Abstract
Purpose of review
The purpose of this review is to present recent data on the effects of substance P (SP) on the development two common pathological conditions, namely obesity and gut inflammation, and elucidate the neuropeptide as a potential regulator between increased adiposity and exacerbated inflammatory responses during IBD.
Recent findings
We present data that demonstrate a role for SP in both obesity and IBD and investigate potential effects on fat tissue that may influence the progression of intestinal inflammation. More specifically, we present new evidence of direct effects of SP on fat tissue that determine fat depot size and overall weight in mice and analyze some of the potential mechanisms. Furthermore, we present data that describe changes in the intestinal sensory innervation, in particular SP-positive inneravation, during gut inflammation and new direct evidence of the effects of pre-established obesity in the outcome of experimental inflammation of the colon in mice. In the end we propose a link between the role of SP in the promotion of obesity and the potential consequences on IBD.
Summary
We propose that SP may promote fat tissue expansion either centrally or peripherally, and thus create a pro-inflammatory environment (as is the case with obesity) which may in turn affect the progression (exacerbate) of gut inflammation. Further studies are required on the effects of “creeping fat” in IBD in order to decipher the role this type of fat depot expansion in the development of the disease.
Keywords: Neuropeptides, Substance P (SP), Obesity, Intestinal inflammation, Inflammatory Bowel Disease (IBD), “creeping fat”
Obesity is quickly becoming one of western society’s great epidemics [1,2]. The well-documented association of obesity with a number of disease states, including atherosclerosis, hypertension, insulin resistance etc (collectively termed as “metabolic syndrome”) [3] and mores recently cancer [4], has increased interest on the mechanisms through which obesity exerts these effects. Earlier studies have demonstrated that chronic obesity in humans is associated with immune dysregulation reminiscent of low grade inflammation characterized by increased macrophage infiltration into adipose tissue as well as increased expression of proinflammatory cytokines from fat cells [5–9]. These observations stimulated discussion suggesting that obesity may affect the outcome or even the development of diseases in which inflammation plays a central role including Inflammatory Bowel Disease (IBD) [10]. IBD is used to describe two pathological conditions, namely Crohn’s disease (CD) and ulcerative colitis (UC), where intestinal inflammation is a major component. Indeed, a number of fat derived molecules, termed adipokines, have been shown to be involved in the development of intestinal inflammation in several animal models [11–14]. Interestingly, in the case of Crohn’s disease the extent of inflammation and histological damage are correlated with the development of a fat mass (of mesenteric origin) that wraps the intestine, termed “creeping fat [15]. As is the case with obesity, CD-associated “creeping fat” is also characterized by infiltration of fat depots with immune cells and increased levels of proinflammatory cytokines, such as IL-6 and TNFα, secreted in part by adipocytes [16].
Substance P (SP), is an eleven amino acid peptide and a member of the tachykinin family of peptides [17]. It interacts with the G protein-coupled neurokinin receptor family, primarily with the high affinity neurokinin-1 receptor (NK-1R) and to a lesser extent with NK-2R and NK-3R. SP-NK-1R interactions mediate neurogenic inflammation [18,19], intestinal motility [20], mucosal permeability [21], and epithelial ion transport and colonocyte proliferation [22,23]. The identification of the SP in fat depots [24,25], representing the first evidence for adipose tissue sensory innervation, as well as the reductions in epididymal and retroperitoneal adiposity after capsaicin-induced sensory neuron desensitization [26], suggest that this neuropeptide may exert effects on adipocytes among other cells within the fat depots. Recently, using an experimental model of colitis (TNBS) reminiscent of CD, we observed significant inflammatory changes in the mesenteric fat depots of mice and demonstrated the potential involvement of the neuropeptide SP in the generation of proinflammatory responses in adipose tissue that may participate in the development of IBD [27]. This represented the first demonstration that gut inflammation is accompanied by profound inflammatory changes in the adjacent mesenteric fat depots characterized by increased inflammatory cell infiltration, and elevated cytokine and NK-1R transcription. These results also provided strong evidence linking SP, adipose tissue, and intestinal inflammation.
Substance P and gut inflammation
The distribution of neurokinin receptors in different cell types, both in the small intestine and colon [28–31] underlines the importance of these neuropeptide receptors in gastrointestinal function. Moreover, changes in the abundance of these receptors coupled with their proinflammatory potential following ligand stimulation are expected to affect physiological functions of this tissue. Indeed, several lines of evidence support an important role for SP in the generation of intestinal inflammation [32–34]. In a recent study, Straub et. al observed a decrease in the number of sympathetic nerve fibers in all layers of the colon of patients with CD as well as the colon of the DSS and IL-10 −/− animal models of intestinal inflammation [35•]. In contrast, the number of SP-positive nerve fibers increased in the same patients along with the concentration of the sympathetic nerve repellent factor SEMA3C in the crypts of these animal models of colitis. In a separate study, the number of SP immunoreactive nerve fibers was increased in rectosigmoid biopsies collected from patients with irritable bowel syndrome (IBS) [36••]. Although the exact role of SP in the development of these diseases is yet to be determined, its ability to induce cytokine expression from different cell types [27,37–39] provides plausible evidence for such a role.
Another possible explanation for the role of SP in the pathogenesis of intestinal inflammatory diseases comes from recent data from our group showing effects of SP on angiogenesis. The importance of angiogenesis in the development of IBD has been established by Danese et al. that demonstrated substantial angiogenic activity in the mucosa of both patients with CD and UC [40]. In our group, Koon et al. have established a link between SP and angiogenesis in the intestine by showing increased expression of the angiogenic factor CCN1 in colonocytes in response to SP treatment [41•]. In the same study, CNN1 was found to be elevated in the in the colon of patients with CD and UC as well as in the DSS mouse model of colitis. Furthermore, inhibition of NK-1R signaling led to decreased CCN1 levels in the colon of DSS-exposed mice. Collectively, these data suggest a pro-angiogenic role (in addition to the already established pro-inflammatory role) for SP during colitis in both humans and animal models. Interestingly, angiogenesis is closely related to the development of adipose tissue and adipocyte-endothelial cell interactions are presumed to be involved in the development and maintenance of adipose tissue [42] [43]. Since human mesenteric preadipocytes express NK-1R [27] it is possible that SP-NK-1R interactions to stimulate release of pro-angiogenic factors that can participate in fat depot expansion during obesity, colitis, and IBD.
SP and Obesity
The control of food intake and energy balance is achieved through complex interactions between numerous hormones, signaling molecules and other intracellular effectors, a variety of neuropeptides and their collective receptors [44,45]. Obesity and its related pathologies are a result of a disruption in this balance. Despites its role in gastric motility and digestion [46], its presence in the stomach, duodenum, and jejunum and the participation of a host of other neuropeptides in the regulation of food intake [44], very little evidence exists to suggest involvement of SP in the development of obesity and the regulation of metabolic processes. In a recent study, we have demonstrated the potential of SP to regulate food intake centrally, as well as its ability to act through NK-1R on fat cells at the peripheral level and exert effects in weight and associated metabolic responses [47••]. We showed that SP had moderate effects on appetite and disruption of SP-NK-1R interactions by a specific NK-1R antagonist, leads to reduced weight gain in a high fat diet feeding mouse model and to weight loss in the diet-induced and ob/ob mouse models of obesity [47]. Furthermore, reduced weight was accompanied with improved responses of these animals to glucose and insulin challenges, thus reversing a prominent obesity-related pathology, namely insulin resistance. As mentioned earlier these effects may be due to both central and peripheral actions of SP on fat tissue components (demonstrated in this study by the discrepancy in fat depot size between the inhibitor-receiving and its pair fed counterpart group, in diet-induced obesity model). Indeed, this potential of SP to directly signal on adipose tissue components has already been demonstrated in our previous study [27].
Our latest data show the ability of SP to affect fat tissue mass through effects on both preadipocyte replication and apoptosis (Gross et al. AJP in revised form). In this study we observed that SP treatment led to increased preadipocyte replication via the activation of a number of intracellular molecules and membrane receptors and that this increase is both Akt and a PKCθ-dependent. Finally, we demonstrate that SP-induced NK-1R-dependent preadipocyte rescue of apoptosis in response to Fas-L is likely due to reduced PARP and caspase-7 cleavage as well as reduction of caspase-3 activation. This is in agreement with the results from a previous study demonstrating that removal of sensory neurons increase adiposity in certain depots suggesting that sensory innervation of white adipose tissue (WAT) is essential for its homeostasis. Along with these data, the ability of SP to signal through its high affinity NK-1R receptor in adipose tissue components underlies its potential to affect fat depot growth overall, with the latter constituting a major physiological change observed in patients with CD.
Gut inflammation and obesity
We have mentioned above the ability of fat cells to produce numerous inflammatory components as well as the ability of fat tissue to recruit inflammatory cells. There is sufficient data that associate certain nutrients as well as dietary habits with the risk of developing IBD. Such habits include consumption of high levels of sugars as well as fat from animal origin [48,49], two major sources of energy associated with obesity. Perhaps the most direct experimental evidence for the effects of increased adiposity in the outcome of intestinal inflammation have been provided by a recent study showing that high fat feeding of mice before induction of experimental colitis disrupted the balance between regulatory T cells and natural killer T cells thus exacerbating the outcome of the disease. In this model animals kept in high fat diet exhibited increased number of natural killer T cells, which, compared to natural killer T cells isolated from animals on normal diet, produced higher amounts of TNFα and IFNγ, while the numbers of the regulatory T cells was significantly decreased [50••]. Furthermore, adoptive transfer of regulatory T cells rescued both increased colitis and increased cytokine levels observed in the high fat diet group. In a similar paradigm of chemically-induced inflammation researchers found that high fat diet promoted caerulein-induced pancreatitis in doses that were non-inflammatory in mice under normal diet [51•]. However, pancreatitis was significantly reduced in animals injected with an adiponectin-expressing adenovirus. Interestingly, adiponectin is an adipocytokine with lower expression in the hypertrophic fat depots of obese individuals [52] and higher expression in the mesenteric fat depots of patients with CD disease [14]. It is thus evident that increases of abdominal fat masses may influence the severity of gut inflammation or even contribute as an initial trigger in the generation of such diseases.
Furthermore, IBD patients exhibit elevated cytokine and endotoxin (LPS) levels in their circulation and these factors are also thought to be central to the activation of inflammatory cells that participate in the development of these diseases. In two separate studies, endotoxemia was related to food intake [53], as well as obesity and insulin resistance in mice [54]. In the later study, high fat diet also increased the numbers of LPS-positive microbiota in the gut. Furthermore, in mice, LPS treatment produced similar changes in adipose tissue, liver size and insulin resistance, as did high fat diet. Therefore, such data reinforce the notion that obesity and changes in fat tissue overall are important factors in the outcome of inflammatory conditions of the intestine.
Conclusion
Based on the studies described in this review, there is persuasive evidence associating the neuropeptide SP with two very common and rapidly growing pathologies of the last two decades, namely obesity and IBD. The mechanisms underlying these potential effects are still unclear, but accumulating evidence demonstrate the ability of SP to stimulate pro-inflammatory responses in different cell types involved in these conditions. In addition, new potential pathways are suggested by its ability to influence angiogenesis and fat tissue growth. The later can be accomplished in multiple ways through the effects of SP on preadipocytes replication and apoptosis. Since increased preadipocyte numbers are essential for fat depot enlargement, the direct effect of SP on these cells strongly favors such a development. Another way SP could affect fat growth is through its ability increase angiogenesis, although these studies have not yet been performed in the context of fat tissue. Furthermore, it is also becoming clear that the pro-inflammatory environment that exists with increased adiposity is favorable for the development of conditions where inflammation represents a central component, such as IBD. Thus, SP seems to be able to influence the development of intestinal inflammation both directly, through the activation of pro-inflammatory pathways, and indirectly through its actions on fat tissue that may disrupt fat tissue homeostasis, including the promotion of obesity. As more of these pathologies continue to be investigated under the scope of pre-existing obesity, changes in responses of fat tissue components to endocrine signals will be of central importance in the efforts to comprehend the mechanisms underlying these diseases and in future attempts for potential treatments. The increasing incidence of IBD and the continuously decreasing age at which symptoms for this disease first appear, point to environmental and habitual factors as potential reason(s) for this phenomenon. Increased energy intake and the subsequent development of obesity (with its pathological consequences) is becoming a major epidemic and factors, such as SP and possibly other neuropeptides, that are common among the two conditions may prove helpful in uncovering etiologies as well as developing future therapeutic approaches.
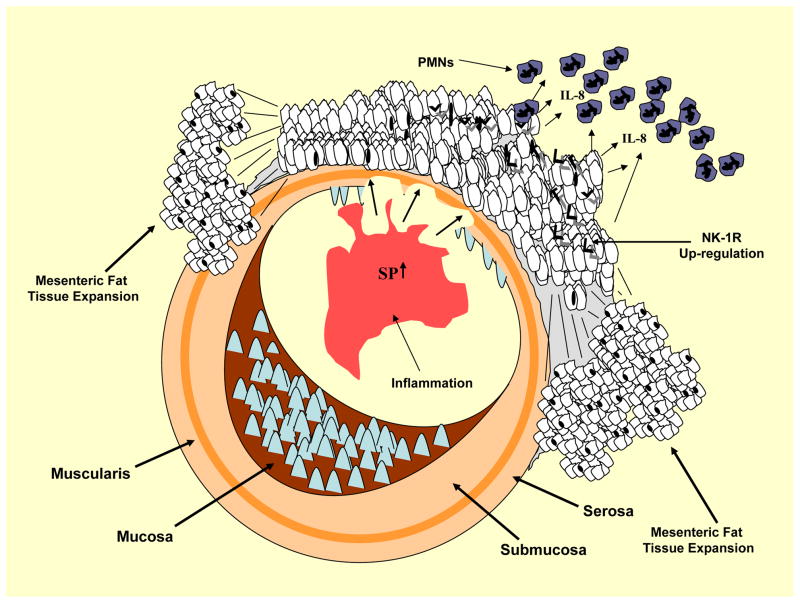
Figure 1.
Schematic description on the sequence of events that may link SP with increased mesenteric adipocity and the severity of intestinal inflammation. SP levels increase in the intestinal mucosa during inflammation and signal to the proximal mesenteric fat depots via the destruction of the intestinal wall (alternatively fat cells may be exposed to SP through the sensory neurons innervating these depots). Such an exposure may than lead to increased preadipocytes proliferation and the subsequent fat depot expansion observed in CD (as well as in our experimental models of TNBS colitis). Finally, SP-induced increases in IL-8 expression may lead to increased neutrophil recruitment that in turn have the potential to induce the recruitment of a number of inflammatory cells through cytokine secretion of their own.
Acknowledgments
This work was supported by a Research Fellowship Award from the “Crohn’s and Colitis Foundation of America, Inc.” to IK, and Grants RO-1 DK60729, RO-1 DK072471, and PO-1 DK 33506 to CP.
References
- 1.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Michaud D. The Role of Obesity and Related Metabolic Disturbances in Cancers of the Colon, Prostate, and Pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 6.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 7.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel Inflammation as Measured by Fecal Calprotectin: A Link between Lifestyle Factors and Colorectal Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279–284. doi: 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
- 11.Sennello JA, Fayad R, Pini M, Gove ME, Fantuzzi G. Transplantation of wild-type white adipose tissue normalizes metabolic, immune and inflammatory alterations in leptin-deficient ob/ob mice. Cytokine. 2007 doi: 10.1016/j.cyto.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 13.Siegmund B, Sennello JA, Jones-Carson J, Gamboni-Robertson F, Lehr HA, Batra A, Fedke I, Zeitz M, Fantuzzi G. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965–972. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Muller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 16.Schaffler A, Herfarth H. Creeping fat in Crohn’s disease: travelling in a creeper lane of research? Gut. 2005;54:742–744. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 18.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 19.De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 20.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 21.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O’Keane JC, Snider RM, Leeman SE. CP-96, 345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem. 2000;275:26545–26550. doi: 10.1074/jbc.M003990200. [DOI] [PubMed] [Google Scholar]
- 23.Riegler M, Castagliuolo I, So PT, Lotz M, Wang C, Wlk M, Sogukoglu T, Cosentini E, Bischof G, Hamilton G, et al. Effects of substance P on human colonic mucosa in vitro. Am J Physiol. 1999;276:G1473–1483. doi: 10.1152/ajpgi.1999.276.6.G1473. [DOI] [PubMed] [Google Scholar]
- 24.Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci. 1998;111 (Pt 17):2587–2594. doi: 10.1242/jcs.111.17.2587. [DOI] [PubMed] [Google Scholar]
- 25.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 26.Cui J, Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. 1992;262:R568–573. doi: 10.1152/ajpregu.1992.262.4.R568. [DOI] [PubMed] [Google Scholar]
- 27.Karagiannides I, Kokkotou E, Tansky M, Tchkonia T, Giorgadze N, O’Brien M, Leeman SE, Kirkland JL, Pothoulakis C. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci U S A. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goode T, O’Connell J, Anton P, Wong H, Reeve J, O’Sullivan GC, Collins JK, Shanahan F. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut. 2000;47:387–396. doi: 10.1136/gut.47.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pothoulakis C, Castagliuolo I, Leeman SE, Wang CC, Li H, Hoffman BJ, Mezey E. Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275:G68–75. doi: 10.1152/ajpgi.1998.275.1.G68. [DOI] [PubMed] [Google Scholar]
- 31.Renzi D, Pellegrini B, Tonelli F, Surrenti C, Calabro A. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agro A, Stanisz AM. Inhibition of murine intestinal inflammation by anti-substance P antibody. Reg Immunol. 1993;5:120–126. [PubMed] [Google Scholar]
- 33.Hon Wai Koon CP. Immunomodulatory Properties of Substance P. Annals of the New York Academy of Sciences. 2006;1088:23–40. doi: 10.1196/annals.1366.024. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock JV, Blum A, Metwali A, Elliott D, Bunnett N, Arsenescu R. Substance P regulates Th1-type colitis in IL-10 knockout mice. J Immunol. 2003;171:3762–3767. doi: 10.4049/jimmunol.171.7.3762. [DOI] [PubMed] [Google Scholar]
- 35••.Straub RH, Grum F, Strauch U, Capellino S, Bataille F, Bleich A, Falk W, Scholmerich J, Obermeier F. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut. 2008;57:911–921. doi: 10.1136/gut.2007.125401. Analysis of IBD patients as well as animal models of colitis demonstrating the importance of a balance between sympathetic and sensory innervation during colitis. The study shows that decreased sympathetic in favor of increased SP-positive sensory innervation is evident in the intestine IBD patients and in mice with either DSS-induced colitis or genetic susceptibility (IL-10−/−) to colitis. [DOI] [PubMed] [Google Scholar]
- 36•.Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. This study demonstrated an increase in SP-immunoreactive nerve fibers in the intestine of IBS patients, suggesting a possible implication of the neuropeptide in this disease state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blum AM, Metwali A, Cook G, Mathew RC, Elliott D, Weinstock JV. Substance P modulates antigen-induced, IFN-gamma production in murine Schistosomiasis mansoni. J Immunol. 1993;151:225–233. [PubMed] [Google Scholar]
- 38.Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci U S A. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurenzi MA, Persson MA, Dalsgaard CJ, Haegerstrand A. The neuropeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand J Immunol. 1990;31:529–533. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 40.Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 41•.Koon HW, Zhao D, Xu H, Bowe C, Moss A, Moyer MP, Pothoulakis C. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am J Pathol. 2008;173:400–410. doi: 10.2353/ajpath.2008.080222. In this study, the involvement of SP in intestinal angiogenesis is demonstrated. It is shown the the expression of the angiogenic factor CCN1 in colonocytes can be regulated by SP. It is also shown that the levels of CCN1 increase in IBD, implicating both molecules in the development of angiogenesis during IBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev. 1983;8:1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 43.Aoki S, Toda S, Sakemi T, Sugihara H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct Funct. 2003;28:55–60. doi: 10.1247/csf.28.55. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson TJ. Central and peripheral neuroendocrine peptides and signalling in appetite regulation: considerations for obesity pharmacotherapy. Obes Rev. 2008;9:108–120. doi: 10.1111/j.1467-789X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 45.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 46.Nicholl CG, Polak JM, Bloom SR. The hormonal regulation of food intake, digestion, and absorption. Annu Rev Nutr. 1985;5:213–239. doi: 10.1146/annurev.nu.05.070185.001241. [DOI] [PubMed] [Google Scholar]
- 47••.Karagiannides I, Torres D, Tseng YH, Bowe C, Carvalho E, Espinoza D, Pothoulakis C, Kokkotou E. Substance P as a novel anti-obesity target. Gastroenterology. 2008;134:747–755. doi: 10.1053/j.gastro.2007.12.032. An orexigenic effect of SP in mice is reported. In addition, inhibition of SP signaling leads to reductions in weight and improves the responses of these mice to obesity-related metabolic disorders such as insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754–760. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K, et al. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11:154–163. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 50••.Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non- CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151:130–138. doi: 10.1111/j.1365-2249.2007.03530.x. In this study, the researchers have demonstrated the importance of obesity in determining the intensity of intestinal inflammation. Using the DSS model of colitis in combination with diet-induced obesity, they showed that obesity increased the numbers of natural killer T cells in the colon of DSS-exposed mice. When these cells were isolated from the animals they were shown to produce higher levels of proinflammatory cytokines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Araki H, Nishihara T, Matsuda M, Fukuhara A, Kihara S, Funahashi T, Kataoka TR, Kamada Y, Kiyohara T, Tamura S, et al. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut. 2008;57:1431–1440. doi: 10.1136/gut.2007.135665. This work elucidates the importance of food content in the development of another inflammatory disease model, the caerulein-induced pancreatitis in mice. In addition, the role of adiponectin, an adipokine, in reversing these effects is also highlighted, demonstrating the participation of fat tissue in the regulation of yet another pathology with an inflammatory basis. [DOI] [PubMed] [Google Scholar]
- 52.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 53.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 54.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]