Abstract
The ability of fat tissue cells to produce proinflammatory cytokines and the concept that obesity represents a low-grade inflammatory response have been well documented during the past decade. The effects of fat-mediated inflammation on metabolic pathologies have also been drawing increasing interest, However, very little is known on the potential effects of adipose tissue in the pathophysiology of gastrointestinal diseases with an inflammatory component, such as Inflammatory Bowel Disease (IBD). The development of large fat masses around the inflamed intestine during Crohn’s disease makes this tissue a candidate for more intense investigation in studies aiming to gain insights in the pathogenesis and progress of the disease. Furthermore, neuropeptides act in many cases in a proinflammatory manner and are shown to participate in the pathogenesis of intestinal inflammation in animal models of IBD However, the potential of these molecules to interact with fat cells in the context of IBD has not been investigated. In this review we describe our most recent data related to the effects of neuropeptides on non-inflammatory fat tissue components. In addition, we include discussion to associate neuropeptide-induced, adipose tissue-mediated responses with the generation of intestinal inflammatory conditions such as Crohn’s disease.
Keywords: Neuropeptides, Substance P (SP), Neurotensin (NT), Intestinal inflammation, Inflammatory Bowel Disease (IBD), Adipose tissue, Mesenteric fat, “creeping fat”
Multiple lines of evidence have now demonstrated that adipose tissue is metabolically active, and a source of a number of hormones and cytokines that are integral parts of numerous physiological processes, as well as several disease states. Such observations have identified fat as candidate tissue for research on therapies, especially of diseases with an inflammatory component. Several prominent intestinal pathologies, such as inflammatory bowel disease (IBD), involve a strong inflammatory reaction and recent observations associating fat tissue with innate immunity suggest a possible link between increased adipocity and gut inflammation. Although there is no direct link between obesity and IBD, increased inflammatory infiltrate as well as enhanced proinflammatory/proangiogenic adipokine production in obese patients may create favorable pathophysiological conditions for the overall progression of intestinal inflammation [1–4]. Perhaps the strongest support of this hypothesis is the observation of the development of a large fat mass that envelopes the intestine during Crohn’s colitis. This mass, named wrapping or “creeping” fat, derives from mesenteric fat depots and might play an important role in the development of the disease. Despite the occurrence of such dramatic changes in fat depots during Crohn’s disease, very little research has been performed to allow characterization of this tissue and examine its potential involvement in intestinal inflammation. One such study is by Desreumaux and colleagues demonstrating increases in the cytokines TNFα and IL-6 in “creeping“ fat of patients with Crohn’s disease [5]. Interestingly, such increases were also observed in fat hypertrophy and obesity [6–8].
Additional lines of evidence suggesting an association between fat tissue and intestinal inflammation comes from studies demonstrating the importance of fat-derived molecules (adipokines), such as leptin and adiponectin, in the development of such conditions. Leptin levels correlate with levels of adipocity, and this appetite-regulating hormone appears is strongly associated with the generation and progress of intestinal inflammation in two mouse IBD models of colitis, DSS and TNBS [9–11], as well as with the Clostridium difficile toxin A acute enteritis model [12]. Adiponectin, on the other hand, has strong anti-inflammatory properties [13–16] and its levels are inversely related to adipocity. It is noteworthy that the levels of this anti-inflammatory adipokine are also increased in the “creeping” fat of Crohn’s disease patients [17] indicating a potential role in the healing phase of the disease. Studies with adiponectin deficient mice in models of colitis, however, demonstrated conflicting results, indicating that this adipokine might stimulate proinflammatory, as well as anti-inflammatory responses in IBD colitis models [18] [19]. It is also possible that secretion of these molecules is related to the physiological state of the cells in this tissue, a subject on which very little is known given the plasticity in properties that fat cells demonstrate between different depots in the body [20, 21]. Finally, another interesting example is that of calprotectin, the levels of which are associated with obesity [22, 23] and at the same time is a marker for intestinal inflammation and a life style risk factor for colorectal cancer [24]. Collectively, these pieces of evidence suggest a strong possibility of mesenteric fat tissue participation in either or both the generation and progression of intestinal inflammation.
Intestinal inflammation and mesenteric fat depots
Prompted by the evidence discussed above we examined whether acute intestinal inflammation elicit changes in the underlying mesenteric fat depot [25]. We induced TNBS colitis in CD1 mice, and the mesenteric fat depot as well as a part of the colon were removed and observed under the light microscope. Increased colonic macroscopic damage score and histological scores in TNBS-treated mice was accompanied with increased inflammatory as well as histological changes such as venular dilatation and congestion, neutrophil margination and diapedesis and perivascular accumulation of neutrophils in the mesenteric fat depots from the same animals (Fig. 1). In addition, TNBS-treated mice also exhibited increased mRNA transcriptions of several proinflammatory cytokines such as TNFα, IL-6, MCP-1 and KC (IL-8 in humans) in these fat depots [25]. Indeed, in a recent study Gambero et al have also demonstrated increased TNFα, leptin and adiponectin levels after chronic TNBS administration [26]. Furthermore, this study also provides the first insight on the morphology (reduced diameter) of “creeping fat” adipocytes. Since the TNBS mouse model of colitis resembles the intestinal changes in Crohn’s disease, these observations suggest o role for mesenteric depots in the generation of inflammatory responses via release of proinflammatory cytokines, and that fat hypertrophy and wrapping of the bowel may be involved in the development and/or progression of Crohn’s disease [5, 27].
Figure 1. TNBS-induced colitis results in increased inflammation in the mesenteric fat depots of CD1 mice.
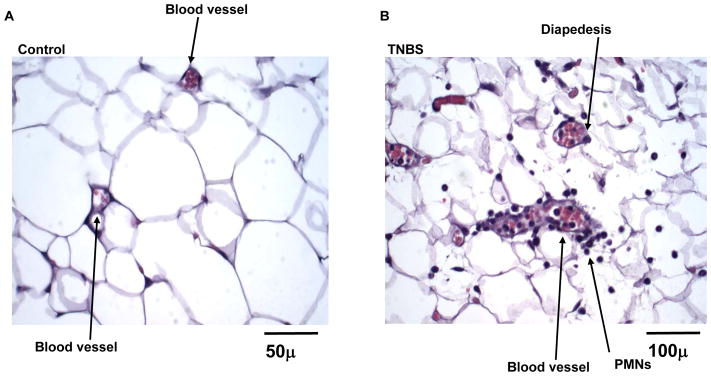
Male CD1 mice received a single intra-colonic dose of TNBS, sacrificed after 48 hrs and mesenteric fat depots proximally to the inflamed intestine were removed for observation under a light microscope. (A) Mesenteric fat depot removed from a control mouse (received a single mock injection of ethanol) showing normal blood vessels without the presence of inflammatory infiltrate. (B) Mesenteric fat depot from a TNBS-treated mouse showing inflammatory changes in this tissue evident by the venular dilatation and congestion, neutrophil margination and diapedesis as well as perivascular accumulation of neutrophils (from Karagiannides et al. 2006. Proc Natl Acad Sci U S A. 103: 5207–5212, by permission).
A potential role for SP in mesenteric fat induced inflammation
The involvement of neuropeptides in the pathogenesis of IBD as well as the prominent role of SP in intestinal inflammation prompted us to investigate the expression of NK-1R (high affinity receptor for SP) in mesenteric fat and for changes in its expression during colonic inflammation [25]. In the same study, we have demonstrated that NK-1R is expressed in mesenteric fat and that its levels increase during TNBS colitis in the same tissue. This observation is consistent with previous studies showing increased expression of NK-1R in tissues with intestinal inflammation both in animals and humans [28–33]. Since fat depots are known to contain other cell types among which are macrophages [34] (which express functional NK-1R when activated [35]) we also isolated primary human mesenteric preadipocytes (fat cell precursors) and showed that they express functional NK-1R and, after SP stimulation, can release IL-8 in an NF-κB-dependent manner [25]. The abundance of preadipocytes in fat depots [36] along with the ability of SP to induce proinflammatory responses in these cells may produce favorable conditions for the recruitment of other inflammatory cells which will then be a source that exacerbates the intestinal inflammation. A potential mechanism for this may be triggered by a sequence of events that involve the presence of increased amounts of SP during intestinal inflammation through sensory neurons that innervate the intestine along with the subsequent NK-1R upregulation and increased IL-8 production. These changes in turn may lead to increased neutrophil infiltration and production of macrophage inflammatory protein- 1 alpha (MIP-1a) which promotes the recruitment of macrophages into the fat depot (perhaps facilitated by ICAM-1 molecules on the surface of preadipocytes). Macrophages can then produce a host of proinflammatory molecules, including TNFα, and thus create a new cycle of cellular activation (including preadipocytes) and inflammatory molecule production with the anticipated consequences. More studies are needed in order to clarify the overall effects of SP on mesenteric fat derived inflammation since even the source for this neuropeptide in fat tissue is yet to be identified.
SP and the generation of “creeping fat” during IBD
In Crohn’s fat tissue hyperplasia correlates with the extent of transmural inflammation [37–39] and is used for the radiographic identification of inflamed bowel segments during surgery. Current evidence suggests that circulating factors and neuronal inputs as well as paracrine/autocrine signals are among the factors that influence preadipocyte proliferation [40, 41]. Such factors are secreted from the various cell types within adipose tissue and their variations between sites may contribute to regional differences in the metabolic and developmental characteristics of different fat depots which receive both sensory and sympathetic innervation [41]. The identification of the neuropeptide substance P (SP) in fat depots [41–43], was the initial indirect proof for adipose tissue sensory innervation which was also suggested to play a role in brown adipose tissue trophic responses [44, 45]. In addition, significant reductions in epididymal and retroperitoneal adiposity were observed after capsaicin-induced sensory neuron desensitization [46]. These observations led us to investigate whether SP has any direct effects in the generation of the mesenteric “creeping” fat phenotype in patients with Crohn’s disease.
Our recent data demonstrate that exposure of primary mesenteric preadipocytes to SP, increases their viability by increasing proliferation and blocking apoptosis in these cells [Gross et. al. JBC, in revision). In the same study we have shown that SP activates both PKCθ and Akt/PKB kinases, two molecules known to be involved in the induction of replication in many other cell types [47], and that SP-induced human mesenteric preadipocyte replication is dependent upon this activation. This notion is further supported by our findings demonstrating that SP increases cyclin D1 expression, thus potentially promoting progression into the cell cycle. In addition, activation of p70 S6 kinase and 4E-BP1 (two molecules that increase translational efficiency) by SP, provides further evidence to that direction. Interestingly, activation of the latter two molecules is also implicated in promotion of adipogenesis in 3T3-L1 mouse preadipocytes [48]. The well documented involvement of IGF-1R and its downstream effector PI-3 kinase in adipocyte proliferation [49, 50] along with the synergistic action of IGF-1R and SP in the promotion of epithelial cell proliferation through entry into the cell cycle [51] led to investigate the effects of SP treatment of human mesenteric preadipocytes on the activation state of these two molecules. Indeed, we have also shown that, in addition to the activation of both IGF-1R and PI3 kinase, SP exposure leads to the activation of EGFR, another molecule that promotes preadipocyte replication [52]. Finally, we demonstrate SP-induced PARP and caspase-7 cleavage as well as reduction of caspase-3 activation as potential pathways for the rescue of preadipocyte apoptosis in response to FasL-.
Collectively our data implicate SP as a potential inducer of “creeping” fat formation, through a combined effect on preadipocyte proliferation and apoptosis. The presence of SP along with its ability to simultaneously increase cell addition while decreasing removal from the overall population may be central to the ability of mesenteric fat depots to expand in such a dramatic fashion during Crohn’s disease.
Neurotensin, fat tissue and intestinal inflammation
Expression of neurotensin (NT) and its receptor NTR1 has been observed in the colonic mucosa of rats and mice and it is modulated during immobilization stress and during both acute and chronic experimental colitis [53–55]. However, the expression of NT and NTR1 in adipose tissue (including the mesenteric fat depot) has not been investigated. Recent studies in our laboratory indicate increased NT and NTR1 mRNA in mesenteric fat depots of mice with TNBS-induced colitis (Koon et al. Gastroenterology, submitted), that persisted throughout the duration of the study (9 days). As in our previous study [25], inflammation in the form of PMN infiltration, and venular dilatation, was present in the mesenteric fat depots of these mice and accompanied by higher gross colitis scores, on day 2 and was partially resolved by day 9 when signs of recovery became evident. In the same study, the use of NT-null mice provided the first evidence for direct involvement of NT in the generation of experimental colitis and the associated mesenteric fat inflammation. These animals also showed diminished inflammatory responses, in both the intestine and mesenteric fat depots, after TNBS administration when compared to wild-type littermates. In addition, NT-knockout (KO) mice also exhibited reduced immunostaining for the p65 subunit of NF-κB and reduced macrophage infiltration in response to TNBS. Levels of the cytokine IL-6, an important component of colitis in mouse models, were also decreased during TNBS colitis in both the colon and mesenteric fat of NT-KO mice. When 3T3-L1 mouse preadipocytes were used we observed that the secretion of this cytokine was induced by NT through protein kinase C (PKC) δ and NF-κB-dependent activation pathways. Further experiments directly demonstrated that both IL-6 transcription as well as NF-κB activation in response to NT required the initial activation of PKCδ. Finally, experiments using Boyden chambers showed that NT can induce the migration of macrophages through effects on preadipocytes and that this response involves release of IL-6.
Collectively our data provides strong evidence on neuropeptide –induced effects of mesenteric fat depots associated with the generation and progression of intestinal inflammation relevant to Finally, our results point to the ability of neuropeptides, such as NT to recruit macrophages into the adipose tissue, a response that has been also observed in models of obesity [1, 2].
Melanin Concentrating Hormone (MCH), fat tissue, and intestinal inflammation
MCH is expressed primarily in the hypothalamus and is involved in the regulation of appetite and energy balance. Very little is known about the potential effects of MCH on intestinal inflammation, apart from early evidence indicating its expression in the intestine [56] and expression of its MCHR1 receptor in lymphocytes [57]. We recently demonstrated the involvement of MCH signaling in the development of experimental colitis (Kokkotou et. al. PNAS, in revision). In these studies anti-MCH antibodies and MCHR1-null mice were used to neutralize MCH-related signaling, resulting in protection of mice from TNBS-induced colitis, suggesting a proinflammatory role for this peptide in intestinal inflammation. In addition, MCH and MCHR1 levels were elevated in epithelial cells isolated by Laser Capture Microdissection from mucosal biopsies of IBD patients. In agreement with its role in the intestine, MCH also increased expression of IL-8 and MCP-1 in primary human mesenteric preadipocytes (Kokkotou et. al. Abstract. Gastroenterology 128, A: 98, 2005) providing another neuropeptide link in the involvement of fat in the generation of inflammatory processes in the intestine.
RELEVANCE TO HUMAN DISEASE
The relevance of these studies in human disease originates from the fact that they implicate fat as new active component in the development or progression of intestinal inflammatory conditions, such as IBD. Such new data implicate adipose tissue as a potentially novel target tissue for studies investigating for factors that influence IBD-related processes, including Crohn’s disease. In combination with observations by other groups of pericardial fat tissue expansion in other conditions with an inflammatory component, such as atherosclerosis, our studies point to this tissue as a significant participant in the maintenance of intestinal physiology and pathophysiology that was not previously recognized. The importance of fat is widely accepted in maintenance of energy homeostasis and metabolic diseases such as insulin resistance and the development of type II diabetes. Our recent findings implicate several neuropeptides as key components in the regulation of adipose tissue-derived effects on intestinal inflammation. Overall, such observations provide important evidence for novel therapeutic strategies against intestinal inflammatory conditions, such as IBD. Considering the ability of fat tissue to produce a series of proinflammatory as well as anti-inflammatory molecules, along with the plasticity in the numbers and phenotype of its cellular components, the physiology of fat depots as it relates to different pathological conditions has received limited attention to date. Based on this evidence further investigation of the potential involvement of pre-existing, hypertrophic visceral fat depots, such as in the case of obesity, in the progression of Crohn’s disease merits consideration. Such studies could provide significant insights in an important and previously not well-recofnized research area that may influence the development and outcome of inflammatory conditions of the gut.
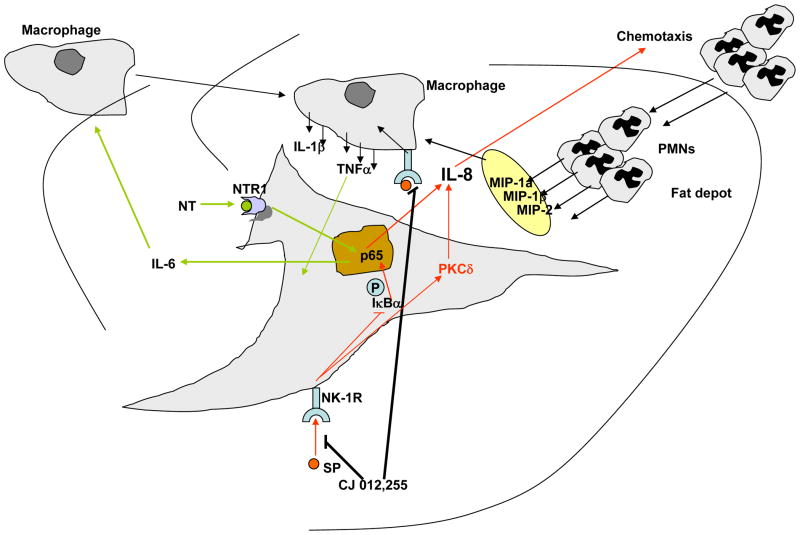
Figure 2. Schematic representation of the Substance P- and Neurotensin-induced inflammatory changes in mesenteric fat depots.
Interaction of both SP and NT with their receptors on mesenteric preadipocytes leads to activation of NF-kB subunit p65 and release of IL-8 and IL-6 respectively. IL-8 acts as a chemotactic agent to attract neutrophils into the fat depot which in turn are capable of releasing a number of chemokines that may lead to increases in mesenteric fat macrophages. Mesenteric fat depot macrophage numbers may also increase via the NT-induced IL-6 release and possibly other mechanisms described previously. Such fat depot inflammatory changes may participate in the development of Crohn’s Disease or even in the healing process of this condition.
Acknowledgments
This work was supported by a Research Fellowship Award from the “Crohn’s and Colitis Foundation of America, Inc.” to IK, and Grants RO-1 DK60729, RO-1 DK072471, and PO-1 DK 33506 to CP.
REFFERENCES
- 1.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasudevan AR, et al. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt CC, et al. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol. 2001;175:81–92. doi: 10.1016/s0303-7207(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 5.Desreumaux P, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- 6.Kern PA, et al. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruun JM, et al. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- 8.Skurk T, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 9.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 10.Siegmund B, et al. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965–972. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sennello JA, et al. Transplantation of wild-type white adipose tissue normalizes metabolic, immune and inflammatory alterations in leptin-deficient ob/ob mice. Cytokine. 2007 doi: 10.1016/j.cyto.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mykoniatis A, et al. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 2003;124:683–691. doi: 10.1053/gast.2003.50101. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi N, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Neumeier M, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 15.Saijo S, et al. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005;334:1180–1183. doi: 10.1016/j.bbrc.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhao T, et al. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238:19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn’s disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fayad R, et al. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara T, et al. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131:853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Tchkonia T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 21.Tchkonia T, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 22.Yesilbursa D, et al. The effect of orlistat-induced weight loss on interleukin-6 and C-reactive protein levels in obese subjects. Acta Cardiol. 2005;60:265–269. doi: 10.2143/AC.60.3.2005002. [DOI] [PubMed] [Google Scholar]
- 23.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27:1699–1705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]
- 24.Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23:661–666. doi: 10.1097/MOG.0b013e3282c8c8d3. [DOI] [PubMed] [Google Scholar]
- 25.Karagiannides I, et al. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci U S A. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambero A, et al. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm Bowel Dis. 2007;13:1357–1364. doi: 10.1002/ibd.20222. [DOI] [PubMed] [Google Scholar]
- 27.Schaffler A, Herfarth H. Creeping fat in Crohn’s disease: travelling in a creeper lane of research? Gut. 2005;54:742–744. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantyh CR, et al. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988;85:3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantyh CR, et al. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology. 1996;111:1272–1280. doi: 10.1053/gast.1996.v111.pm8898641. [DOI] [PubMed] [Google Scholar]
- 30.Pothoulakis C, et al. Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275:G68–75. doi: 10.1152/ajpgi.1998.275.1.G68. [DOI] [PubMed] [Google Scholar]
- 31.Renzi D, et al. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blum AM, et al. Interleukin 12 and antigen independently induce substance P receptor expression in T cells in murine schistosomiasis mansoni. Faseb J. 2001;15:950–957. doi: 10.1096/fj.00-0379. [DOI] [PubMed] [Google Scholar]
- 33.Goode T, et al. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut. 2000;47:387–396. doi: 10.1136/gut.47.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouloumie A, et al. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 35.Castagliuolo I, et al. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci U S A. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkland JL, et al. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol. 1994;49:B31–35. doi: 10.1093/geronj/49.1.b31. [DOI] [PubMed] [Google Scholar]
- 37.Herlinger H, Furth EE, Rubesin SE. Fibrofatty proliferation of the mesentery in Crohn disease. Abdom Imaging. 1998;23:446–448. doi: 10.1007/s002619900377. [DOI] [PubMed] [Google Scholar]
- 38.Knutson H, Lunderquist A. Vascular changes in Crohn’s disease. Am J Roentgenol Radium Ther Nucl Med. 1968;103:380–385. doi: 10.2214/ajr.103.2.380. [DOI] [PubMed] [Google Scholar]
- 39.Aaronson SA. Growth factors and cancer. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 40.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 41.Hausman DB, et al. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 42.Giordano A, et al. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25:125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 43.Giordano A, et al. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci. 1998;111(Pt 17):2587–2594. doi: 10.1242/jcs.111.17.2587. [DOI] [PubMed] [Google Scholar]
- 44.De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27:877–886. doi: 10.1023/a:1006996922657. [DOI] [PubMed] [Google Scholar]
- 45.Cui J, Zaror-Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol. 1990;259:R324–332. doi: 10.1152/ajpregu.1990.259.2.R324. [DOI] [PubMed] [Google Scholar]
- 46.Cui J, Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. 1992;262:R568–573. doi: 10.1152/ajpregu.1992.262.4.R568. [DOI] [PubMed] [Google Scholar]
- 47.Bauer B, Baier G. Protein kinase C and AKT/protein kinase B in CD4+ T-lymphocytes: new partners in TCR/CD28 signal integration. Mol Immunol. 2002;38:1087–1099. doi: 10.1016/s0161-5890(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 48.El-Chaar D, Gagnon A, Sorisky A. Inhibition of insulin signaling and adipogenesis by rapamycin: effect on phosphorylation of p70 S6 kinase vs eIF4E-BP1. Int J Obes Relat Metab Disord. 2004;28:191–198. doi: 10.1038/sj.ijo.0802554. [DOI] [PubMed] [Google Scholar]
- 49.Wright JT, Hausman GJ. Insulinlike growth factor-1 (IGF-1)-induced stimulation of porcine preadipocyte replication. In Vitro Cell Dev Biol Anim. 1995;31:404–408. doi: 10.1007/BF02634290. [DOI] [PubMed] [Google Scholar]
- 50.Nougues J, et al. Differentiation of adipocyte precursors in a serum-free medium is influenced by glucocorticoids and endogenously produced insulin-like growth factor-I. Int J Obes Relat Metab Disord. 1993;17:159–167. [PubMed] [Google Scholar]
- 51.Lorenzo M, et al. IGF-I is a mitogen involved in differentiation-related gene expression in fetal rat brown adipocytes. J Cell Biol. 1993;123:1567–1575. doi: 10.1083/jcb.123.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koellensperger E, et al. Human serum from platelet-poor plasma for the culture of primary human preadipocytes. Stem Cells. 2006;24:1218–1225. doi: 10.1634/stemcells.2005-0020. [DOI] [PubMed] [Google Scholar]
- 53.Castagliuolo I, et al. A neurotensin antagonist, SR 48692, inhibits colonic responses to immobilization stress in rats. Proc Natl Acad Sci U S A. 1996;93:12611–12615. doi: 10.1073/pnas.93.22.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagliuolo I, et al. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–849. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brun P, et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G621–629. doi: 10.1152/ajpgi.00140.2004. [DOI] [PubMed] [Google Scholar]
- 56.Hervieu G, Nahon JL. Pro-melanin concentrating hormone messenger ribonucleic acid and peptides expression in peripheral tissues of the rat. Neuroendocrinology. 1995;61:348–364. doi: 10.1159/000126857. [DOI] [PubMed] [Google Scholar]
- 57.Verlaet M, et al. Human immune cells express ppMCH mRNA and functional MCHR1 receptor. FEBS Lett. 2002;527:205–210. doi: 10.1016/s0014-5793(02)03232-5. [DOI] [PubMed] [Google Scholar]