Abstract
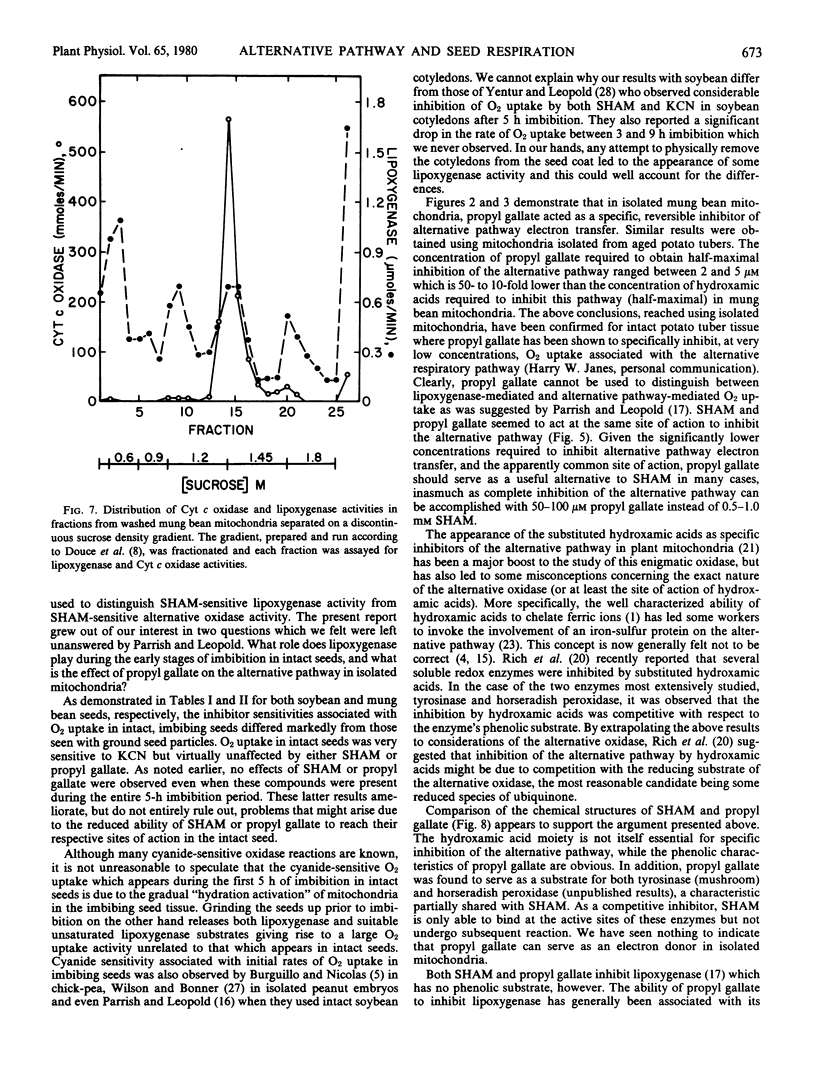
Oxygen uptake during the first hours of imbibition in intact soybean and mung bean seeds showed a marked sensitivity to potassium cyanide but was unaffected by addition of either salicylhydroxamic acid or propyl gallate. However O2 uptake by finely ground seed particles was very sensitive to the addition of either compound. The results indicated that O2 uptake in intact, imbibing seeds was associated with a cyanide-sensitive process, most probably mitochondrial mediated respiration, and not the result of the cyanide-insensitive lipoxygenase activity which was readily detectable in ground seed particles.
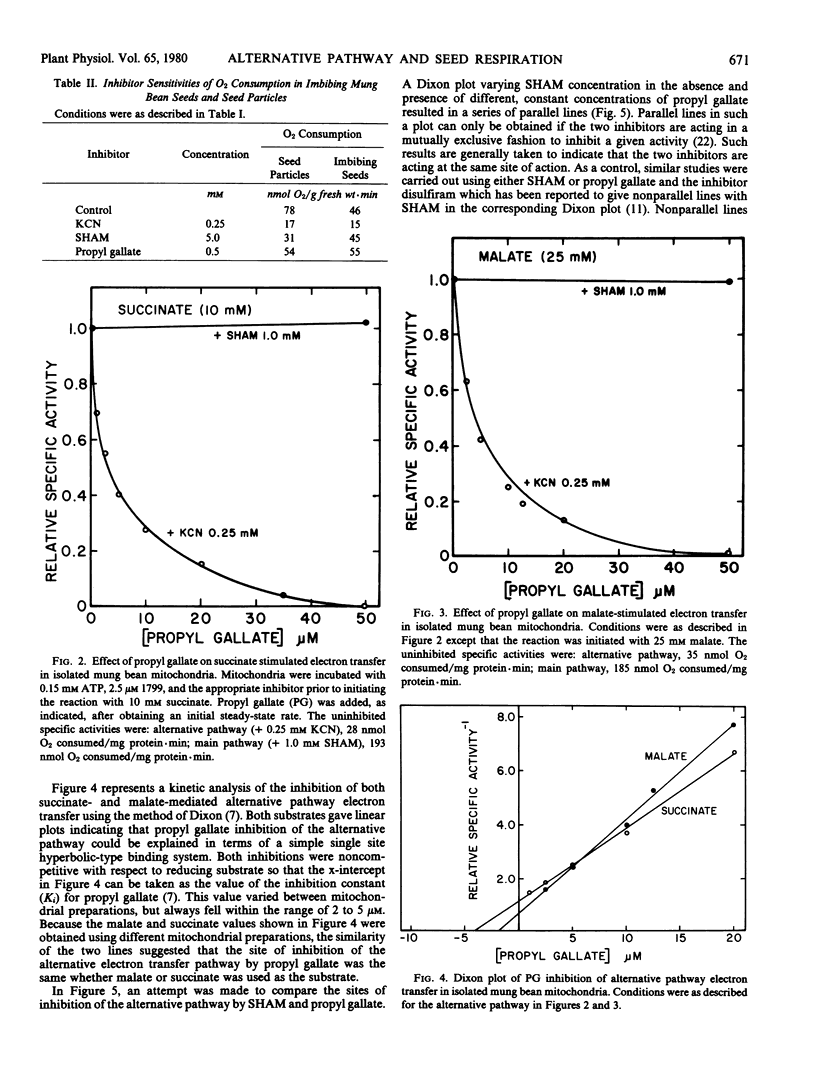
The antioxidant propyl gallate was found to inhibit specifically alternative pathway electron transfer in isolated mung bean mitochondria. Half-maximal inhibition occurred with 2 to 5 micromolar propyl gallate. Kinetic analysis indicated that propyl gallate inhibition of the alternative pathway occurred at, or very near, the site of inhibition of the alternative pathway by salicylhydroxamic acid.
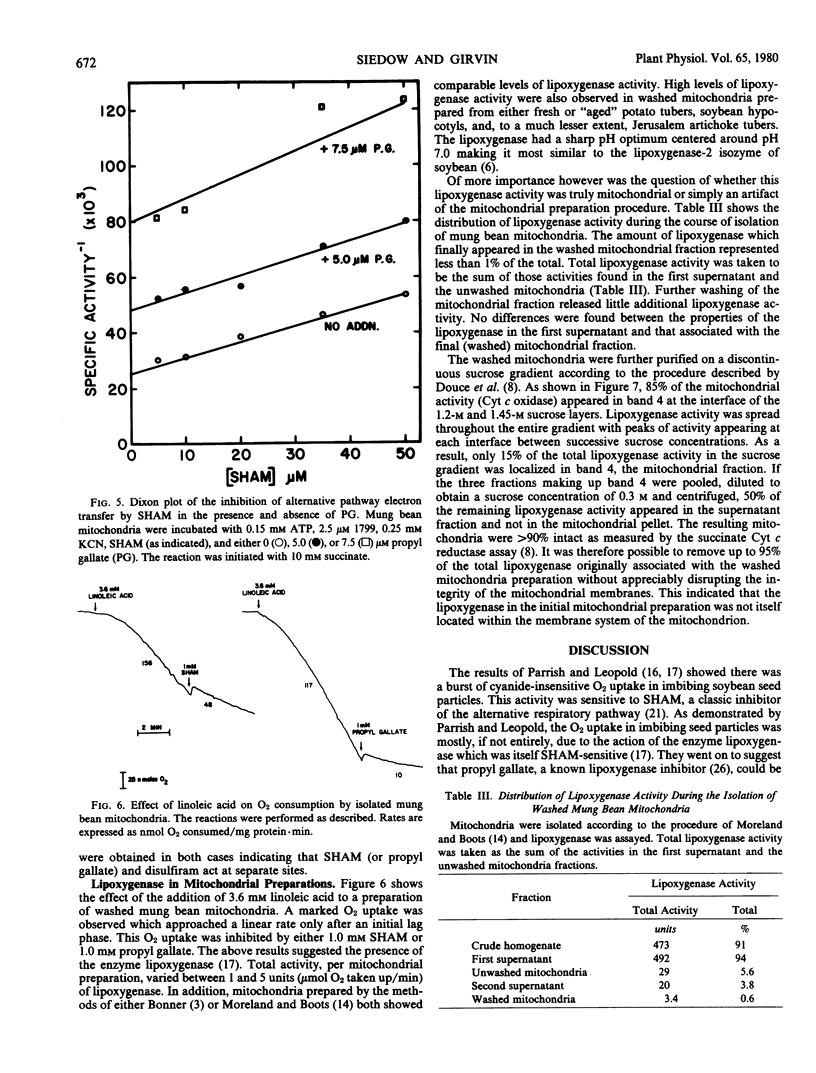
A high level of lipoxygenase activity was found to be associated with washed mitochondria isolated from a variety of etiolated plant tissues. Most of this lipoxygenase activity could be eliminated from mung bean mitochondria if the mitochondria were purified on a discontinuous sucrose gradient. This indicated that the mitochondrial-associated activity was probably the result of nonspecific adsorption of lipoxygenase onto the mitochondrial membranes during isolation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burguillo P. de L., Nicolás G. Appearance of an Alternate Pathway Cyanide-resistant during Germination of Seeds of Cicer arietinum. Plant Physiol. 1977 Oct;60(4):524–527. doi: 10.1104/pp.60.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher J., Pistorius E., Axelrod B. Isolation of an isozyme of soybean lipoxygenase. Biochim Biophys Acta. 1970 Jan 14;198(1):12–19. doi: 10.1016/0005-2744(70)90028-8. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Eskin N. A., Grossman S., Pinsky A. Biochemistry of lipoxygenase in relation to food quality. CRC Crit Rev Food Sci Nutr. 1977 Apr;9(1):1–40. doi: 10.1080/10408397709527229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moreland D. E., Boots M. R. Effects of optically active 1-(alpha-methylbenzyl)-3-(3,4-dichlorophenyl)urea on reactions of mitochondria and chloroplasts. Plant Physiol. 1971 Jan;47(1):53–58. doi: 10.1104/pp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M. The "uniqueness" of plant mitochondria. Biochem Soc Trans. 1979 Feb;7(1):246–252. doi: 10.1042/bst0070246. [DOI] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Confounding of alternate respiration by lipoxygenase activity. Plant Physiol. 1978 Sep;62(3):470–472. doi: 10.1104/pp.62.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish D. J., Leopold A. C. Transient changes during soybean imbibition. Plant Physiol. 1977 Jun;59(6):1111–1115. doi: 10.1104/pp.59.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L., Ingledew W. J., Bonner W. D., Jr EPR studies of higher plant mitochondria. I Ubisemiquinone and its relation to alternative respiratory oxidations. Biochim Biophys Acta. 1977 Dec 23;462(3):501–514. doi: 10.1016/0005-2728(77)90097-4. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Wiegand N. K., Blum H., Moore A. L., Bonner W. D., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978 Aug 7;525(2):325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloway S., Rosen P. Estimation of Basicity with a Novel Thermochromic Indicator. Science. 1955 Jan 21;121(3134):99–100. doi: 10.1126/science.121.3134.99. [DOI] [PubMed] [Google Scholar]
- Surrey K. Spectrophotometric Method for Determination of Lipoxidase Activity. Plant Physiol. 1964 Jan;39(1):65–70. doi: 10.1104/pp.39.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. B., Bonner W. D. Studies of electron transport in dry and imbibed peanut embryos. Plant Physiol. 1971 Sep;48(3):340–344. doi: 10.1104/pp.48.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yentur S., Leopold A. C. Respiratory Transition during Seed Germination. Plant Physiol. 1976 Feb;57(2):274–276. doi: 10.1104/pp.57.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


