Abstract
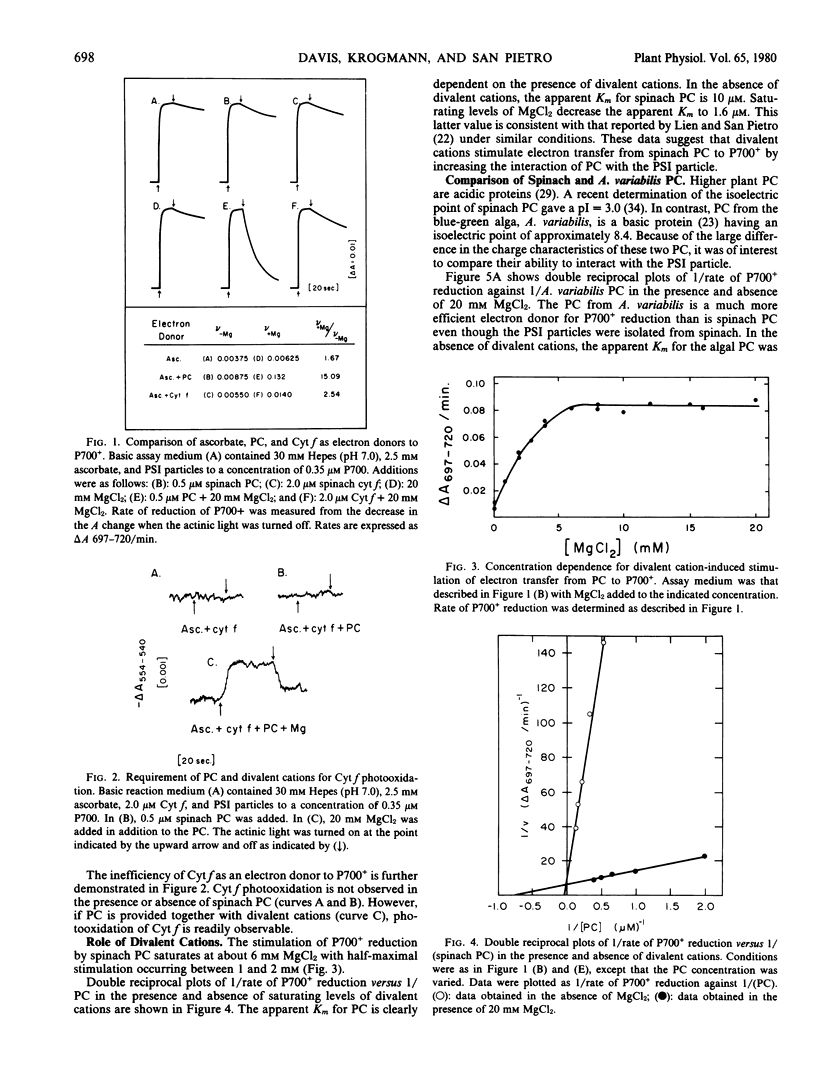
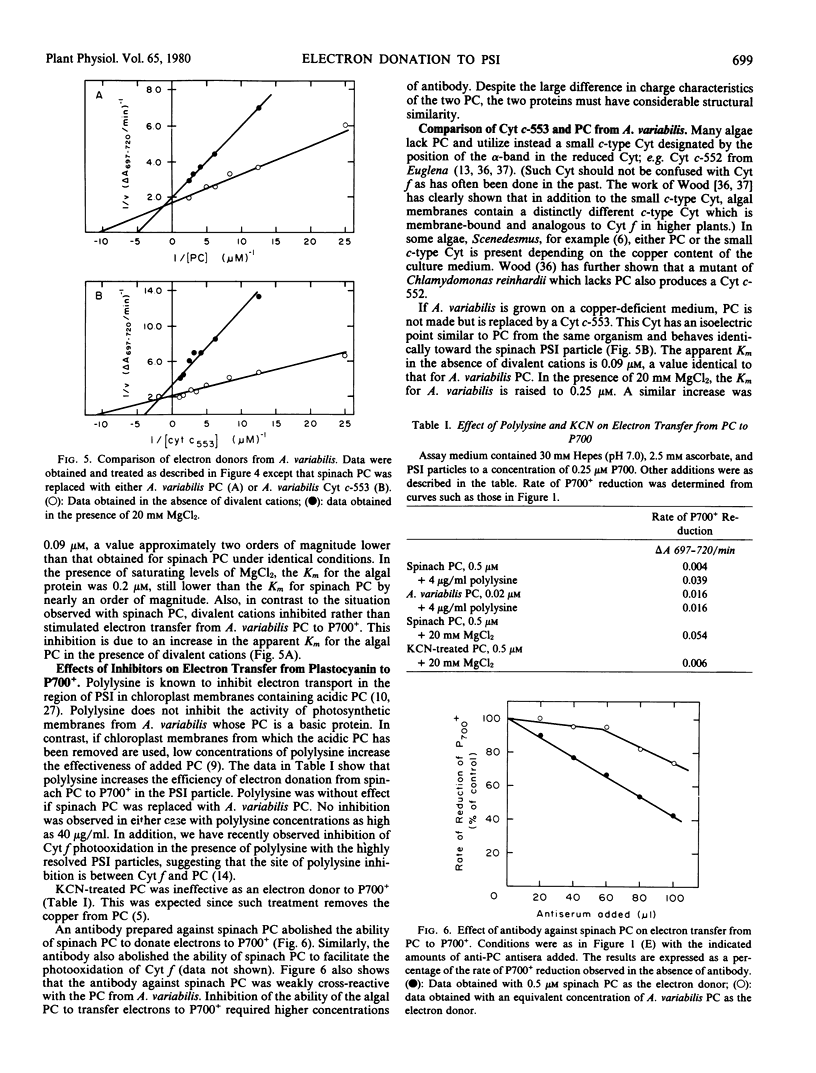
Electron donation to photosystem I was studied in highly resolved particles from spinach. Divalent cations increased the efficiency of electron donation from spinach plastocyanin to P700+ through a decrease in the apparent Km for plastocyanin. Cytochrome f was not an efficient electron donor for P700+ in the presence or absence of divalent cations. Cytochrome f photooxidation could be observed in the presence of both plastocyanin and divalent cations.
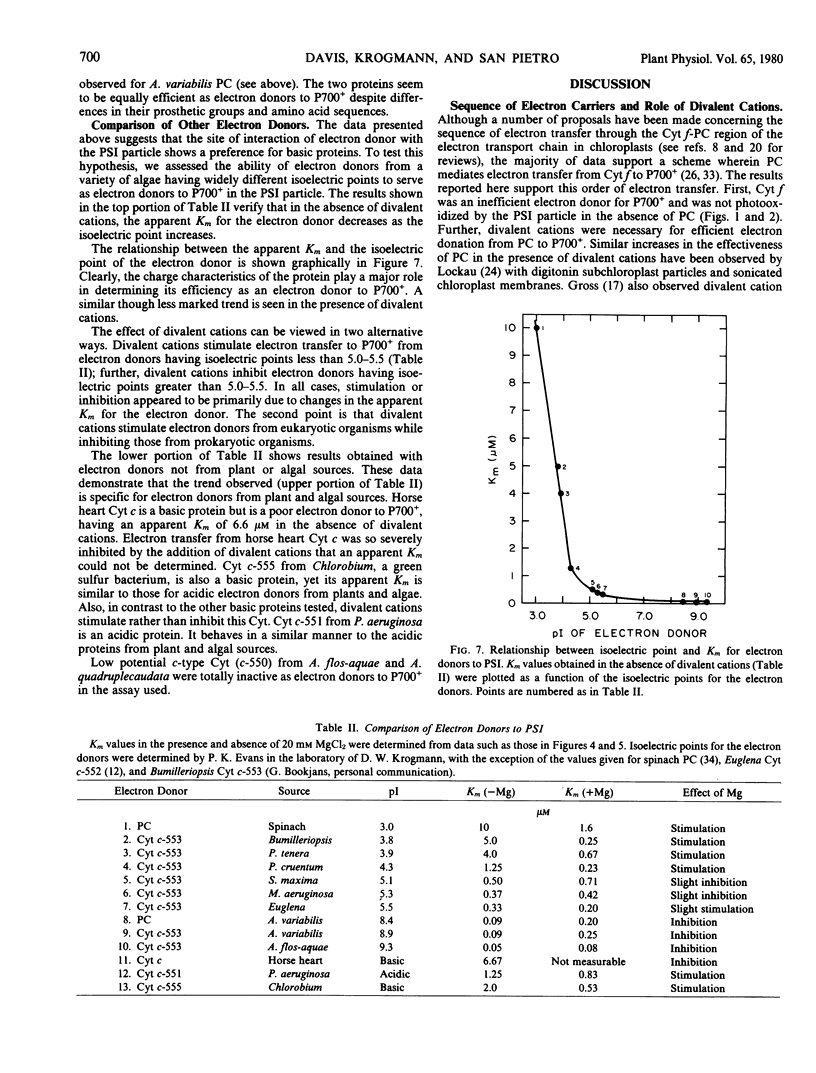
The efficiencies of electron donors from eukaryotic and prokaryotic algae to P700+ were also examined. Divalent cations enhanced the effectiveness of electron donors from eukaryotic organisms, while inhibiting electron donors from prokaryotic organisms. The prokaryotic electron donors were also much more efficient donors than were the electron donors from eukaryotic organisms. A correlation between the Km for the electron donor and its isoelectric point suggests that the net charge on the donor protein is a major determinant of the efficiency for electron donation. The data presented raise interesting questions with respect to the evolution of electron donation to photosystem I and the possibility of an additional electron carrier between plastocyanin and P700+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A. Prokaryote-eukaryote relationship and the amino acid sequence of plastocyanin from Anabaena variabilis. Biochem J. 1975 Sep;149(3):675–683. doi: 10.1042/bj1490675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A. Protein evolution in cyanobacteria. Nature. 1976 Oct 28;263(5580):793–796. doi: 10.1038/263793a0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Bartsch R. G. Amino acid sequence similarity between cytochrome f from a blue-green bacterium and algal chloroplasts. Nature. 1975 Jan 24;253(5489):285–288. doi: 10.1038/253285a0. [DOI] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Berg S. P., Krogmann D. W. Mechanism of KCN inhibition of photosystem I. J Biol Chem. 1975 Dec 10;250(23):8957–8962. [PubMed] [Google Scholar]
- Bohner H., Böger P. Reciprocal formation of cytochrome c-553 and plastocyanin in Scenedesmus. FEBS Lett. 1978 Jan 15;85(2):337–339. doi: 10.1016/0014-5793(78)80486-4. [DOI] [PubMed] [Google Scholar]
- Brand J., San Pietro A., Mayne B. C. Site of polylysine inhibition of photosystem I in spinach chloroplasts. Arch Biochem Biophys. 1972 Sep;152(1):426–428. doi: 10.1016/0003-9861(72)90233-0. [DOI] [PubMed] [Google Scholar]
- Brand J., San Pietro A. Polylysine-enhanced effectiveness of plastocyanin in photosystem. I. Biochim Biophys Acta. 1973 Nov 22;325(2):255–265. doi: 10.1016/0005-2728(73)90101-1. [DOI] [PubMed] [Google Scholar]
- Davis D. J., Krogmann D. W., San Pietro A. Localization of polylysine inhibition in a photosystem I subchloroplast particle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):110–116. doi: 10.1016/0006-291x(79)91596-1. [DOI] [PubMed] [Google Scholar]
- Davis D. J., San Pietro A. Preparation and characterization of a chemically modified plastocyanin. Anal Biochem. 1979 May;95(1):254–259. doi: 10.1016/0003-2697(79)90214-8. [DOI] [PubMed] [Google Scholar]
- Gross E. L. Cation-induced increases in the rate of P700 recovery in photosystem I particles. Arch Biochem Biophys. 1979 Jun;195(1):198–204. doi: 10.1016/0003-9861(79)90341-2. [DOI] [PubMed] [Google Scholar]
- Gross E. L., Grenier J. Regulation of excitation energy distribution in subchloroplast particles: photosystem I. Arch Biochem Biophys. 1978 Apr 30;187(2):387–398. doi: 10.1016/0003-9861(78)90049-8. [DOI] [PubMed] [Google Scholar]
- Ho K. K., Ulrich E. L., Krogmann D. W., Gomez-Lojero C. Isolation of photosynthetic catalysts from cyanobacteria. Biochim Biophys Acta. 1979 Feb 8;545(2):236–248. doi: 10.1016/0005-2728(79)90203-2. [DOI] [PubMed] [Google Scholar]
- Kunert K. J., Böhme H., Böger P. Reactions of plastocyanin and cytochrome 553 with photosystem I of Scenedesmus. Biochim Biophys Acta. 1976 Dec 6;449(3):541–553. doi: 10.1016/0005-2728(76)90163-8. [DOI] [PubMed] [Google Scholar]
- Lien S., San Pietro A. Interaction of plastocyanin and P700 in PSI reaction center particles from C. reinhardi and spinach. Arch Biochem Biophys. 1979 Apr 15;194(1):128–137. doi: 10.1016/0003-9861(79)90602-7. [DOI] [PubMed] [Google Scholar]
- Lightbody J. J., Krogmann D. W. Isolation and properties of plastocyanin from Anabaena variabilis. Biochim Biophys Acta. 1967 May 9;131(3):508–515. doi: 10.1016/0005-2728(67)90010-2. [DOI] [PubMed] [Google Scholar]
- Lockau W. The inhibition of photosynthetic electron transport in spinach chloroplasts by low osmolarity. Eur J Biochem. 1979 Mar;94(2):365–373. doi: 10.1111/j.1432-1033.1979.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ulrich E. L., Berg S. P., Krogmann D. W. Nuclear magnetic resonance studies of the copper binding sites of blue copper proteins: oxidized, reduced, and apoplastocyanin. Biochemistry. 1975 Oct 7;14(20):4428–4433. doi: 10.1021/bi00691a014. [DOI] [PubMed] [Google Scholar]
- Nelson N., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. X. Purification of spinach cytochrome f and its photooxidation by resolved photosystem I particles. J Biol Chem. 1972 Jun 25;247(12):3848–3853. [PubMed] [Google Scholar]
- Ort D. R., Izawa S., Good N. E., Krogmann D. W. Effects of the plastocyanin antagonists KCN and poly-L-lysine on partial reactions in isolated chloroplasts. FEBS Lett. 1973 Apr 1;31(1):119–122. doi: 10.1016/0014-5793(73)80087-0. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The purification and amino acid sequence of cytochrome C-552 from Euglena gracilis. Biochem J. 1974 May;139(2):449–459. doi: 10.1042/bj1390449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J. A., Brown R. H., Scawen M. D., Boulter D. Higher plant plastocyanin. Biochim Biophys Acta. 1973 Apr 20;303(2):269–273. doi: 10.1016/0005-2795(73)90357-7. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Nisonoff A., Margoliash E. Immunological activity of cytochrome c. 3. Enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970 Mar 10;245(5):947–954. [PubMed] [Google Scholar]
- Scawen M. D., Ramshaw J. A., Boulter D. The amino acid sequence of plastocyanin from spinach. (Spinacia oleracea L.). Biochem J. 1975 May;147(2):343–349. doi: 10.1042/bj1470343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Siedow J. N., Curtis V. A., San Pietro A. Studies on photosystem I. I. Relationship of plastocyanin, cytochrome f and P700. Arch Biochem Biophys. 1973 Oct;158(2):889–897. doi: 10.1016/0003-9861(73)90583-3. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., Rasched I. R., Kunert K. J., Kroneck P., Böger P. Plastocyanin: possible significance of quaternary structure. Eur J Biochem. 1976 Apr 15;64(1):131–140. doi: 10.1111/j.1432-1033.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. The roles of c-type cytochromes in algal photosynthesis. Extraction from algae of a cytochrome similar to higher plant cytochrome f. Eur J Biochem. 1977 Feb;72(3):605–612. doi: 10.1111/j.1432-1033.1977.tb11283.x. [DOI] [PubMed] [Google Scholar]



