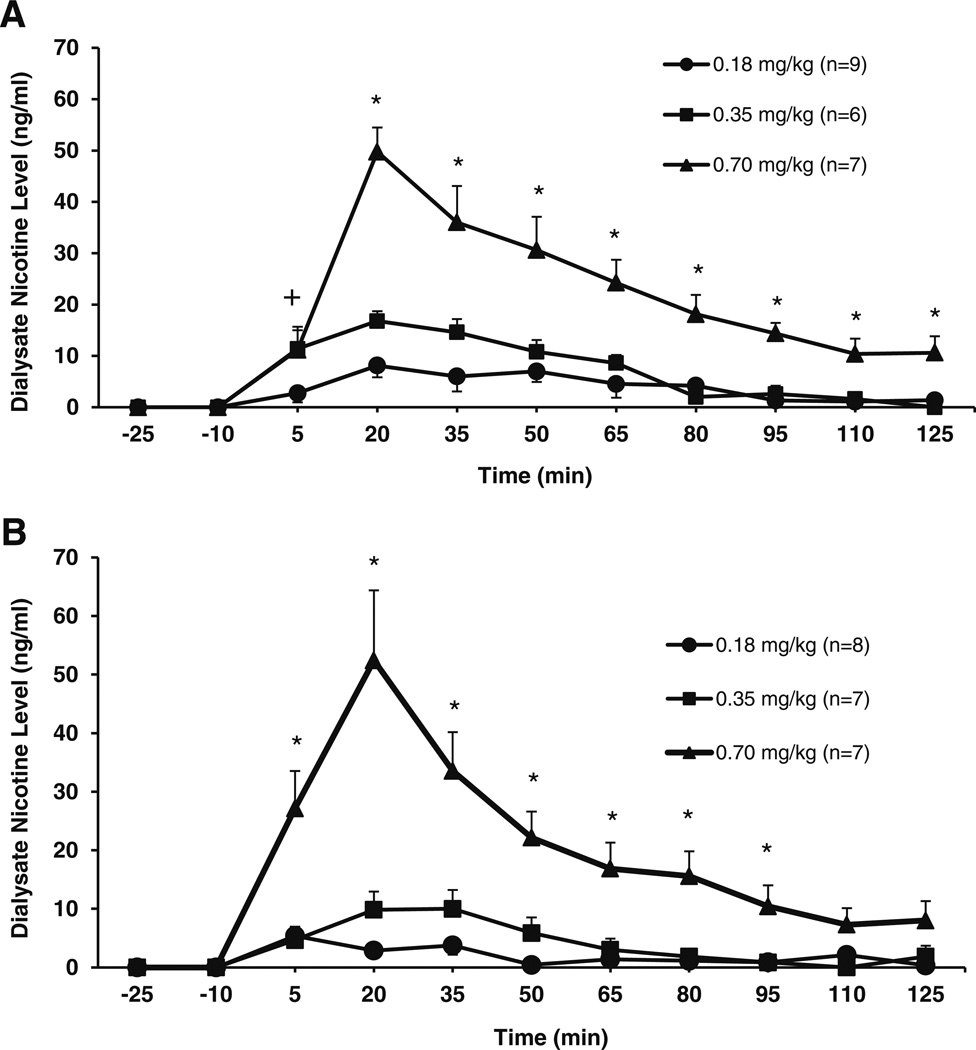
Dose and line effects on brain nicotine and cotinine levels were assessed in P and Wistar rats after SC administration of nicotine. A dose-response effect was observed on nicotine concentrations in the NACsh following SC injection of nicotine in P (Fig. 1a) or Wistar (Fig. 1b) rats. A three-way repeated measures ANOVA was performed on nicotine concentrations (ng/ml; line × dose × time). A main effect of time (F8,34 = 23.8; p < 0.001), a time × dose interaction (F16,70 = 4.0; p < 0.001), no time × line interaction (F8,34 = 2.1; not significant (ns)), and no time × line × dose interaction (F16,70 = 1.2; ns) were observed. Tests of between-subjects effects found a main effect of dose (F2,41 = 40.9; p < 0.001), no main effect of line (F1,41 = 1.1; ns), and no line × dose interaction (F2,41 = 0.009; ns). Since a main effect of dose and a dose × time interaction were observed in the three-way ANOVA, planned comparisons were conducted for each line using a two-way repeated measures ANOVA on dose × time. For P rats, this analysis revealed a main effect of time (F8,15 = 18.9; p < 0.001), a time × dose interaction (F16,28 = 3.1; p < 0.001), and a main effect of dose (F2,22 = 27.2; p < 0.001) on nicotine concentrations. The interaction of time × dose allowed for further analysis of the effect of dose at each time point. One-way ANOVAs showed significant effects of dose after nicotine administration for P rats (F > 4.7; p < 0.05; at each time point). For Wistar rats, the two-way analysis revealed a main effect of time (F8,12 = 14.4; p < 0.001), a time × dose interaction (F16,22= 6.7; p < 0.001), and a main effect of dose (F2,19 = 15.6; p < 0.001) on nicotine concentrations. The interaction of time × dose allowed for further analysis of the effect of dose at each time point. One-way ANOVAs showed significant effects of dose after nicotine administration for Wistar rats (F > 7.1; p < 0.05; at each time point up to 95 min). Post hoc tests revealed that from the 20-min time point on, only the 0.7 mg/kg dose was significantly different from the other doses for both strains (up to the 95-min time point for Wistar rats; p < 0.05; see Fig. 1a, b).
Fig. 1.
Mean (±SEM) concentrations of nicotine (ng/ml) in the NACsh of P (a) andWistar (b) rats following subcutaneous (−)nicotine administration (0.18, 0.35, and 0.70 mg/kg). For P rats (a), two-way ANOVA revealed a main effect of time (F8,15 = 18.9; p < 0.001), a time × dose interaction (F16,28 = 3.1; p < 0.001), and a main effect of dose (F2,22= 27.2; p < 0.001). The follow-up one-way ANOVAs showed significant effects of dose after nicotine administration for P rats (F > 4.7; p < 0.05; at each time point), and post hoc tests showed that, from the 20-min time point on, the 0.7 mg/kg dose was significantly different from the other doses. For Wistar rats (b), the two-way analysis revealed a main effect of time (F8,12 = 14.4; p < 0.001), a time × dose interaction (F16,22 = 6.7; p < 0.001), and a main effect of dose (F2,19 = 15.6; p < 0.001). The follow-up one-way ANOVAs showed significant effects of dose after nicotine administration forWistar rats (F > 7.1; p < 0.05; at each time point up to 95min). Post hoc tests revealed that from the 5-min time point on, only the 0.7 mg/kg dose was significantly different from the other doses for both strains (up to the 95 min time point for Wistar rats; p < 0.05). * = 0.7 significantly different from 0.18 and 0.35 mg/kg doses; + = 0.7 significantly different from 0.18 mg/kg; p < 0.05
The elimination rate of nicotine following the 0.70 mg/kg SC dose did not differ between P and Wistar rats (−4.0±1.0 and −4.3±0.7 ng/ml/15 min, respectively; t(14) = 0.21; ns). Furthermore, the AUC (210±26 in P rats; 190±39 in Wistar; t(14) = 0.45; ns), maximal concentration (Cmax; 50.8± 4.3 ng/ml in P rats; 53.7±11.8 ng/ml in Wistar; t(14)=−0.26; ns), and time to Cmax (Tmax; 33.3±3.3 min in P rats; 25.7± 2.8 min inWistar; t(14) = 1.7; ns) did not differ between P and Wistar rats for the 0.70 mg/kg SC nicotine dose.
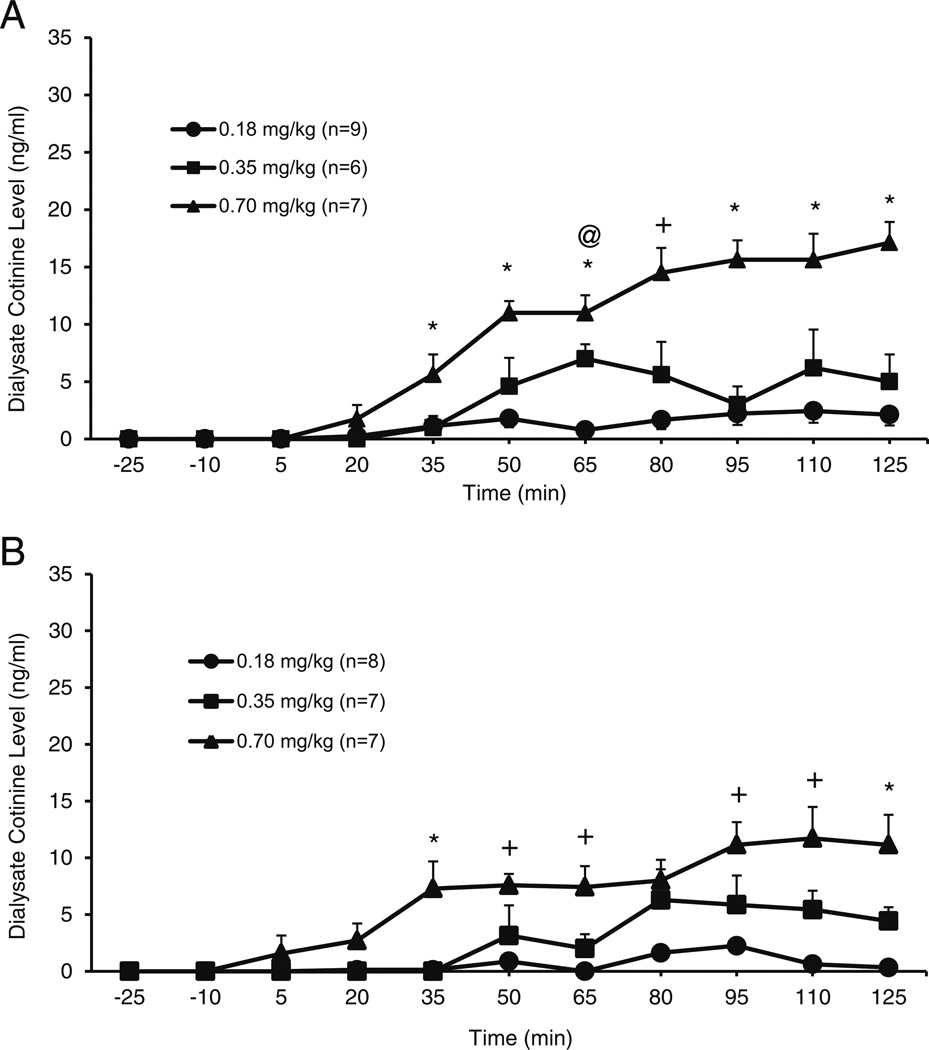
A dose-response effect was observed on cotinine concentrations in the NACsh following SC injection of nicotine in P (Fig. 2a) or Wistar (Fig. 2b) rats. A three-way repeated measures ANOVA was performed on cotinine concentrations (ng/ml; line × dose × time). A main effect of time (F8,34 = 15.9; p < 0.001) and a time × dose interaction (F16,70 = 4.7; p < 0.001) were found, but no time × line interaction (F8,34= 0.9; ns) and no time × line × dose interaction (F16,70 = 1.0; ns) were observed. Tests of between-subjects effects found a main effect of dose (F2,41 = 39.7; p < 0.001), but no main effect of line (F1,41 = 2.7; ns) and no line × dose interaction (F2,41 = 1.3; ns). Since a main effect of dose and a dose × time interaction were observed in the three-way ANOVA, planned comparisons were conducted for each line using a two-way repeated measures ANOVA on dose × time. For P rats, this analysis revealed a main effect of time (F8,17 = 13.4; p < 0.001), a time × dose interaction (F16,32 = 5.6; p < 0.001), and a main effect of dose (F2,24 = 43.9; p < 0.001) on cotinine concentrations. The interaction of time × dose allowed for further analysis of the effect of dose at each time point. One-way ANOVAs showed significant effects of dose on cotinine concentrations after nicotine administration for P rats (F > 4.7; p < 0.05; at each time point). For Wistar rats, the two-way analysis revealed a main effect of time (F8,12 = 10.0; p < 0.001), a time × dose interaction (F16,22 = 3.4; p < 0.005), and a main effect of dose (F2,19 = 7.4; p < 0.002) on cotinine concentrations. The interaction of time × dose allowed for further analysis of the effect of dose at each time point. One-way ANOVAs showed significant effects of dose after nicotine administration for Wistar rats (F > 7.1; p < 0.05; at each time point up to 95 min). Post hoc tests revealed that from the 35-min time point on, the 0.7 mg/kg dose was significantly different from at least one of the other doses for both strains at almost every time point (up to 125 min postadministration; p < 0.05; see Fig. 2a, b) on cotinine concentrations.
Fig. 2.
Mean (±SEM) concentrations of cotinine (ng/ml) in the NACsh of P (a) andWistar (b) rats following subcutaneous (−)nicotine administration (0.18, 0.35, and 0.70 mg/kg). For P rats (a), the two-way ANOVA revealed a main effect of time (F8,17 = 13.4; p < 0.001), a time × dose interaction (F16,32 = 5.6; p < 0.001), and a main effect of dose (F2,24= 43.9; p < 0.001). The follow-up one-way ANOVAs showed significant effects of dose after nicotine administration for P rats (F > 4.7; p < 0.05; at each time point). For Wistar rats, the two-way analysis revealed a main effect of time (F8,12 = 10.0; p < 0.001), a time × dose interaction (F16,22= 3.4; p < 0.005), and a main effect of dose (F2,19 = 7.4; p < 0.002) on cotinine concentrations. The follow-up one-way ANOVAs showed significant effects of dose after nicotine administration for Wistar rats (F > 7.1; p < 0.05; at each time point up to 95 min). Post hoc tests revealed that from the 35-min time point on, the 0.7 mg/kg dose was significantly different from at least one of the other doses for both strains at almost every time point (up to 125 min postadministration; p < 0.05). * = 0.7 different from 0.18 and 0.35 mg/kg doses; + = 0.7 significantly different from 0.18 mg/kg; @ = 0.35 significantly different from 0.18 mg/kg; p < 0.05
IG nicotine administration
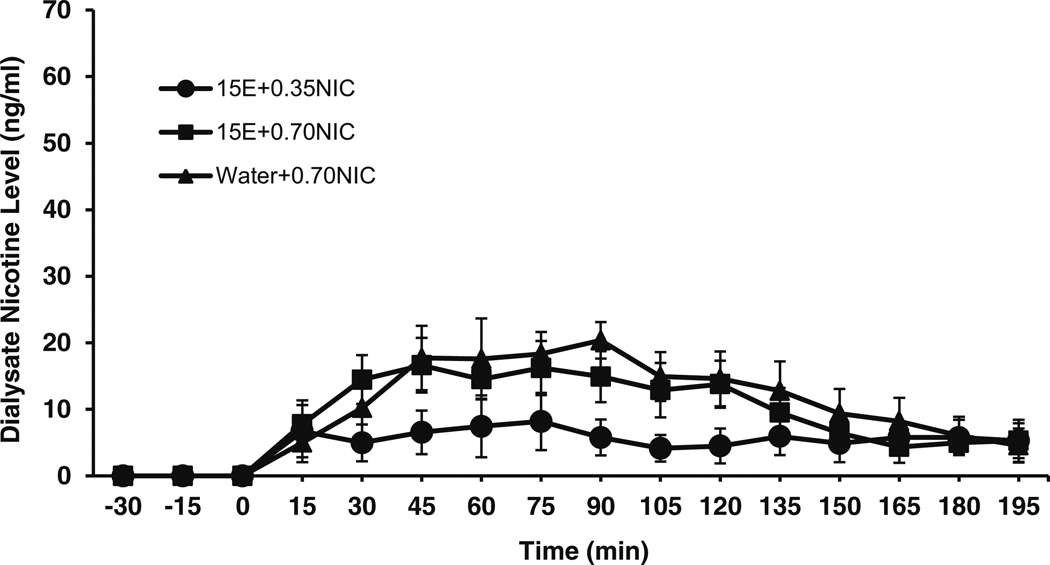
Effects of IG nicotine and nicotine+ethanol on brain nicotine and cotinine levels were assessed in P rats. Significant nicotine concentrations were observed in the NACsh following IG administration of nicotine in P rats (Fig. 3). A two-way ANOVA, assessing the nicotine dose effect on brain nicotine levels showed that there was a main effect of time on nicotine levels for the 15E+0.35NIC and 15E+0.70NIC groups (F13,104 = 4.1; p < 0.05). However, there was no main effect of treatment (F1,8 = 1.7; ns) and no significant interaction between time and treatment (F13,104 = 1.9; ns) on nicotine levels for the 15E+0.35NIC and 15E+0.70NIC groups. Post hoc tests found significant differences between 0 min and nearly all time points on nicotine levels after IG nicotine. In addition, independent t tests found no significant effects of treatment on maximal concentration (13.2±3.4 ng/ml for 15E+0.35NIC; 25.9±2.8 ng/ml for 15E+0.70NIC) between groups.
Fig. 3.
Mean (±SEM) concentrations of nicotine (ng/ml) in the NACsh of P rats following intragastric administration of 15 % EtOH+0.35 mg/kg nicotine (15E+0.35NIC; n = 4), 15 % EtOH+0.70 mg/kg nicotine (15E+ 0.70NIC; n = 6), and water+0.70 mg/kg nicotine (water+0.70NIC; n = 8) solutions. A two-way ANOVA found a main effect of time on nicotine levels for 15E+0.35NIC and 15E+0.70NIC treatments (F13,104 = 4.1; p < 0.05). A two-way ANOVA found a main effect of time on nicotine levels for 15E+0.70NIC and water+0.70NIC treatments (F13,156 = 8.0; p < 0.001). However, no main effects of dose or treatment nor interactions with time were observed for nicotine levels (p > 0.05)
Brain levels of nicotine and cotinine were compared after IG administration of 0.7 mg/kg in the presence or absence of 15 % ethanol. The results of a two-way ANOVA revealed a main effect of time on nicotine levels for 15E+0.70NIC and water+0.70NIC rats (F13,156 = 8.0; p < 0.001). However, there was no main effect of treatment (F1,12 = 0.22; ns) and no significant interaction between time and treatment (F13,156= 0.4; ns) on nicotine levels for the 15E+0.70NIC and water+ 0.70NIC groups. Post hoc tests found significant differences between 0 min and almost all time points on nicotine levels after IG nicotine. In addition, independent t tests found no significant effects of treatment on maximal concentrations (20.8±3.6 ng/ml for 15E+0.70NIC; 25.9±2.8 ng/ml for water+ 0.70NIC) between groups.
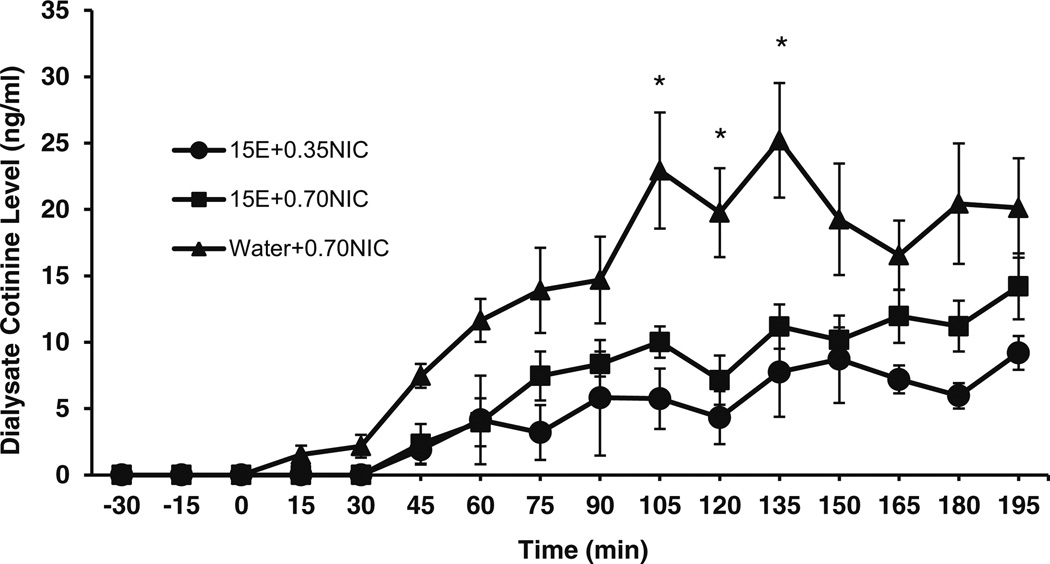
Significant cotinine levels were observed in the NACsh 45 min after IG administration of nicotine in P rats (Fig. 4). For the two-way ANOVA, there was a main effect of time on cotinine levels for 15E+0.35NIC and 15E+0.70NIC groups (F13,104 = 10.0; p < 0.001). However, there was no main effect of treatment (F1,6 = 2.4; ns) and no significant interaction between time and treatment (F13,104 = 3.8; ns) on cotinine levels for the 15E+0.35NIC and 15E+0.70NIC rats. Post hoc tests found significant differences between 0 min and most time points on cotinine levels after IG nicotine (p < 0.05). In addition, independent t tests found no significant effects of treatment on maximal concentration (12.5± 2.1 ng/ml for 15E+0.35NIC; 17.9±1.8 ng/ml for 15E+ 0.70NIC) between groups.
Fig. 4.
Mean (±SEM) concentrations of cotinine (ng/ml) in the NACsh of P rats following intragastric administration of 15 % EtOH+0.35 mg/kg nicotine (15E+0.35NIC; n = 4), 15 % EtOH+0.70 mg/kg nicotine (15E+ 0.70NIC; n = 6), and water+0.70 mg/kg nicotine (water+0.70NIC; n = 8) solutions. A two-way ANOVA revealed a main effect of time on cotinine levels for 15E+0.35NIC and 15E+0.70NIC treatments (F13,104 = 10.0; p < 0.001). A two-way ANOVA found a main effect of time on cotinine levels for 15E+0.0.70NIC and water+0.70NIC treatments (F13,156 = 20.0; p < 0.001), a main effect of treatment (F1,12 = 8.9; p < 0.02), and an interaction between time and treatment (F13,156 = 3.0; p < 0.001). Independent sample t tests examining the effect of treatment on cotinine levels at each time point found significant differences between treatment conditions at 105, 120, and 135min (p < 0.05). * = water+0.7NIC significantly different from 15E+0.7NIC; p < 0.05
For the two-way ANOVA, there was a main effect of time on cotinine levels for 15E+0.70NIC and water+0.70NIC groups (F13,156 = 20.0; p < 0.001). There was also a main effect of treatment (F1,12 = 8.9; p < 0.02) and a significant interaction between time and treatment (F13,156 = 3.0; p < 0.001) on cotinine levels for the 15E+0.70NIC and water+0.70NIC groups.
The significant interaction between time and treatment allowed for assessment of the effects of treatment on cotinine levels at each collection time point. Independent sample t tests examining the effect of treatment on cotinine levels at each time point found significant differences between treatment conditions at 105, 120, and 135 min (p < 0.05).
Post hoc tests examining the effect of time for the 15E+ 0.70NIC treatment condition found significant differences at the 0 versus almost all time points after 75 min on cotinine levels. Post hoc tests examining the effect of time for the water+0.70NIC treatment found significant differences at the 0 versus almost all time points after 45 min on cotinine levels. Independent t tests found significant effects of treatment on maximal concentrations (16.8±1.8 ng/ml 15E+ 0.70NIC; 29.3±4.5 ng/ml water+0.70NIC) (t(12) = 5.0; p < 0.05) of cotinine levels.
Comparison of SC versus IG nicotine administration
Although a direct comparison of SC and IG administration is not possible in this study due to differences in collection time points, some distinct features of each administration are evident. As indicated in Fig. 1, SC administration of 0.70 mg/kg nicotine resulted in peak extracellular nicotine levels of approximately 50 ng/ml within 20 min of injection, which then declined relatively rapidly. IG nicotine administration also led to significant extracellular concentrations of nicotine in the brain (Fig. 3), producing a rise to near maximal concentration by 45 min and increasing to the nominal maximal concentration in excess of 20 ng/ml at 90min postadministration. These differences are likely due to the effects of extensive first-pass metabolism and possible delay of absorption due to the presence of food that is associated with oral routes of nicotine administration (Benowitz et al. 2009). In contrast, cotinine levels followed a similar time-course after SC and IG administration (Figs. 2 and 4).
Discussion
The present findings indicate that both the SC and IG routes of administration produce significant nicotine levels and a gradual escalation of cotinine levels in the central nervous system (CNS). Consistent with the respective routes of administration and differences in first-pass metabolism, the rise in levels was more modest and delayed after IG administration as compared with SC administration. The accumulation of high concentrations of cotinine in the brain, together with its potential pharmacological activity, suggests that cotinine should be examined for its possible involvement in nicotine addiction. No significant strain differences were observed between P and Wistar rats in terms of brain nicotine and cotinine levels after SC nicotine administration. The addition of ethanol to an IG-administered nicotine solution reduced microdialysate levels of cotinine collected from the NACsh, indicating that ethanol may attenuate the accumulation of cotinine in the CNS.
Following SC nicotine administration, nicotine concentrations in the brain peaked sharply (within 20 min) and declined rapidly, suggesting that nicotine is rapidly removed from the CNS. In contrast, cotinine concentrations in the brain increased slowly and gradually over the course of the sampling period, indicating that cotinine penetrates the brain slowly and remains in the brain for a longer period of time than nicotine. These findings are consistent with several studies in which ex vivo brain tissue concentrations were analyzed for nicotine and cotinine levels. For example, SC nicotine administration produced a rapid rise in brain nicotine levels and a gradual and prolonged increase in brain cotinine levels in Sprague-Dawley rats (Crooks et al. 1997), and in a similar study, peak nicotine levels were achieved within 15 to 20 min following SC nicotine administration in Charles River CD rats (Rosecrans and Schechter 1972; Rosecrans 1972). Although the absolute level of nicotine appears higher in theWistar versus the P rats at the 5-min time point at the 0.7 mg/kg dose (Fig. 1), the levels were not statistically different (p > 0.05; independent t test). In depth, analyses of the ascending limb of the curve are restricted by the time resolution limits integral to brain microdialysis studies. Additional studies using more rapid sampling techniques may better assess possible strain differences on this metric in systemic tissues.
Crooks et al. (1997) found that following SC nicotine, administration of cotinine is able to cross the blood brain barrier (BBB) and access the CNS but is not biotransformed in the brain. Nicotine increased then decreased rapidly in the brain, while cotinine took much longer to reach peak levels in the CNS. Similar results were obtained in rats administered with nicotine or cotinine by various routes of administration (Riah et al. 1998). Nicotine uptake into the brain is believed to be much more rapid than cotinine due to the relative polarities of the two compounds (Crooks et al. 1997; Riah et al. 1998). Peripheral cotinine formed by first-pass metabolism of nicotine may contribute part or all of the brain cotinine observed after SC nicotine (Crooks et al. 1997). Recent work in humans suggests that the time-course of nicotine in arterial blood appears to reflect brain levels while smoking (Berridge et al. 2010). This suggests that brain nicotine may be in equilibrium with arterial nicotine levels and measurement of nicotine in arterial bloodmay provide an indirectmethod tomonitor brain nicotine levels. Additional studies will be necessary to determine if this relationship is also present with other routes of administration.
The current data indicate that IG nicotine administration produces significant brain levels of nicotine and sustained brain levels of cotinine. These findings suggest that oral self-administration of nicotine solutions may produce pharmacologically relevant levels of nicotine and cotinine in the brain. IG ethanol and nicotine coadministration resulted in similar brain levels of nicotine but reduced cotinine levels compared with nicotine alone (Figs. 3 and 4). The time-course changes in brain cotinine levels may be of particular significance since cotinine has been shown to have multiple pharmacological functions including effects on serotonin metabolism (Essman 1973), the release of brain neurotransmitters, and regulation of enzymes involved in the synthesis of estrogen and testosterone (Fuxe et al. 1979; Barbieri et al. 1986; Yeh et al. 1989; Patterson et al. 1990). Cotinine also reduces vascular resistance and blood pressure in animals (Dominiak et al. 1985). Importantly, cotinine administration to abstinent smokers attenuated the desire to smoke and decreased irritability, restlessness, anxiety, tension, and insomnia associated with nicotine abstinence (Benowitz and Jacob 1993; Keenan et al. 1994). Thus, brain cotinine levels may play an important role in nicotine use and/or ethanol/nicotine coadministration.
The mechanism for the observed effect of ethanol on nicotine pharmacokinetics is unknown. However, several factors could be responsible for the greater brain cotinine levels produced by IG administration of water+0.7NIC compared to 15E+0.7NIC. First, it is possible that ethanol may have decreased the ability of cotinine to pass the blood brain barrier. Ethanol has been shown to modify cellular membrane structure and composition (Gruber et al. 1977; Chin and Goldstein 1977). Acute and chronic ethanol exposure has also been shown to modify membrane permeability (Isselbacher 1977) and alter brain or tissue distribution of various compounds (Paul and Whitehouse 1977; Hetland and Couri 1974; Seidel 1967; Siemens et al. 1977). Second, ethanol may have reduced the metabolism of nicotine by the liver. However, this seems unlikely since there was no elevation in brain nicotine levels after 15E+0.7NIC versus water+0.7NIC. Third, it is possible that ethanol may have affected the gastric absorption of nicotine due to the observations indicating that nicotine can apparently affect alcohol absorption with IG administration (Parnell et al. 2006). However, this possibility also seems unlikely, since cotinine levels reflect nicotine levels and lower nicotine levels would be expected if ethanol reduced gut absorption of nicotine. Lastly, ethanol may have increased the metabolism and/or clearance of cotinine relative to nicotine. However, the two compounds are largely metabolized by the same enzymes (Hukkanen et al. 2005) reducing the likelihood of a differential effect of ethanol on metabolism. To best address these questions and more fully understand the pharmacokinetics of nicotine after nicotine and ethanol coadministration, additional dose-effect studies examining the arterial plasma concentration of nicotine and cotinine over this same time-course are indicated.
The use of females in this study adds an additional factor to consider in evaluating the results. Females have been shown to have increased metabolism of nicotine as compared to males (Benowitz et al. 2006, 2009), and to date, few studies have examined the interaction of sex and EtOH coadministration on the time-course of extracellular nicotine and cotinine levels in the brain. Thus, caution should be used when comparing data generated in the current study with data from male rats. In summary, the overall results of this study indicate that nicotine can be readily detected in the NACsh following IG administration, indicating that consumption of nicotine-laced drinks will produce pharmacologically active levels of nicotine in the brain. In addition, administration of nicotine produces elevated levels of cotinine in the brain, which persist long after nicotine levels in the brain have returned to baseline. The persisting levels of cotinine in the brain coupled with its potential pharmacological activity support the idea that this metabolite may have a role in the development and/or maintenance of nicotine addiction.
Acknowledgments
The skillful technical assistance of Joseph A. McClaren and Curtis D. Bard is gratefully acknowledged. This work was supported by NIH-NIAAA grants AA07611, AA019366, and AA020396.
Footnotes
Conflict of interest The authors declare no conflicts of interest
References
- Applegren LE, Hansson E, Schmiterlo CG. The accumulation and metabolism of [14C]-labeled nicotine in the brain of mice and cats. Acta Physiol Scand. 1962;56:249–257. doi: 10.1111/j.1748-1716.1962.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. J Clin Invest. 1986;77:1727–1733. doi: 10.1172/JCI112494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., III Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., 3rd . Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine psychopharmacology. Oxford: Oxford University Press; 1990. pp. 112–157. [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology. 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- Chin JH, Goldstein DB. Drug tolerance in biomembranes: a spin label study of the effect of ethanol. Science. 1977;196:684–685. doi: 10.1126/science.193186. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. Determination of nicotine metabolites in rat brain after peripheral radiolabeled nicotine administration: detection of nornicotine. Drug Metab Dispos. 1995;23:1175–1177. [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. Metabolites of nicotine in rat brain after peripheral nicotine administration: cotinine, nornicotine, and norcotinine. Drug Metab Dispos. 1997;25:47–54. [PubMed] [Google Scholar]
- Deutsch J, Hegedus L, Greig NH, Rapoport SI, Soncrant TT. Electron-impact and chemical ionization detection of nicotine and cotinine by gas chromatography-mass spectrometry in rat plasma and brain. J Chromatogr. 1992;579:93–98. doi: 10.1016/0378-4347(92)80366-x. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak P, Fuchs G, von Toth S, Grobecker H. Effects of nicotine and its major metabolites on blood pressure in anaesthetized rats. Klin Wochenschr. 1985;63:90–92. doi: 10.1007/BF01733074. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, O’Brien CE, McBride WJ, Murphy JM. Effect of housing conditions on sulpiride induced increases in extracellular dopamine (DA) levels in the nucleus accumbens of alcohol-preferring (P) rats. Brain Res. 2004;1022:247–250. doi: 10.1016/j.brainres.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. doi: 10.1126/science.159.3816.739. [DOI] [PubMed] [Google Scholar]
- Eriksson K. Factors affecting voluntary alcohol consumption in the albino rat. Ann Zool Fenn. 1969;6:227–265. [Google Scholar]
- Essman WB. Nicotine-related neurochemical changes: some implications for motivational mechanisms and differences. In: Dunn WL Jr, editor. Smoking behavior: motives and incentives. Washington, DC: Winston and Sons; 1973. pp. 51–65. [Google Scholar]
- Fuxe K, Everitt BJ, Hokfelt T. On the action of nicotine and cotinine on central 5-hydroxytryptamine neurons. Pharmacol Biochem Behav. 1979;10:671–677. doi: 10.1016/0091-3057(79)90319-8. [DOI] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li W-K, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-14C]nicotine. Drug Metab Dispos. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Gorrod JW, Jacob P., III . Analytical determination of nicotine and related compounds and their metabolites. Oxford: Elsevier; 1999. [Google Scholar]
- Gorrod JW, Wahren J. Nicotine and related alkaloids, absorption, distribution, metabolism, excretion. London: Chapman and Hall; 1993. [Google Scholar]
- Gruber B, Dinovo EC, Noble EP, Tewari S. Ethanol induced conformational changes in rat brain microsomal membranes. Biochem Pharmacol. 1977;26:2181–2185. doi: 10.1016/0006-2952(77)90272-6. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr, Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA. Development of an oral operant nicotine-ethanol co-use model in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland LB, Couri D. Effects of ethanol on glutethimide absorption and distribution in relationship to a mechanism for toxicity enhancement. Toxicol Appl Pharmacol. 1974;30:26–35. [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Isselbacher KJ. Metabolic and hepatic effects of alcohol. New Engl J Med. 1977;296:612–616. doi: 10.1056/NEJM197703172961106. [DOI] [PubMed] [Google Scholar]
- Keenan RM, Hatsukami DK, Pentel PR, Thompson TN, Grillo ME. Pharmacodynamic effects of cotinine in abstinent cigarette smokers. Clin Pharmacol Ther. 1994;55:581–590. doi: 10.1038/clpt.1994.72. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Tuomainen P, Makova N, Seppä T, Mikkola JA, Petteri-Piepponen T, Ahtee L, Hyytiä P. The effects of nicotine on locomotor activity and dopamine overflow in the alcohol-preferring AA and alcohol-avoiding ANA rats. Eur J Pharmacol. 2000;407:293–302. doi: 10.1016/s0014-2999(00)00759-7. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Vesell ES. Metabolism of nicotine. Drug Metab Rev. 1991;23:3–41. doi: 10.3109/03602539109029754. [DOI] [PubMed] [Google Scholar]
- Lee AM, Messing RO. Protein kinase C epsilon modulates nicotine consumption and dopamine reward signals in the nucleus accumbens. Proc Natl Acad Sci U S A. 2011;108:16080–16085. doi: 10.1073/pnas.1106277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30(8):1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Patterson TR, Stringham JD, Meikle AW. Nicotine and cotinine inhibit steroidogenesis in mouse Leydig cells. Life Sci. 1990;46:265–272. doi: 10.1016/0024-3205(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Paul CJ, Whitehouse LW. Metabolic basis for the supra-additive effect of ethanol-diazepam combination in mice. Br J Pharmacol. 1977;60:83–96. doi: 10.1111/j.1476-5381.1977.tb16751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Perry KW, Fuller RW. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50:1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- Peterson DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Riah O, Courrière P, Dousset JC, Todeschi N, Labat C. Nicotine is more efficient than cotinine at passing the blood-brain barrier in rats. Cell Mol Neurobiol. 1998;18:311–318. doi: 10.1023/A:1022501131709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11:863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Schechter MD. Brain area nicotine levels in male and female rats of two strains. Arch Int Pharmacodyn Ther. 1972;196:46–54. [PubMed] [Google Scholar]
- Schmiterlöw CG, Hansson E, Andersson G. Distribution of nicotine in central nervous system. Ann NY Acad Sci. 1967;142:2–14. [Google Scholar]
- Seidel G. Distribution of pentobarbital, barbital and thiopental under ethanol. Arch Pharmakol Exp Pathol. 1967;257:221–229. [PubMed] [Google Scholar]
- Siemens AJ, Jatinder M, Khanna JM. Acute metabolic interaction between ethanol and cannabis. Alcohol Clin Exp Res. 1977;1:343–348. [Google Scholar]
- Sziraki, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Yeh J, Barbieri RL, Friedman AJ. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. J Steroid Biochem. 1989;33:627–630. doi: 10.1016/0022-4731(89)90051-4. [DOI] [PubMed] [Google Scholar]