Abstract
Administration of intra-articular medications, including corticosteroids and analgesics, is common clinical practice for knee pathology and dysfunction. Non-steroidal anti-inflammatory drugs (NSAIDs) are another category of medication commonly prescribed for their analgesic and anti-inflammatory properties. Recent studies have demonstrated the efficacy of injectable NSAIDs in the treatment of intra-articular pathology and postoperative analgesia.1–3 However, little data exist regarding the safety of intra-articular injection, despite the increase in its application.4 Therefore, the objective of this study was to investigate the effects of intra-articular NSAID injection on articular cartilage, the anterior cruciate ligament (ACL), and joint function in the rat. Sixty-four Sprague-Dawley rats were divided into either saline (SAL) or ketorolac (NSAID) tibiofemoral single injection treatment groups. Animals were sacrificed at 2, 7, 28, and 84 days post-injection for histological and mechanical analyses. Additionally, a subset of animals underwent longitudinal ambulatory evaluation to determine joint functional properties. We hypothesized that intra-articular ketorolac injection would result in no detrimental mechanical, histological, or functional changes. No differences were reported between the NSAID and SAL groups in any of the parameters measured at any time point, demonstrating the potential safety of intra-articular NSAID administration. Therefore, NSAID injection could be further considered for clinical application in humans.
Keywords: NSAID, intra-articular, injection, knee, mechanics
INTRODUCTION
Every year, more than 28 million Americans incur musculoskeletal injury, with an estimated annual cost to society of $254 billion.5 Included in these injuries are knee pathology and dysfunction, which can also result from non-traumatic cause. Abnormalities of the bony anatomy, articular cartilage, soft-tissues, and neurologic structures about the knee have all been implicated in joint and lower extremity dysfunction. As with many orthopaedic conditions, both operative and non-operative treatment options exist for these various diagnoses. While surgical treatment options are quite varied, there are a limited number of non-operative treatments, including immobilization, activity modification, physical therapy, and/or pharmacologic intervention. Medications can be administered by several routes, with local administration via injection being a frequent option for the knee as it mitigates unwanted systemic effects while delivering the medication directly to the joint space. Due to the presence of both pain and inflammation associated with joint injuries and dysfunction, non-steroidal anti-inflammatory drugs (NSAIDs) are often prescribed due to their anti-inflammatory and analgesic effects. However, little data exists supporting the safety of intra-articular injection of NSAIDs.
NSAIDs are commonly prescribed peri-operatively to reduce inflammation and alleviate pain. Oral administration of these agents is most common, but may be associated with systemic side effects.6–8 This has resulted in the investigation of intra-articular administration of NSAIDs for the treatment of various orthopaedic conditions as well as management of post-operative pain. Multiple studies have demonstrated the efficacy of intra-articular NSAIDs for postoperative pain management following orthopaedic surgery.1–3; 9 Additional work has demonstrated the beneficial effects of intra-articular NSAID administration for the treatment of osteoarthritis in both human and animal models.10; 11
Following early reports of intra-articular injection of NSAIDs, concern regarding the safety of such practice was documented.12; 13 Several resultant studies investigated the local effects of intra-articular NSAID injection in human and animal models, providing mixed results. In a rat knee model, histological evaluation indicated that intra-articular injection of ketorolac increased inflammation in the joint tissues.14 Additionally, intra-articular injection of ketorolac into the healthy rabbit knee showed mild histophathological changes, although these changes were considered non-detrimental.15 Human patients that received intra-articular ketorolac after knee arthroscopy demonstrated increased levels of glycerol, a marker of cell damage, in the synovial membrane, suggesting a potentially toxic effect of this NSAID on the joint soft tissues.16 Conversely, bufexamac was shown to cause no systemic or local changes in healthy horse intercarpal joint articular cartilage from histological analysis.17 Similarly, intra-articular injection of ketorolac into a healthy rabbit knee showed no histological changes, and injection into the rabbit patellar tendon showed no detrimental biomechanical or histological changes.4 These studies provide conflicting data both supporting and questioning the safety of intra-articular NSAID injection.
Most of the aforementioned studies only provide a histological evaluation at early or few time points after NSAID injection. In addition to this data, longitudinal evaluation of the mechanical properties of the joint tissues as well as the kinematic functional performance could provide support and further insight into the safety of intra-articular NSAID use. Because joint motion, cartilage structure and health, and soft tissue function are intricately related, damage or disruption to any one of these aspects may have profound impacts on the others. Therefore, the objective of this study was to investigate the safety of intra-articular tibiofemoral NSAID injection by evaluating a comprehensive set of structural and mechanical properties of articular cartilage and the ACL in the knee, as well as assess functional ambulation changes caused by NSAID injection into the rat knee. In this study, ketorolac, a commonly used NSAID in orthopaedic surgery, was investigated due to its potential anti-inflammatory and analgesic properties. We hypothesized that intra-articular ketorolac injection would not alter articular cartilage or ACL mechanical or structural properties, nor would it change functional ambulation parameters.
METHODS
Study Design and Animal Use
This study was approved by the University of Pennsylvania Institute for Animal Care and Use Committee (protocol 804452). Sixty-four Sprague-Dawley rats were used to investigate the effects of intra-articular injection of NSAIDs. Buprenorphine was administered subcutaneously to all animals (0.05 mg/kg) prior to intra-articular injection. Following anesthetization, the lower extremities were shaved, treated with hair removal cream (Nair) to improve visualization of surface anatomy, and sterilized with a betadine and alcohol solution. Bilateral tibiofemoral joints were injected with either saline (0.1 mL) or ketorolac tromethamine (Toradol, Bedford Laboratories, 3 mg/0.1 mL) (0.1mL), with both knees of an animal receiving the same injection. A lateral para-patellar injection was performed with the needle oriented towards the intercondylar notch. Injection was confirmed by visual distension of the joint. All rats were returned to cage activity for the remainder of the study. Sixteen rats (8 ketorolac, 8 saline) were sacrificed at each of four time points (2, 7, 28, and 84 days) (Figure 1). Immediately following sacrifice, the left tibia was isolated and frozen (−20°C) for cartilage indentation testing and the left femur with attached ACL was placed in formalin for histological processing. The right hindlimb was frozen (−20°C) for ACL mechanical testing. Additionally, the 84 day experimental group underwent knee kinematic evaluation.
Figure 1.

Group numbers correspond to each time point (−1, 2, 7, 28, and 84 days) for both NSAID and SAL groups. Gait analysis (GA) measurements were taken at each time point. Histological and mechanical evaluations were performed on the articular cartilage and ACL.
Quantitative Ambulatory Assessment
Hindlimb gait and ground reaction forces were measured in the 84 day time point animals using an instrumented walkway18 1 day prior to knee injection (baseline) and at 2, 7, 28, and 84 days post-injection. For each measurement, ground reaction force data (medial/lateral, braking, propulsion, and vertical forces), paw placement data (stride width and length), and ambulation timing data (speed, rate of loading, and stance time) was acquired. All parameters were averaged across walks on a given day for each animal, and all force data was normalized to the body weight of each animal at each time point. All data was collected using a custom LabVIEW program (National Instruments, Austin, TX) and parameters of knee function were analyzed using a custom MATLAB program (MathWorks, Inc., Natick, MA).
ACL Mechanical Testing
The right hindlimb was thawed at room temperature and dissected by removing surrounding tissue from the tibia and femur keeping the ACL intact to isolate it between its bony attachments (Figure 2A). Three Verhoeff stain lines were placed along the ACL for optical strain tracking. Cross-sectional area was determined by imaging coronal and sagittal planes of the ACL using a stereomicroscope. Specifically, the tibia-ACL-femur unit was placed at 45° flexion, and a ruler was placed in the image view in the same plane as the ACL for calibration. MIPAV software was used to determine the thickness in both planes and the area was calculated assuming an elliptical cross-section. Both the tibia and femur were embedded in holding fixtures using polymethylmethacrylate (PMMA) and inserted into a custom fixture with the joint in 45° flexion (Figure 2B). The specimen was immersed in a phosphate-buffered saline (PBS) bath at 37°C, and tensile testing was performed as follows: preload to 0.1N, preconditioning (10 cycles of 0.1N – 0.5N at 1% strain/sec), stress relaxation (ramp to 5% strain at 5% strain/sec, hold for 600 sec) followed by a return to initial displacement for 60 sec, and a ramp to failure (0.3% strain/sec). Stress was calculated as force divided by initial area, and two-dimensional Lagrangian strain was determined from stain line displacements using texture tracking software in MATLAB.
Figure 2.
ACL mechanical testing set-up. (A) ACL isolated between the tibia and femur embedded in PMMA. Stain lines were placed along the ligament for optical tracking. (B) Sagittal view of testing set-up with custom fixtures to hold at 45° knee flexion.
Histological Evaluation
The left hind limb was dissected as described for ACL mechanical preparation. Special care was taken not to damage the femoral or tibial cartilage. The ACL was detached at the tibial attachment site, and the femur was cut at the base of the femoral head to isolate the femoral condyles with attached ACL. The femur-ACL unit was fixed and processed using standard protocols and sagittal sections (7μm) were collected. Sections containing ACL were stained with hematoxylin and eosin (H&E) and those containing articular cartilage were stained with safranin-O, fast green, and iron hematoxylin (Saf-O/FG). ACL sections were imaged (Nikon Eclipse 90i, Melville, NY) at the femoral attachment site and the midsubstance of the ligament, and cartilage sections were imaged at the anterior load-bearing region (identified by anatomical location, section depth, and relative cartilage thickness and stain intensity) at 200× magnification. All images were analyzed for cell shape (aspect ratio: 0–1, with 1 being a circle) and cell density (number of cells/mm2) using a bioquantification software system (Bioquant Osteo II; BIOQUANT Image Analysis Corp, Nashville, TN, USA). All cellularity quantification analyses were performed by the same blinded investigator. The cartilage samples were qualitatively evaluated for amount of staining, surface fibrillation, and tissue integrity by the same blinded investigator.
Cartilage Thickness Measurement
The tibia was dissected to remove surrounding tissue, embedded in PMMA, and immersed in PBS containing a protease inhibitor cocktail (5 mM benzamidine hydrochloride, 1 mM phenylmethylsulfonyl fluoride, 1 M N-ethylmaleimide) at room temperature. The articular cartilage surface of the medial tibial plateau was scanned in 0.25 mm increments using a 55 MHz ultrasound probe (VisualSonics, Inc, Toronto, Ontario, Canada) in coronal and sagittal planes. Using methods consistent with previous studies,19 captured B-Mode images of each scan were manually segmented by selecting the cartilage and bony surfaces of the tibia (Figure 3A,B). The 3D positions of these surfaces were reconstructed with a custom MATLAB program and used to determine cartilage thickness maps by subtracting the cartilage and bony surfaces. The average thickness was computed for each thickness map in a 0.5 mm diameter region at the center region of the tibia (Figure 3C), and this center thickness was averaged between the coronal and sagittal images for each specimen. Following ultrasound scanning, specimens were re-wrapped in soft tissue and PBS soaked gauze and refrozen (−20°C) until mechanical testing.
Figure 3.

Ultrasound image of medial tibial plateau articular cartilage segmentation. (A) Sagittal and (B) coronal ultrasound images with markers defining the cartilage and bone surfaces. (C) Thickness map of cartilage with center region of interest defined.
Cartilage Mechanical Testing
The tibia was thawed and immersed in PBS containing a protease inhibitor cocktail at room temperature. Indentation testing was performed using a 0.5 mm diameter, non-porous spherical indenter tip in the center region of the medial tibial plateau. Cartilage was positioned under the indenter using angular, rotational, and linear stages such that the indenter tip was perpendicular to the cartilage surface. The PBS in the bath was used as a level plane for reference. Positioning was performed by the same blinded investigator for all specimens. A stress-relaxation test was performed, with a preload of 0.005N followed by a ramp to 20% strain at −0.05 mm/sec and a 300 second hold. Equilibrium elastic modulus was calculated as described 20 assuming Poisson’s ratio (ν=0.3).
Statistics
For the ambulatory assessment, significance was assessed using a two-way ANOVA with repeated measures on time. In this data set, points were occasionally absent for a specific animal on a specific day (approximately 5% of the total data). Therefore, multiple imputations were conducted using the Markov chain Monte Carlo method on the ambulation data to allow for a repeated measures analysis. Tissue mechanics and histology were assessed using two-way ANOVAs to evaluate the effect of NSAID injection and time post-injection. Cartilage histology tissue integrity and staining was evaluated qualitatively, so no statistical test was performed. Significance was set at p<0.05, and data is presented as mean ± standard deviation. We used the statistical program SPSS for Windows (Version 20.0; IBM Corp, Armonk, NY, USA) for data analysis.
To develop a pre-study power analysis, we used data from previous studies evaluating joint damage in the presence of altered loading in our lab using methods similar to those of the current study. Those studies demonstrated significant differences in articular cartilage equilibrium modulus of 1±0.31 MPa and 1.45±0.45 MPa between groups,21 and tendon modulus of 173.25±25.26 MPa and 312.89±137.12 MPa between groups.22 Due to similarities in testing methods, tissue types, and experimental parameters, we assumed that the current study would have similar variance and effect sizes as these previous data, therefore supporting the necessary sample size to achieve appropriate power. As a result, we determined that eight animals in each group were sufficient to achieve a power of 80% with p=0.05.
RESULTS
There were no differences between the ketorolac (NSAID) and saline (SAL) injection groups in any measured parameter at any time point. Specifically, for knee kinematics evaluation, we measured forces (propulsion, vertical, medial and lateral, and braking), paw placement (stride width and length), and timing (speed, rate of loading, and stance time) of ambulation longitudinally at all time points for both NSAID and SAL groups (Figure 4). There were no differences due to the NSAID compared to the SAL in any of these parameters, although walking speed did change over time.
Figure 4.
Measurements from force plate and paw placement analysis showed no differences between NSAID and SAL groups. Changes over time were statistically significant in speed.
For ACL mechanical evaluation, we measured maximum load, stiffness, percent relaxation, modulus, maximum stress, and cross-sectional area (Figure 5) at each time point post-injection. There were no changes between treatment groups, but changes were observed over time in maximum load, percent relaxation, maximum stress, and cross-sectional area in both groups.
Figure 5.
No differences in ACL mechanics between NSAID and SAL groups. Changes in time were statistically significant in max load, percent relaxation, max stress, and cross-sectional area.
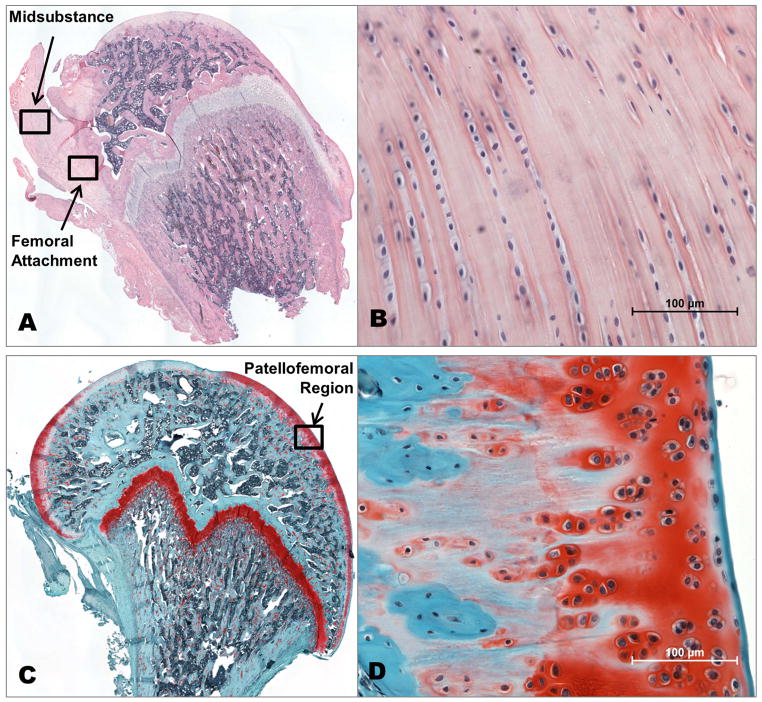
For histological evaluation, we measured cell shape and cell density for a load bearing region of femoral articular cartilage and for both the ACL femoral attachment and midsubstance. We found no differences between groups in any of the regions for either cell shape or density, however, all parameters showed changes over time (Table 1). The regions of analysis are depicted in the stitched images of the full femoral head in (Figure 6A,C), with corresponding representative 200× images of the ACL and cartilage (Figure 6B,D) used for quantitative cellular analysis. Additionally, the cartilage samples were qualitatively evaluated for GAG staining intensity and surface fibrillation, and all samples appeared to have normal cartilage structure.
Table 1.
Histological evaluation of cell shape and cell density for cartilage and ACL femoral attachment and midsubstance regions. There are no statistically significant differences between NSAID and SAL groups for any parameter in any region (p ≥ 0.25). Time was a statistically significant effect (*p < 0.05) for all parameters.
| Region | Time Point (days) | Group | Cell Density* (cells/mm^2) | Cell Shape* (Aspect Ratio) |
|---|---|---|---|---|
| ACL Midsubstance | 2 | NSAID | 277 ± 41 | 0.54 ± 0.09 |
| SAL | 284 ± 17 | 0.55 ± 0.06 | ||
| 7 | NSAID | 234 ± 34 | 0.67 ± 0.07 | |
| SAL | 241 ± 18 | 0.64 ± 0.09 | ||
| 28 | NSAID | 245 ± 57 | 0.63 ± 0.07 | |
| SAL | 235 ± 42 | 0.52 ± 0.11 | ||
| 84 | NSAID | 223 ± 69 | 0.67 ± 0.07 | |
| SAL | 181 ± 54 | 0.72 ± 0.10 | ||
|
| ||||
| ACL Femoral Attachment | 2 | NSAID | 374 ± 82 | 0.60 ± 0.03 |
| SAL | 426 ± 31 | 0.59 ± 0.12 | ||
| 7 | NSAID | 335 ± 41 | 0.72 ± 0.04 | |
| SAL | 318 ± 14 | 0.69 ± 0.03 | ||
| 28 | NSAID | 331 ± 101 | 0.61 ± 0.03 | |
| SAL | 335 ± 68 | 0.58 ± 0.03 | ||
| 84 | NSAID | 328 ± 100 | 0.72 ± 0.06 | |
| SAL | 221 ± 38 | 0.72 ± 0.05 | ||
|
| ||||
| Cartilage | 2 | NSAID | 667 ± 103 | 0.78 ± 0.06 |
| SAL | 665 ± 81 | 0.77 ± 0.03 | ||
| 7 | NSAID | 546 ± 113 | 0.82 ± 0.02 | |
| SAL | 574 ± 110 | 0.81 ± 0.02 | ||
| 28 | NSAID | 551 ± 84 | 0.78 ± 0.02 | |
| SAL | 511 ± 49 | 0.82 ± 0.01 | ||
| 84 | NSAID | 407 ± 95 | 0.83 ± 0.04 | |
| SAL | 536 ± 74 | 0.82 ± 0.02 | ||
Figure 6.
Representative images of (A,B) ACL stained with H&E and (C,D) cartilage stained with Saf-O/FG. (A,C) Stitched images of the femur to demonstrate the regions for quantitative evaluation. (B,D) Representative 200× images for quantitative cellular evaluation.
Finally, for cartilage mechanical evaluation, we measured cartilage thickness and equilibrium elastic modulus (Figure 7). There were no changes due to treatment group in either parameter, but both showed changes over time.
Figure 7.

(A) Equilibrium modulus of cartilage obtained from indentation testing and (B) cartilage thickness obtained from high-frequency ultrasound showed no differences between the NSAID and SAL groups. Changes over time were statistically significant.
DISCUSSION
Many studies have demonstrated the efficacy of injectable NSAIDs in the treatment of intra-articular pathology. However, little data exists supporting the safety of these injections in the joint space. Given the significant potential of both application and benefit for these injections, the likelihood for widespread routine use exists. However, prior to the customary use of intra-articular NSAIDs for the treatment and management of joint pathology, the effects and safety of their use must be well established. While there have been a small number of studies evaluating the safety of intra-articular NSAID administration,4; 14–17 most of these studies lack a longitudinal evaluation of both the structural and mechanical properties of cartilage and intra-articular ligaments as well as the kinematic functional performance of the joint, which could provide support and further insight into the safety of this NSAID administration strategy. Therefore, we examined a comprehensive set of parameters that allowed us to evaluate the mechanical and structural properties of the cartilage and ACL, as well as functional ambulation changes due to NSAID injection. Our study demonstrates that tibiofemoral intra-articular injection of ketorolac does not cause damage to the articular cartilage and ACL, or cause detrimental ambulatory changes in a native rat model when compared to a saline control. These findings suggest that intra-articular administration of NSAIDs could be safe for clinical use for pain management.
If the intra-articular injection of ketorolac was determined to be detrimental to joint health, we would have expected to see significant decreases in the mechanical properties of both the articular cartilage and the ACL. The cartilage mechanical properties of both the NSAID and saline injection groups are consistent with the healthy control groups in previous literature.19; 23 The injury models in these studies (joint instability or corticosteroid intra-articular injection) show decreases in cartilage modulus well below the measures in this study, indicating that our study does not show properties consistent with injury. Additionally, if damage was caused by this treatment, we would have expected to see a decrease in proteoglycan content in the cartilage histological analysis indicated by decreased safranin-O staining and the development of surface fibrillation.24 Since none of these measures, among others, showed any significant or abnormal changes, we are confident in the safety of intra-articular injection of ketorolac in this model.
The volume of fluid injected into each knee joint was determined after preliminary studies demonstrated 0.1 mL of injected fluid resulted in uniform distribution throughout the knee joint, without disruption of the joint capsule. Based on the standard dose of ketorolac available and utilized in this study, and the maximum volume allowable in the rat knee without capsule damage, 3 mg of ketorolac was delivered. This would represent an approximately 30× supratherapeutic dose in humans. Given the consistent results at this high dosage, it is unlikely that a lower, therapeutic ketorolac dose would result in tissue damage. While changes were observed over time in some parameters, these changes were the same in both treatment groups, and are therefore most likely due to changes in animal age and/or weight over time as is expected in such a longitudinal study. Therefore, with no changes in a comprehensive set of structural, mechanical, and ambulatory parameters noted in this study, this data strongly supports the conclusion of no detrimental effects of intra-articular injection of ketorolac.
This study is not without limitations. First, this study was performed on healthy tissue. It is possible that healing, damaged, or degraded tissue would respond differently to ketorolac exposure than healthy tissue. However, the results of this study are an important first step to determining the safety of intra-articular NSAID injection. Future studies could evaluate the safety and efficacy of intra-articular NSAID administration in the setting of damaged joint tissues. Additionally, this study did not evaluate the toxicity of ketorolac on a cellular level. While this analysis would aid in the conclusions of the study, evaluating the effect of NSAIDs in an in vivo model using functional, mechanical, and structural measures is more relevant to the human clinical response than an in vitro experiment. Since no detrimental effects were observed due to the administration of NSAIDs, we can state that any cellular toxicity is negligible, and does not impact the health or function of the joint tissues.
This study demonstrates no detrimental effects of intra-articular ketorolac injection on articular cartilage, ligaments, and kinematic function of the native knee in an in vivo rat model. This data adds to the limited existing literature, supporting the safety of intra-articular NSAID administration, thus further supporting the use of intra-articular NSAIDs for anti-inflammatory and analgesic therapies. Findings from this study support future studies aimed to examine the therapeutic effects of injectable NSAIDs on intra-articular pathologies.
Acknowledgments
The authors thank Adam Caro and Sarah Yannascoli for their contributions. This study was funded by a pilot grant from the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders and a NSF Graduate Research Fellowship.
References
- 1.Convery PN, Milligan KR, Quinn P, et al. Low-dose intra-articular ketorolac for pain relief following arthroscopy of the knee joint. Anaesthesia. 1998;53:1125–1129. doi: 10.1046/j.1365-2044.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Axelsson K, Allvin R, et al. Postoperative pain following knee arthroscopy: the effects of intra-articular ketorolac and/or morphine. Reg Anesth Pain Med. 1999;24:225–230. doi: 10.1016/s1098-7339(99)90132-3. [DOI] [PubMed] [Google Scholar]
- 3.Reuben SS, Connelly NR. Postoperative analgesia for outpatient arthroscopic knee sugery with intraarticular bupivacaine and ketorolac. Anesth Analg. 1995;80:1154–1157. doi: 10.1097/00000539-199506000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro PS, Rohde RS, Froimson MI, et al. The effect of local corticosteroid or ketorolac exposure on histologic and biomechanical properties of rabbit tendon and cartilage. Hand (N Y) 2007;2:165–172. doi: 10.1007/s11552-007-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Praemer A, Furner S, Rice DP, et al. Musculoskeletal conditions in the United States. Park Ridge, Ill: American Academy of Orthopaedic Surgeons; 1992. [Google Scholar]
- 6.Hudson N, Hawthorne AB, Cole AT, et al. Mechanisms of gastric and duodenal damage and protection. Hepatogastroenterology. 1992;39(Suppl 1):31–36. [PubMed] [Google Scholar]
- 7.Rainsford KD, Stetsko PI, Sirko SP, et al. Gastrointestinal mucosal injury following repeated daily oral administration of conventional formulations of indometacin and other non-steroidal anti-inflammatory drugs to pigs: a model for human gastrointestinal disease. J Pharm Pharmacol. 2003;55:661–668. doi: 10.1211/002235703765344577. [DOI] [PubMed] [Google Scholar]
- 8.Sanghi S, MacLaughlin EJ, Jewell CW, et al. Cyclooxygenase-2 inhibitors: a painful lesson. Cardiovasc Hematol Disord Drug Targets. 2006;6:85–100. doi: 10.2174/187152906777441803. [DOI] [PubMed] [Google Scholar]
- 9.Reuben SS, Steinberg RB, Kreitzer JM, et al. Intravenous regional anesthesia using lidocaine and ketorolac. Anesth Analg. 1995;81:110–113. doi: 10.1097/00000539-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Unlu Z, Ay K, Tuzun C. Comparison of intra-articular tenoxicam and oral tenoxicam for pain and physical functioning in osteoarthritis of the knee. Clin Rheumatol. 2006;25:54–61. doi: 10.1007/s10067-005-1136-3. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Zou J, Huang L, et al. Efficacy of intra-articular injection of celecoxib in a rabbit model of osteoarthritis. Int J Mol Sci. 2010;11:4106–4113. doi: 10.3390/ijms11104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson DJ. Intraarticular ketorolac. Anesth Analg. 1996;82:433. doi: 10.1097/00000539-199602000-00057. [DOI] [PubMed] [Google Scholar]
- 13.Cook TM, Nolan JP, Tuckey JP. Postarthroscopic meniscus repair analgesia with intraarticular ketorolac or morphine. Anesth Analg. 1997;84:466–467. doi: 10.1097/00000539-199702000-00046. [DOI] [PubMed] [Google Scholar]
- 14.Irwin MG, Cheung KM, Nicholls JM, et al. Intra-articular injection of ketorolac in the rat knee joint: effect on articular cartilage and synovium. Br J Anaesth. 1998;80:837–839. doi: 10.1093/bja/80.6.837. [DOI] [PubMed] [Google Scholar]
- 15.Dogan N, Erdem AF, Gundogdu C, et al. The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can J Physiol Pharmacol. 2004;82:502–505. doi: 10.1139/y04-066. [DOI] [PubMed] [Google Scholar]
- 16.Stalman A, Tsai JA, Segerdahl M, et al. Ketorolac but not morphine exerts inflammatory and metabolic effects in synovial membrane after knee arthroscopy: a double-blind randomized prospective study using the microdialysis technique. Reg Anesth Pain Med. 2009;34:557–564. doi: 10.1097/aap.0b013e3181bfbd9f. [DOI] [PubMed] [Google Scholar]
- 17.Suominen MM, Tulamo RM, Anttila MO, et al. Effects of intra-articular injections of bufexamac suspension in healthy horses. Am J Vet Res. 2001;62:1629–1635. doi: 10.2460/ajvr.2001.62.1629. [DOI] [PubMed] [Google Scholar]
- 18.Sarver JJ, Dishowitz MI, Kim SY, et al. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 2010;43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuther KE, Sarver JJ, Schultz SM, et al. Glenoid cartilage mechanical properties decrease after rotator cuff tears in a rat model. J Orthop Res. 2012;30:1435–1439. doi: 10.1002/jor.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes WC, Keer LM, Herrmann G, et al. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 21.Reuther KE, Thomas SJ, Sarver JJ, et al. Effect of return to overuse activity following an isolated supraspinatus tendon tear on adjacent intact tendons and glenoid cartilage in a rat model. J Orthop Res. 2013;31:710–715. doi: 10.1002/jor.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas SJ, Reuther KE, Tucker JJ, et al. Biceps Detachment Decreases Joint Damage in a Rotator Cuff Tear Rat Model. Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-013-3422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray RC, DeBowes RM, Gaughan EM, et al. The effects of intra-articular methylprednisolone and exercise on the mechanical properties of articular cartilage in the horse. Osteoarthritis Cartilage. 1998;6:106–114. doi: 10.1053/joca.1997.0100. [DOI] [PubMed] [Google Scholar]
- 24.Teeple E, Elsaid KA, Jay GD, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39:164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]