Abstract
Despite the well-documented importance of paternal caregiving for positive child development, little is known about the neural changes that accompany the transition to fatherhood in humans, or about how changes in hormone levels affect paternal brain function. We compared fathers of children aged 1–2 with non-fathers in terms of hormone levels (oxytocin and testosterone), neural responses to child picture stimuli, and neural responses to visual sexual stimuli. Compared to non-fathers, fathers had significantly higher levels of plasma oxytocin and lower levels of plasma testosterone. In response to child picture stimuli, fathers showed stronger activation than non-fathers within regions important for face emotion processing (caudal middle frontal gyrus [MFG]), mentalizing (temporo-parietal junction [TPJ]) and reward processing (medial orbitofrontal cortex [mOFC]). On the other hand, non-fathers had significantly stronger neural responses to sexually provocative images in regions important for reward and approach-related motivation (dorsal caudate and nucleus accumbens). Testosterone levels were negatively correlated with responses to child stimuli in the MFG. Surprisingly, neither testosterone nor oxytocin levels predicted neural responses to sexual stimuli. Our results suggest that the decline in testosterone that accompanies the transition to fatherhood may be important for augmenting empathy toward children.
Keywords: Fathers, Testosterone, Oxytocin, Empathy, Inferior Frontal Gyrus, Orbitofrontal Cortex, FMRI
Introduction
Considerable evidence now attests to the importance of hormone changes for the onset and maintenance of paternal caregiving (reviewed in (Rilling 2013)). In particular, studies indicate that both oxytocin (OT) and testosterone (T) levels are altered when men become fathers (Gettler et al. 2011; Gordon et al. 2010), and that these changes are important for paternal nurturance (Feldman et al. 2011; Gettler et al. 2011; Gordon et al. 2010; Mascaro et al. 2013). However, it remains unclear how parenting-induced changes in OT and T influence brain function to support engaged fathering. Three plausible hypotheses have been offered.
One hypothesis is that these hormonal changes augment empathic responding to children in ways that promote positive child outcomes, and there is considerable indirect evidence to support the idea that a reduction in T and increase in OT may serve such a function in new fathers. Exogenous testosterone impairs the ability to read emotional facial expressions (van Honk et al. 2011). In addition, men with higher testosterone levels report less sympathy in response to unknown newborn infant cries (Fleming, Corter, Stallings, & Steiner, 2002), exhibit less affectionate touch towards their infant (Weisman et al. 2014), and are less involved in parental care (Alvergne et al. 2009; Gettler et al. 2011; Mascaro et al. 2013), which may reflect decreased empathic responding to children’s needs. Oxytocin is also involved in empathy and emotion processing more generally (Bartz et al. 2011), and perhaps with paternal empathy more specifically. For example, baseline plasma oxytocin levels predict infant-father synchrony, social engagement, and interaction styles of fathers with infants aged 4–6 months and 7 weeks (Feldman et al. 2011; Gordon et al. 2010). Furthermore, intranasal OT treatment caused fathers to touch their infants more and to engage in more social reciprocity with them (Weisman et al. 2012b), and altered the physical proximity and movement of fathers toward their infants during a play session (Weisman et al. 2013a). Intranasal OT also enhanced sensitivity and decreased hostility of fathers during play with their toddlers (Naber et al. 2010; Naber et al. 2013), and augmented the cortisol responses to a stressful stillface paradigm in fathers observed to have synchronous relationships with their infant, a finding interpreted as suggesting that OT enhanced the salience of infant social cues in available fathers (Weisman et al. 2013b).
If T or OT modulate empathy, they likely act on neural systems known to mediate empathic responding. Consistently, both the perception (audio and visual) and contemplation of the suffering of another elicits activation in the anterior insula (AI), particularly on the right side (Lamm et al. 2010), thought to represent a simulated mapping of the observed individual’s body state onto one’s own (Tania Singer, Critchley, & Preuschoff, 2009). The anterior insula is also consistently activated in response to infant cry stimuli (Mascaro et al. 2013; Rilling 2013), suggesting that AI activity may be important for paternal empathy. In addition to the anterior insula, more basic motor simulation supports the ability to read emotional facial expressions (Carr et al. 2003; Jabbi and Keysers 2008), so it is possible that changes in OT or T alter a father’s simulation of children’s emotional facial expressions. This hypothesis predicts that T will be negatively correlated with responses to child stimuli in the AI and putative mirror system pathways, whereas OT will be positively correlated with these responses.
A second hypothesis is that hormone changes in new fathers may support paternal nurturance by enhancing the reward value of child stimuli in ways that motivate caregiving. The ventromedial prefrontal cortex (vmPFC)/OFC is broadly implicated in reward (Rolls 2000), and in parental reward more specifically (Parsons et al. 2013), and the medial portion of the OFC is thought to trigger innate and specific responses to children’s faces (Kringelbach et al. 2008). In addition to the OFC, rodent models of maternal behavior point to the importance of the mesolimbic dopamine (DA) system in the appetitive drive to nurture offspring, with DA-producing cell bodies in the ventral tegmental area (VTA) projecting to the nucleus accumbens (NA) to motivate proactive care of infants (Numan and Stolzenberg 2009). Oxytocin acts at the VTA to facilitate DA release in NA (Numan and Stolzenberg 2009), which facilitates maternal behavior in rats presumably by enhancing parental motivation and the reward value of offspring. Recent research showing the effects of intranasal OT administration on the VTA response to social stimuli in nulliparous women supports the idea that OT interacts with dopamine to enhance social reward processing in humans (Groppe et al. 2013), and it may be that increases in OT in new fathers function similarly in the VTA to enhance the reward value of child stimuli (Mascaro et al. 2013).
A third hypothesis for understanding hormone changes in new fathers is derived from Life History Theory (LHT), which posits a trade-off between mating and parenting effort and proposes that the hormonal changes that accompany fatherhood bias men’s efforts and resources away from mating and toward parenting (Kaplan and Gangestad 2005; Wingfield et al. 1990). A large body of evidence supports the role of testosterone in mediating this trade-off. For example, experimental elevation of T increased mating effort and decreased parenting effort in socially monogamous birds (Hunt et al. 1999). In mammals, there is evidence that male parental behavior is more flexible with respect to hormonal changes. For example, male cotton-top tamarins increase T in response to their ovulating partner, but maintain high levels of parenting (Ziegler et al. 2004). Despite this added complexity, there is evidence that T supports mating effort at the expense of parenting effort among human males. As outlined above, men with higher testosterone levels tend to be less involved in parental caregiving. Furthermore, low levels of testosterone are associated with reduced libido among men (Wang et al. 2000). Within married couples, testosterone levels are negatively correlated with relationship quality and amount of time spent with one’s partner (Gray et al. 2002) and high levels predict divorce (Booth and Dabbs 1993) as well as polygyny (Alvergne et al. 2009). While there is less data to support the role of oxytocin in mediating LHT trade-offs, a recent study showed that self-administration of intranasal OT decreases approach behavior toward an unknown, attractive woman in partnered (but not single) males (Scheele et al. 2012). Taken together, these data suggest that changes in testosterone and oxytocin may alter neural responses to sexually provocative stimuli of unknown women in fathers compared to non-fathers in ways that decrease sexual pursuits that could conflict with parenting.
With these three functional explanations in mind, the current study investigated hormonal and neural differences between fathers and non-fathers and examined whether hormone differences predicted differences in brain function. To this end, we tested the following hypotheses: 1) Fathers will have significantly lower plasma testosterone levels and significantly higher plasma levels of oxytocin than non-fathers, 2) Fathers will have significantly more neural activity in response to unknown child photo stimuli in regions of the brain important for empathy and reward, 3) Non-fathers will have significantly more neural activity in response to sexually provocative photographs of unknown women in regions of the brain important for reward and sexual motivation, and 4) Plasma T and OT will be correlated with brain responses to child and sexual stimuli.
Materials and Methods
Subjects
We recruited 88 heterosexual, biological fathers of children age 1 or 2 who were currently cohabitating with the child’s mother using flyers posted around the Emory campus, at local parks, daycare centers, and with an electronic advertisement on Facebook. We also recruited 50 heterosexual non-fathers that were unmarried and at least 25 years of age using flyers posted around the Emory campus. The study was approved by the Emory Institutional Review Board, and all participants gave written informed consent. Participants had normal or corrected-to-normal (with contact lenses) vision and were screened and excluded for self-reported history of head trauma, seizures or other neurological disorder, psychiatric illness, alcoholism or any other substance abuse, serious medical illness, claustrophobia, and for ferrous metal in any part of body.
Fathers were between the age of 21 and 55 (M = 33.2, SD = 5.70) and had between 1 and 4 children, with 2 as the modal number (M = 1.80, SD = 0.80) (see table 1 for descriptive statistics). 83 of the fathers were married to their partner. Three married fathers did not indicate how long they had been married, but for those that provided that information (n = 80), the average amount of time married fathers had been married was 5.90 years (SD = 3.55). For the 5 fathers that were not married to their partner, the average amount of time they had lived with their partner was 2.3 years (SD = 0.45). Non-fathers were between the age of 25 and 53 (M = 30.4, SD = 6.11). There was a significant difference in age between the fathers and non-fathers (t(133) = 2.63, p < 0.05). There was not a significant difference in education status.
Table 1.
Descriptive statistics of fathers and non-fathers.
| Fathers | Non-fathers | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | N | Min | Max | Mean | |
| Total number enrolled | 88 | − | − | − | 50 | − | − | − |
| Number scanned | 63 | − | − | − | 30 | − | − | − |
| Age | 85 | 21 | 55 | 33.2 | 50 | 25 | 53 | 30.4 |
| Years education | 79 | 12 | 21 | 17.2 | 42 | 12 | 21 | 16.6 |
| T (ng/dL) | 82 | 157.8 | 850.2 | 413.8 | 49 | 179.6 | 893 | 520.8 |
| OT (pg/ml) | 82 | 4.6 | 22 | 8.8 | 48 | 4.6 | 18.5 | 6.6 |
| Number of children | 88 | 1 | 4 | 1.8 | − | − | − | − |
| 1 child | 36 | − | − | − | − | |||
| 2 children | 37 | − | − | − | − | |||
| 3 children | 12 | − | − | − | − | |||
| 4 children | 3 | − | − | − | − | |||
| Years married | 80 | 0 | 17 | 5.9 | − | − | − | − |
We were unable to obtain blood samples for 5 fathers and 1 non-father due to difficulties in vascular access. Blood samples were acquired from 83 fathers and 49 non-fathers, however there was insufficient sample to measure plasma oxytocin values for one non-father (n = 48). In addition, testosterone values for one father were excluded because he mentioned after the blood draw that he took testosterone supplements (n = 82). Blood was drawn between 7:30 and 15:25, and there was not a significant correlation between T or OT and time of blood draw. 63 fathers and 30 non-fathers completed fMRI scanning.
Photograph Stimuli
Unknown adult photographs were acquired from male and female trained actors who were asked to generate happy, sad, and neutral facial expressions. Unknown child photographs were obtained from male and female children. We captured eight pictures of unknown children making each facial expression during a play session. If the child did not make one of the facial expressions naturally, sad faces were elicited by the mother leaving the room or taking a favorite toy or cell phone from the child, and happy faces were elicited with singing, dancing, or tickling. Men viewed ethnicity-matched unknown adults and children, and fathers viewed an unknown child that was the same sex as their own child. See supplementary figure S1 for examples of each condition. For the sexual task, photographs of men, women in sexually provocative clothing and poses, and women in non-provocative clothing or poses were selected from a Google image search. Prior to scanning, 10 male volunteers were recruited from the Emory University community to rate the photographs on arousal and attractiveness using a 7 point Likert scale (1 = very unattractive/unarousing, 7 = very attractive/arousing). The sexually provocative images were rated as both significantly more attractive (p < 0.001) and as more arousing (p < 0.001) than the non-provocative images (attractiveness: provocative M = 5.69, SD = 0.11; non-provocative M = 3.73, SD = 0.15; arousal: provocative M = 5.88, SD = 0.11; non-provocative M = 3.06, SD = 0.12).
Hormones Assays
Blood samples were centrifuged at 4 °C within 20 min of blood draw. Plasma was collected and frozen at − 80 °C until assayed. Assays were performed in duplicate by the Biomarkers Core Lab of the Yerkes National Primate Research Center at Emory University using a coated-tube radioimmunoassay kit for testosterone (Coat-A-Count Total Testosterone, Cat No. TKTT1, Siemens, Los Angeles, CA). On the day of the assay, frozen plasma samples were thawed, centrifuged for 30 minutes at 3,000 revolutions per minute, and assayed according to the protocol provided by the manufacturer. The assay range for testosterone was 6.0–1667 ng/dL with a 50 µL sample size. Quality control samples were included with all assays and yielded an inter-assay CV% range of (low) 4.05% at 134.73ng/dL and (high) 4.37% at 788.19 ng/dL and an intra-assay CV% range of (low) 2.07% at 136.11 ng/dL and (high) 2.28% at 785.81 ng/dL.
Oxytocin was assayed by ELISA in accordance with the manufacturer’s recommended procedure (Enzo Life Science, Farmingdale, NY) after solid phase extraction and reconstitution of extracts in sample buffer provided by the manufacturer. Quality control samples were included in each assay and extraction set and the inter- and intra-assay CV% was 7.16% at 26.99 pg/mL and 4.41% at 27.30 pg/mL respectively. The assay range for OT was 15.60–1,000.00 pg/mL using a 100uL per well of the ELISA. Extraction of larger sample volumes (>250uL) allowed greater sensitivity. Oxytocin results from one participant (father) were omitted as his value (33.36 pg/mL) was 6 standard deviations greater than the mean of the fathers.
Anatomical image acquisition
Subjects were positioned in the Siemens Trio 3T MRI scanner. Subjects lay motionless in a supine position in the scanner with padded head restraint to minimize head movement during scanning. Each scanning session began with a 15 s scout, followed by a 5 min T1-weighted MPRAGE scan (TR = 2600 ms, TE = 3.02 ms, matrix = 256 × 256, FOV = 256 mm, slice thickness = 1.00 mm, gap = 0 mm).
fMRI image acquisition
Functional scans used an EPI sequence with the following parameters: TR = 2000 ms, TE = 28 ms, matrix =64 × 64, FOV = 224 mm, slice thickness = 2.5 mm, gap thickness = 1.05 mm, 34 axial slices. TE was minimally decreased from the typical value (32 ms) in order to reduce magnetic susceptibility artifact in the orbitofrontal region. For the child task, subjects were imaged while viewing pictures of happy (H), sad (S), and neutral (N) facial expressions in two different people: 1. an unknown child (C), and 2. an unknown adult (A). Participants were instructed to “please observe each picture and try to share the emotions of the person in the picture”. For each expression, men viewed 8 different pictures of the person making that expression over the course of 4 blocks, and each picture was viewed twice. During a single block, 4 photographs of the same type were shown, each for 3 seconds. There was a 0.5 second fixation between each photograph. Thus, the duration of each block was 14 seconds. After every 6 blocks, subjects viewed a fixation block of equal duration. The total duration of the task was 7 minutes (24 face blocks + 6 fixation blocks×14 s per block). Photographs were presented in pseudo-random order. After viewing all blocks of unknown child and adult faces, fathers viewed pictures of their own children in order to explore variation in fathers’ neural response to their OWN child, as reported previously (Mascaro et al. 2013).
Sexual Stimuli were presented following the procedure of Hamann et al (Hamann et al. 2004). Subjects viewed sexually provocative images of women (S), non-provocative images of women (NS), non-provocative images of men (M), and a visual fixation cross. Stimuli were presented in 15 second blocks. Within each block, five exemplars of each stimulus type were presented. Each stimulus was presented for 3 seconds. Subjects viewed eight blocks of each stimulus type. The total duration of the task was 8 minutes.
Functional Image Analysis
Image preprocessing was conducted with Brain Voyager QX (version 2.0.8) software (Brain Innovation, Maastricht, The Netherlands). The first 8 volumes of each run were discarded in order to allow the tissue magnetization to equilibrate. Preprocessing involved image realignment by six-parameter 3-D motion
correction, slice scan time correction using linear interpolation, spatial smoothing with a 8-mm full width at half maximum (FWHM) Gaussian kernel, and temporal smoothing with voxel-wise linear detrending and high-pass filtering of frequencies below three cycles per run length. Data were excluded subsequent to movement greater than 2 mm in the x, y, or z direction. This was the case for 3 subjects for the child task and 3 subjects for the sexual task. In addition, complete data from 2 non-fathers were excluded from the sexual task due to consistent movement greater than 2 mm in the x, y, or z direction. Images were subsequently normalized into Talairach space (Talairach and Tournoux 1988).
A separate general linear model (GLM) was defined for each subject that examined the neural response to the following six regressors for the child task: child’s happy face (CH), child’s sad face (CS), child’s neutral face (CN), adult’s happy face (AH), adult’s sad face (AS), and adult’s neutral face (AN), and the following three regressors for the sexual task: male images (M), sexually provocative female images (S), and non-sexual female images (NS). Each regressor was convolved with a standardized model of the hemodynamic response. For each subject, contrasts of parameter estimates for various predictors were computed at every voxel of the brain. To investigate the main effects of child and sexual stimuli across all men in our sample, in a second-level analysis, we constructed a random-effects GLM combining all fathers and non-fathers, and paired t tests were used to investigate the contrasts [Child – Adult] and [Sexual – Non-sexual]. Results were thresholded at p < 0.0001, uncorrected for multiple comparisons. To evaluate if there are differences between fathers’ and nonfathers’ neural response to child and sexual stimuli, we utilized a dual approach. First, in a whole brain exploratory analysis we identified brain regions in which fathers differed significantly from non-fathers for the contrasts [Child - Adult] and [Sexual – Non-sexual]. Results were thresholded at p< 0.001. Second, we performed functional ROI analyses, identifying ROIs as follows. A subset of fathers were randomly selected to generate a group GLM that contained equal numbers of fathers and non-fathers for both the child (n = 30 fathers and non-fathers, total n = 60) and sexual tasks (n = 28 fathers and non-fathers, total n = 56). For the child task GLM, we specified the contrast [Child-Adult] and applied a threshold of p<0.005 to identify the following functional regions of interest (ROIs): right MFG, right inferior orbital cortex and anterior insula, and vmPFC extending into the medial OFC. The VTA was not significantly active at this threshold, and thus was not included in the ROI analysis for the child task. For the sexual task GLM, we specified the contrast [Sexual-Nonsexual] and applied a threshold of p<0.005 to identify the following functional ROIs in regions important for reward and sexual motivation: hypothalamus, nucleus accumbens, caudate, and substantia nigra/VTA (Stoléru et al. 2012). See table 2 for a list of all functional ROIs tested.
Table 2.
Functional ROIs tested for differences between fathers and nonfathers<comma> and for correlations with OT and T. For each ROI<comma> we list the voxel of peak activation<comma> the number of functional voxels contained within the ROI<comma> and note whether there is a significant difference between fathers and non-fathers<comma> and a significant partial correlation with OT or T (controlling for age).
| X | Y | Z | voxels | Fathers vs. Non-fathers |
OT | T | |
|---|---|---|---|---|---|---|---|
| Child–Adult (n = 60);p ≤ 0.005 | |||||||
| Empathy and emotion processing | |||||||
| R middle frontal gyrus | 44 | 22 | 30 | 176 | + | − | +++ |
| R medial orbital gyrus<comma> extending into ventral anterior insula | 14 | 28 | −18 | 84 | − | − | − |
| Reward | |||||||
| R middle frontopolar gyrus | 11 | 52 | −9 | 68 | +++ | − | − |
| Sexual–Non-sexual (n = 56);p ≤ 0.005 | |||||||
| Reward and sexual motivation | |||||||
| Hypothalamus | −7 | −5 | −3 | 45 | + | − | − |
| L NAcc | −4 | 10 | 3 | 7 | ++ | − | − |
| R Caudate | 14 | 25 | 9 | 27 | − | − | − |
| R substantia nigra/VTA<comma> extending to the left | 5 | −23 | −9 | 79 | − | − | − |
To determine whether fathers differed significantly from non-fathers, individual subject contrast values from the functional ROIs were compared using independent samples t-tests. To determine whether neural responses were correlated with hormone levels, individual subject contrast values from the ROIs were explored in bivariate correlation analyses with oxytocin. Because testosterone varies by age (Harman et al. 2001) and because the fathers were slightly older than non-fathers (Father M = 33.2, Non-father M = 30.4), individual subject contrast values from these ROIs were explored in partial correlation analyses with testosterone, controlling for age.
Finally, we conducted whole brain exploratory analyses using testosterone and oxytocin as covariates for the contrast [Child - Adult] and [Sexual – Non-sexual]. These exploratory analyses were run on the entire sample set and also separately in fathers and non-fathers. Results were thresholded at p < 0.001, uncorrected for multiple comparisons.
Results
Hormones
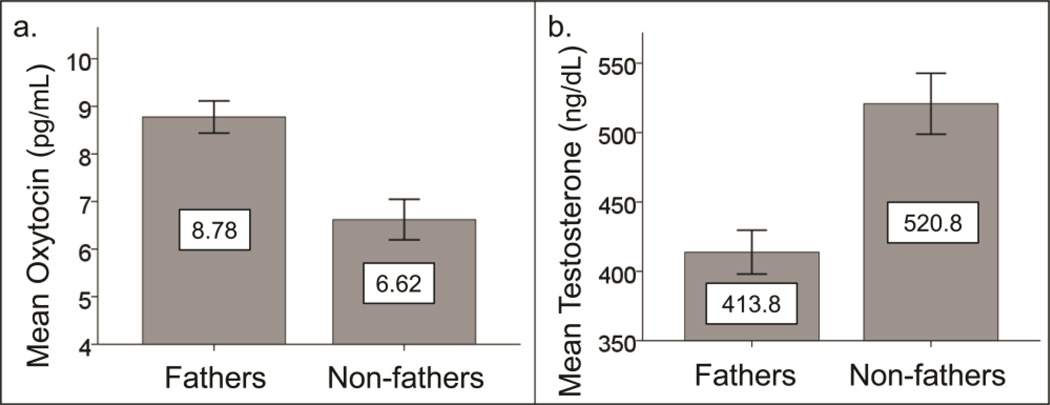
Non-fathers had significantly higher levels of plasma T (t(129) = 4.02, p < 0.0001; Mean fathers = 413.8, SD = 143.3; Mean non-fathers = 520.8, SD = 154.0), and significantly less plasma oxytocin (t(128) = −3.93, p < 0.0005; Mean fathers = 8.78, SD = 3.06; Mean non-fathers = 6.62, SD = 2.96) (see figure 1). Age was weakly correlated with testosterone for the entire sample (r(127) = −0.22, p < 0.05) but not within the fathers and non-fathers samples considered separately. Given the negative correlation between T and age and the significant difference in age between the two groups, we conducted a multiple linear regression analysis with T as the dependent variable and fatherhood status and age as the independent variables in order to determine whether fatherhood status predicted variance in testosterone values when controlling for age differences. Fatherhood status was a significant unique predictor of testosterone levels, β = −.29, t(128) = 3.43, p < .001, while there was only a trend for age to uniquely predict testosterone levels β = −.16, t(128) = 1.94, p = 0.06). There was no correlation between T or OT and age of child within the sample of fathers (T: r(81) = 0.07, p = 0.55, OT: r(82) = 0.11, p = 0.93). Neither was there a correlation between number of children and fathers’ T or OT (T: r(81) = 0.03, p = 0.82; OT: r(82) = −0.04, p = 0.70).
Figure 1.
Differences between fathers and non-fathers in plasma levels of a. Oxytocin (t(128) = −3.93, p < 0.0005), and b. Testosterone ((t(129) = 4.02, p < 0.0001).
Neuroimaging
Main effect of the Child Task (child – adult)
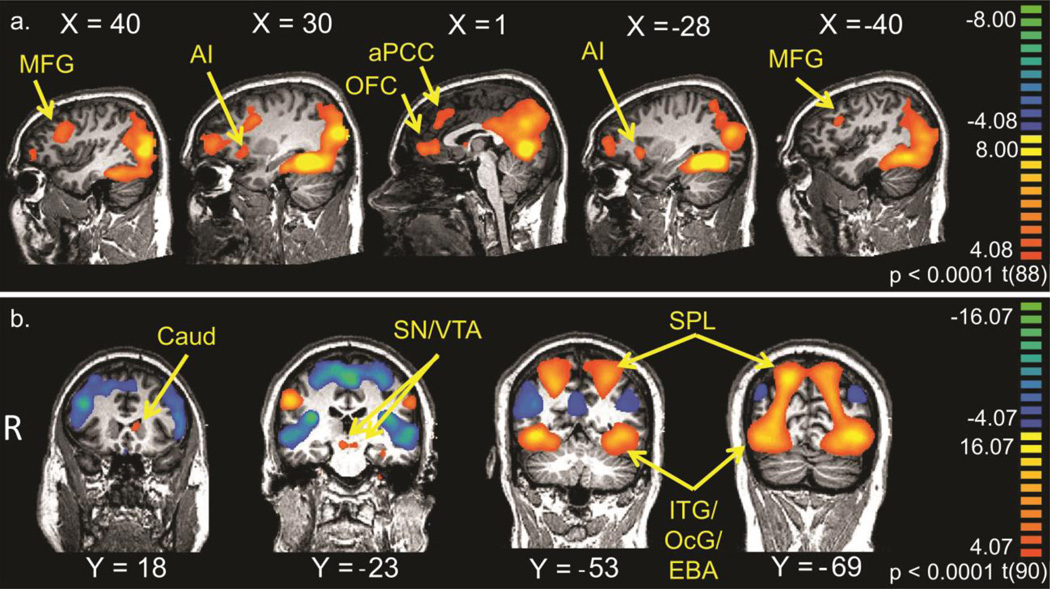
Viewing child picture stimuli robustly activated a brain region known to be important for face identity processing (fusiform gyrus) (Kanwisher et al. 1997), likely because men were attending to child emotion faces more than to adult faces, since attention is known to modulate activation in this region (e.g. (Wojciulik et al. 1998). It also activated regions important for face emotion processing (middle frontal gyrus extending into inferior frontal gyrus) (Carr et al. 2003), empathy (anterior insula) (Lamm et al. 2010), mentalizing (right temporoparietal junction [TPJ] and anterior paracingulate cortex) (Gallagher and Frith 2003; Lieberman 2007; Saxe and Kanwisher 2003), and reward processing (vmPFC/mOFC) (Rolls 2000) (see figure 2a and supplementary table 1).
Figure 2.
Main effect for all subjects of a. Child task for the contrast [Child – Adult] and b. Sexual task for the contrast [Sexual – Non-sexual], thresholded at p < 0.0001, uncorrected.
Main effect of the Sexual Task (sexual – non-sexual)
Viewing visual sexual stimuli robustly activated visual cortex, including the extrastriate body area (Downing et al. 2001), bilaterally. These stimuli also activated regions involved in reward processing (substantia nigra and ventral tegmental area) and sexual function (hypothalamus) (Stoléru et al. 2012) (see figure 2b and supplementary table S2). This contrast also revealed deactivations in the bilateral posterior insula, bilateral inferior frontal gyrus (orbitalis, BA 47), bilateral superior temporal sulcus extending into the middle temporal gyrus, the supplementary motor area (medial frontal gyrus, BA 6 on the medial surface), and posterior cingulate gyrus extending into the precuneus.
Whole brain investigation of neural differences between fathers and non-fathers
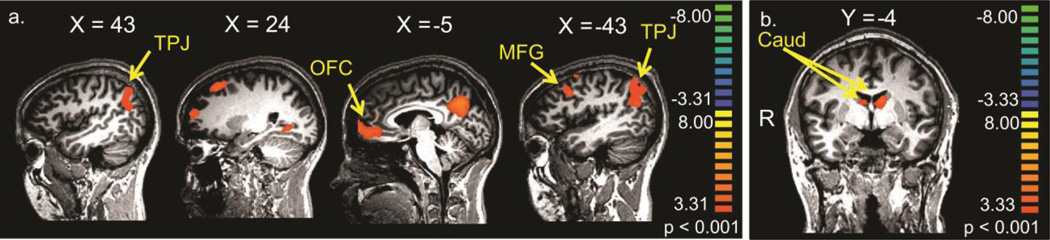
A whole brain exploratory analysis of the child task revealed several areas in which activation was greater for fathers than for non-fathers, including in the bilateral TPJ, middle frontal gyrus, lateral superior frontal gyrus, medial OFC/vmPFC, and the precuneus (see figure 3a and supplementary table S3). For the sexual task, the whole brain analysis revealed areas in the bilateral dorsal caudate and in the cerebellum that were significantly more active for non-fathers than for fathers (figure 3b and supplementary table S3).
Figure 3.
Comparison of fathers’ and non-fathers’ responses. a. Positive t values indicate areas in which fathers have more activity than non-fathers in response to the Child task for the contrast [Child – Adult]; and b. Positive t values indicate areas in which non-fathers have more activity than fathers in response to the Sexual task for the contrast [Sexual – Non-sexual]. All results are thresholded at p < 0.001, uncorrected.
Region of interest analysis
For the child task, the targeted ROI analysis revealed several regions in which fathers had significantly more activation than non-fathers, including regions important for face emotion processing (right middle frontal gyrus; t(91) = 2.50, p < 0.05) and for reward processing (medial OFC; t(91) = 2.97, p < 0.005). The difference between fathers and non-fathers for the rMFG was driven primarily by differential responses to happy [CH – AH] and neutral [CN – AN] child photos, whereas the medial OFC effects were driven largely by differential responses to sad [CS – AS] and neutral [CN – AN] child photos (see supplemental figure S2). There was not a significant difference in the anterior insula (see table 2 for summary). For the sexual task, non-fathers had significantly more activity in regions of the brain important for sexual motivation and function (Stoléru et al. 2012) (hypothalamus; t(89) = 2.41, p < 0.05) and for reward (NAcc; t(89) = 2.81, p < 0.01) in response to sexual stimuli (table 2).
Relationship between brain activity and hormones
Whole brain exploratory analysis
The results of a whole brain exploratory analysis using OT and T as covariates in the child task revealed a small cluster in the hippocampus that was positively correlated with plasma oxytocin levels (peak coordinates: x = 29, y = −41, z = 3; peak r = 0.40; number of functional voxels = 11) (see supplementary figure S3). There were no regions in which activation during the child task covaried with testosterone. Neither did the whole brain analysis reveal any regions of activation during the sexual task [sexual – non-sexual] that were significantly related to OT or T levels. The whole brain analyses conducted separately as a function of parental status did not reveal any effect of hormone on either task.
Region of interest analysis
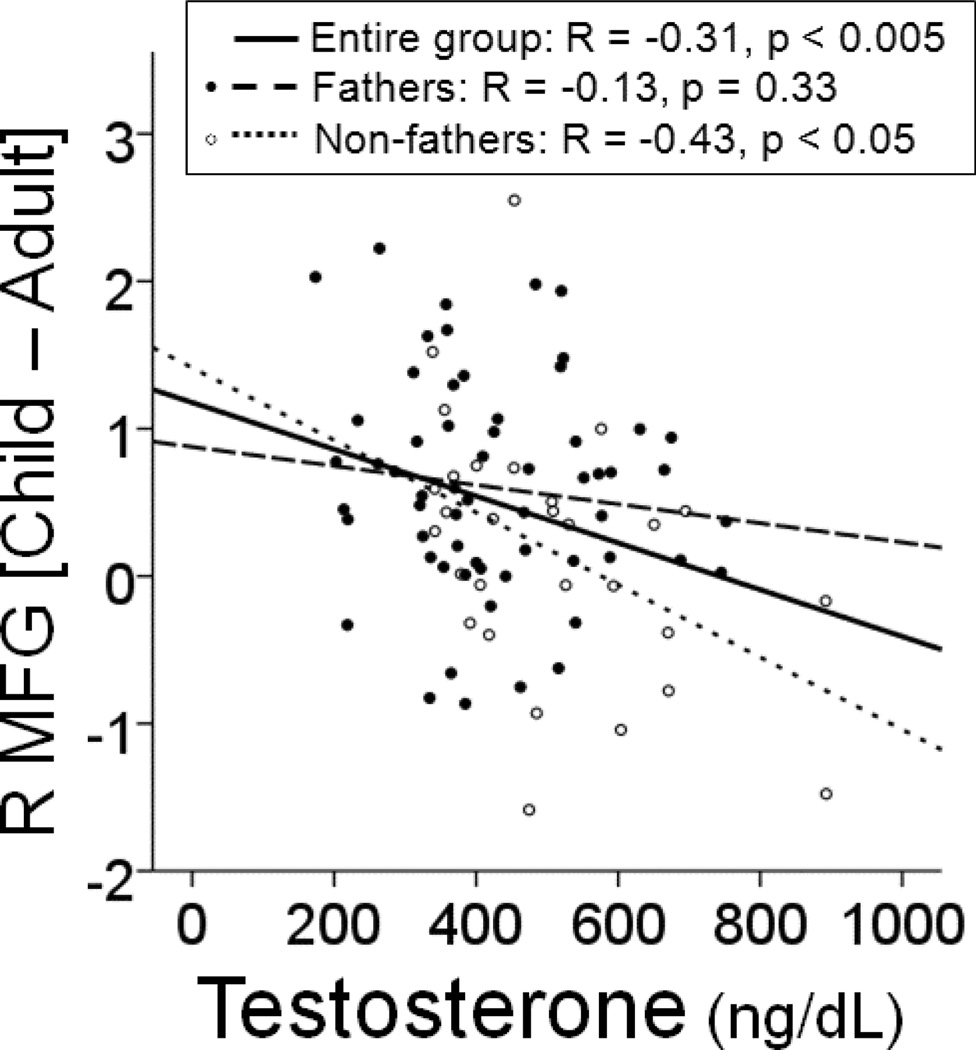
ROI analysis revealed one functional region in which activity in response to child stimuli significantly covaried with testosterone levels after controlling for age: the caudal middle frontal gyrus (r = −0.31, p < 0.005; Fathers: r = −0.13, p = 0.33; Non-fathers: r = −0.43, p < 0.05). The correlation between T and R MFG was stronger within the group of non-fathers (see figure 4), and the effects were driven by the correlation between T and responses to happy child faces for the contrast [CH – AH] (r = −0.55, p < 0.005) rather than for the contrast [CS – AS] (r = −0.27, p = 0.16). There were no regions in which oxytocin significantly correlated with the response to child stimuli. Neither were there any ROIs in which responses to sexual stimuli covaried with either testosterone or oxytocin.
Figure 4.
Scatter plot of testosterone levels and beta contrast values derived from the R MFG ROI for the contrast [Child – Adult], plotted as a function of group status.
Discussion
The current study investigated neural and hormonal systems supporting fatherhood by testing three functional explanations for hormone changes observed when men become fathers. In support of our first hypothesis, fathers in our study had significantly higher oxytocin levels and lower testosterone levels compared with non-fathers. In support of the second and third hypotheses, fathers had more robust neural responses to visual child stimuli in regions of the brain important for face emotion processing (MFG), reward processing (vmPFC/OFC) and mentalizing (TPJ), and less neural reactivity to sexual stimuli in regions important for reward and motivation (caudate and NAcc). For child stimuli, testosterone levels were inversely correlated with neural activity in a region of right MFG implicated in face emotion processing, partially supporting the fourth hypothesis. Neither T nor OT predicted neural responses to sexual stimuli.
Hormones
The findings presented here are consistent with a large body of studies showing that men’s testosterone is impacted by fatherhood (Gettler et al. 2011; Gray et al. 2002; Muller et al. 2009). While we are hesitant to compare the mean values found in this study with those of other labs because of differences in assay protocols, our values fall within normal clinical range and the difference between fathers and non-fathers (fathers had 20.5% less T than non-fathers) is comparable in magnitude to the differences previously observed in a U.S. sample (Gray et al. 2002). Interestingly, in contrast with previous studies suggesting that fathers’ testosterone begins to rise again as their children get older (Gettler et al. 2011; Muller et al. 2009), we do not find a correlation between age of the child and testosterone levels in fathers. While it may be that the child age range in the current study was too restrictive to identify such an effect, the largest longitudinal study to date showed that much of the decline in testosterone experienced by new fathers had recovered by the time the child was age 1 (Gettler et al. 2011). Because all fathers in our sample cohabitated with their child and because T is negatively correlated with instrumental caregiving as reported previously (Mascaro et al. 2013) our data suggest that when men engage heavily in childcare and cohabitate with their child, decreases in testosterone may be maintained for a longer period of time. This idea is consistent with a recent comparative study, which showed that testosterone differences between fathers and non-fathers are only evident in cultural groups in which men typically invest heavily in direct childcare (Muller et al. 2009). In addition to having lower testosterone levels, fathers in our study had significantly higher oxytocin levels than non-fathers. To the best of our knowledge, this is the first study to show a significant difference in OT between fathers and non-fathers; however, this finding is consistent with a previous study showing that plasma OT level increases in men across the first 6 months of fatherhood (Gordon et al. 2010). The difference between fathers and non-fathers reported here (fathers had 33.3% more OT than did non-fathers) are greater than the change reported by Gordon and colleagues over the course of infancy (fathers’ OT increased by 8.0%), perhaps due to the fact that men in the Gordon study were already new fathers at the baseline assessment and may have already experienced some changes in OT levels.
Neural activity
A whole brain analysis revealed that fathers had significantly more activity than non-fathers when viewing pictures of children prominently in the bilateral temporoparietal junction (TPJ), vmPFC/OFC, precuneus, and MFG. Though its exact function is debated (e.g. (Decety and Lamm 2007; Scholz et al. 2009), the TPJ is active during mentalizing tasks, and given our explicit instructions to share the emotions of the unknown child and adult, this result suggests that fathers may be more apt to consider the unknown child’s mental states. Fathers also activate the vmPFC/OFC to a greater extent than non-fathers, a finding consistent with many recent studies highlighting the importance of the OFC within the “parental brain” (Kringelbach et al. 2008; Parsons et al. 2013). Finally, fathers had a stronger response in the MFG. Interestingly, the group difference in the MFG was driven primarily by father’s differential responses to happy faces, whereas group differences in regions important for reward (medial OFC) were driven by differential responses to sad and neutral faces. While admittedly speculative, the latter result may suggest that fathers differ from non-fathers in their propensity to maintain reward processing toward children during times of distress or ambiguity, when sustaining motivation to interact may be more challenging.
There was one region that both differed significantly by parental status (father > non-father) and covaried with testosterone levels: the right MFG. Both the group differences and the correlation with testosterone were driven by responses to happy faces rather than sad faces. The functional ROI used for this analysis includes the peak voxel identified in a seminal fMRI study of the simulation of emotion facial expressions (peak coordinates: 48, 8, 28) (Carr et al. 2003) as well as the peak voxel from a recent meta-analytic investigation of the mirror system using fMRI (peak coordinates: 44, 10, 28) (Molenberghs et al. 2012). Given the role of this region in this component of empathy, we interpret our finding to suggest that the testosterone decrease observed in new fathers may function to enhance empathy. The whole brain exploratory analysis revealed that hippocampal activation in response to children covaried with plasma oxytocin levels, a finding that is of interest given the role of oxytocin in facilitating social memory (Bielsky and Young 2004). Future studies might investigate whether fathers with high levels of oxytocin have enhanced recall or recognition of children.
Thus far, we have considered the regions more active for fathers in response to children as isolated functional activations engaged in disparate processes, but it may be more appropriate to view the functional activations as a network that is more engaged for fathers than non-fathers. The pattern of activation in the bilateral TPJ, vmPFC/OFC, and precuneus overlaps with a network commonly referred to as the default mode, which is active during mind-wandering, self-reflective mentation, episodic memory retrieval, prospection, and mentalizing (Buckner et al. 2008; Spreng et al. 2009). It may be that viewing other children’s emotional faces prompts fathers to recall similar emotional episodes with their own child. Similarly, this network is thought to be modulated by self-relevance (Moran et al. 2009) and it is possible that fathers find even unknown children’s emotional facial expressions more self-relevant than non-fathers.
While there were no regions that were more active for non-fathers in response to child stimuli, non-fathers activated several regions more robustly in response to sexually provocative photographs, including the bilateral dorsal caudate. The dorsal head of the caudate is active for tasks involving visual sexual stimuli and is postulated to mediate the motivation to approach such stimuli (Redouté et al. 2000; Stoléru et al. 2012). Our more targeted ROI analysis also revealed group differences in the nucleus accumbens and hypothalamus, regions that are critically important for reward and sexual function, respectively (Stoléru et al. 2012). While these findings support the idea that fathers down-regulate reward system responses to sexual stimuli, it is important to note that differential responses may have been a function of relationship-status rather than parenting status since both of these co-vary in our sample. If parenting status drives the effect, then the result is consistent with the life history theory prediction that increased investment in parenting should result in decreased investment in mating. Neither can we rule out the effects of sleep deprivation, which is known to affect reward processing (for example, (Gujar et al. 2011)). However, the fact that fathers maintained strong responses to children within putative reward regions mitigates the possibility that group differences in sleep deprivation explain differential reward responses to sexual stimuli. Future studies assessing the role of sleep loss in changes in parental brain function will be an important next step.
Given the role of T and OT in sexual behavior, it is surprising that we did not observe a relationship between hormone levels and neural responses to sexual stimuli. However, the impact of hormones on neural function will depend not only on the circulating levels of the hormones, but also on the brain’s sensitivity to them as reflected in receptor density. Moreover, in the case of testosterone, brain function may depend on the bioavailability of the hormone (i.e. T that is not bound by sex hormone binding globulin (SHGB)), which may be better represented by salivary levels (Rilling et al. 1996). With respect to oxytocin, these null results may be due to the potential limitations of plasma measures, which may not accurately reflect OT levels in the brain (Kagerbauer et al. 2013). One recent study measured the effects of intranasal OT administration on neural responses to infant and sexual stimuli in postpartum and nulliparous women and, while postpartum women with infants 3–6 months of age reported less arousal and exhibited decreased amygdala responses to sexual stimuli, intranasal OT did not impact their amygdala responses to either infant or sexual stimuli (Rupp et al. 2013). The authors interpreted the finding as suggesting that postpartum changes in sexual arousal are relatively more trait-like and may be independent from the acute neuroendocrine changes experienced with parturition. While fathers do not undergo the hormone flux of parturition, our findings in fathers are consistent with such an idea insofar as there was no correlation between hormone levels and the neural response to sexual stimuli. It is also possible that using more naturalistic or ecologically valid sexual stimuli would reveal effects of T or OT on brain function. Finally, some men may have found the blood draw stressful, introducing additional variation to the plasma T and OT measures and obscuring relationships between hormones and subsequent brain function. This may be particularly true for OT since the kinetics of its release are more rapid (e.g. (Zak et al. 2005) than the stress-induced response of testosterone (Sapolsky et al. 2000).
Limitations
We interpret the current findings as the first indication that the testosterone reduction consistently observed in new fathers may serve to enhance empathic and reward-based neural responses to children; however, caution is warranted given the following limitations. First and most importantly, two characteristics differentiate the fathers and non-fathers in the current study: parenting status and relationship status. While we interpret the current findings as differences between fathers and non-fathers that are caused by parenting, relationship status may also be causal to the findings presented here. Both T and OT are known to vary with relationship status (Burnham et al. 2003; Gray et al. 2002; Schneiderman et al. 2012). Related, OT is enhanced by sexual activity (Carmichael et al. 1987), which may have differed between the two groups. We cannot rule out the possibility that the effects presented here are better explained by relationship differences. In fact, one recent ERP study suggests that newly partnered males have a greater neural response to infant stimuli than un-partnered males (Weisman et al. 2012a). Future studies should include two additional groups for comparison: non-partnered fathers, and partnered non-fathers.
For heuristic purposes, the functional ROIs tested here were described at the outset as being important for discrete and singular processes. However, each of these functional regions has been implicated in multiple neural processes and the interpretations offered here are not the only possible interpretations. For example, while we suggest that the difference in vmPFC/ OFC responses between fathers and non-fathers may support enhanced reward processing, the OFC is also important for emotion regulation (Ochsner and Gross 2005). It is possible that fathers engage in more emotion regulation when viewing children’s emotional facial expressions than do non-fathers, though we do not consider the child task used here to demand high levels of emotion regulation. Likewise, while the MFG functional ROI tested here encompassed a region previously observed to be important for the simulation of emotional facial expressions, this region is also active during tasks that require impulse and executive control (Wood and Grafman 2003).
A final limitation arises with the cross-sectional design of the study that does not allow a determination of causation, and it may be the case that fathers had lower T levels, higher OT levels, and differential responses to children and sexual images prior to becoming a father. Similarly, while we suggest that changes in T alter neural responses in the MFG, it could be the case that men who have a greater response in the MFG to child stimuli experience a greater drop in T when becoming fathers, though the likelihood of this is diminished by the fact that the inverse correlation is strongest within the non-fathers. Longitudinal studies assessing within subject changes in hormone levels and brain structure and function will be invaluable for understanding the biology of paternal caregiving. Related to this, a recent study revealed that OT administration elevates T, leading to greater father-infant vocal synchrony (Weisman et al. 2014), and future studies can investigate whether OT-induced elevation of T leads to differential patterns of neural responsivity to children than are reported here. The findings presented here provide the first evidence in support of the hypothesis that new fathers experience a decrease in testosterone that augments the neural processes supporting empathic responding toward children, as well as the first evidence that fathers show less activation of reward system pathways in response to sexually provocative stimuli.
Supplementary Material
Acknowledgements
This work was supported by a Positive Neuroscience Award from the John Templeton Foundation. Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. This facility is supported by the Yerkes National Primate Research Center Base Grant 2P51RR000165-51. Blood draws were provided by the Atlanta Clinical and Translational Science Institute’s Clinical Research Network, supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Alvergne A, Faurie C, Raymond M. Variation in testosterone levels and male reproductive effort: Insight from a polygynous human population. Hormones and Behavior. 2009;56(5):491–497. doi: 10.1016/j.yhbeh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men's marriages. Social Forces. 1993;72(2):463–477. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burnham TC, Chapman JF, Gray PB, McIntyre MH, Lipson SF, Ellison PT. Men in committed, romantic relationships have lower testosterone. Hormones and Behavior. 2003;44(2):119–122. doi: 10.1016/s0018-506x(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. Journal of Clinical Endocrinology & Metabolism. 1987;64(1):27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A Cortical Area Selective for Visual Processing of the Human Body. Science. 2001;293(5539):2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional Imaging of "theory of mind". Trends in Cognitive Sciences. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proceedings of the National Academy of Sciences. 2011;108(39):16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23(3):193–201. [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, Spreckelmeyer KN. Oxytocin Influences Processing of Socially Relevant Cues in the Ventral Tegmental Area of the Human Brain. Biological Psychiatry. 2013;74(3):172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Gujar N, Yoo S-S, Hu P, Walker MP. Sleep Deprivation Amplifies Reactivity of Brain Reward Networks, Biasing the Appraisal of Positive Emotional Experiences. The Journal of Neuroscience. 2011;31(12):4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7(4):411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. Journal of Clinical Endocrinology & Metabolism. 2001;86(2):724–731. 31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hunt KE, Hahn TP, Wingfield JC. Endocrine Influences on Parental Care during a Short Breeding Season: Testosterone and Male Parental Care in Lapland Longspurs (Calcarius lapponicus) Behavioral Ecology and Sociobiology. 1999;45(5):360–369. [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8(6):775–780. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma Oxytocin and Vasopressin do not Predict Neuropeptide Concentrations in Human Cerebrospinal Fluid. Journal of Neuroendocrinology. 2013;25(7):668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. The Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Gangestad SW. Life history theory and evolutionary psychology. The handbook of evolutionary psychology. 2005:68–95. [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, Green AL, Aziz TZ, Hansen PC, Cornelissen PL, et al. A Specific and Rapid Neural Signature for Parental Instinct. PLoS One. 2008;3(2):e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2010;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Testicular volume is inversely correlated with nurturing-related brain activity in human fathers. Proceedings of the National Academy of Sciences. 2013;110(39):15746–15751. doi: 10.1073/pnas.1305579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social Neuroscience. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1655):347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber F, van Ijzendoorn MH, Deschamps P, van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: A double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35(10):1583–1586. doi: 10.1016/j.psyneuen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Naber FB, Poslawsky IE, van IJzendoorn MH, Van Engeland H, Bakermans-Kranenburg MJ. Brief report: oxytocin enhances paternal sensitivity to a child with autism: a double-blind within-subject experiment with intranasally administered oxytocin. Journal of Autism and Developmental Disorders. 2013;43(1):224–229. doi: 10.1007/s10803-012-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Stark EA, Young KS, Stein A, Kringelbach ML. Understanding the human parental brain: A critical role of the orbitofrontal cortex. Social Neuroscience. 2013;8(6):525–543. doi: 10.1080/17470919.2013.842610. [DOI] [PubMed] [Google Scholar]
- Redouté J, Stoléru S, Grégoire M-C, Costes N, Cinotti L, Lavenne F, Le Bars D, Forest MG, Pujol J-F. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11(3):162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. The Neural and Hormonal Bases of Human Parental Care. Neuropsychologia. 2013;32 doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Worthman CM, Campbell BC, Stallings JF, Mbizva M. Ratios of plasma and salivary testosterone throughout puberty: Production versus bioavailability. Steroids. 1996;61(6):374–378. doi: 10.1016/0039-128x(96)00043-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Orbitofrontal Cortex and Reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Lower sexual interest in postpartum women: Relationship to amygdala activation and intranasal oxytocin. Hormones and Behavior. 2013;63(1):114–121. doi: 10.1016/j.yhbeh.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocrine reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in "theory of mind". Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM, Hurlemann R. Oxytocin modulates social distance between males and females. The Journal of Neuroscience. 2012;32(46):16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: Relations to couples’ interactive reciprocity. Psychoneuroendocrinology. 2012;37(8):1277–1285. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct Regions of Right Temporo-Parietal Junction Are Selective for Theory of Mind and Exogenous Attention. PLoS One. 2009;4(3):e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of cognitive neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2012;36(6):1481–1509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Bos PA, Kruijt AW, Lentjes EG, Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(8):3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Journal of Clinical Endocrinology & Metabolism. 2000;85(8):2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- Weisman O, Delaherche E, Rondeau M, Chetouani M, Cohen D, Feldman R. Oxytocin shapes parental motion during father–infant interaction. Biology Letters. 2013a;9(6) doi: 10.1098/rsbl.2013.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Feldman R, Goldstein A. Parental and romantic attachment shape brain processing of infant cues. Biological Psychology. 2012a;89(3):533–538. doi: 10.1016/j.biopsycho.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin Administration to Parent Enhances Infant Physiological and Behavioral Readiness for Social Engagement. Biol Psychiatry. 2012b doi: 10.1016/j.biopsych.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration alters HPA reactivity in the context of parent–infant interaction. European Neuropsychopharmacology. 2013b;23(12):1724–1731. doi: 10.1016/j.euroneuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Oxytocin administration, salivary testosterone, and father–infant social behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;49(0):47–52. doi: 10.1016/j.pnpbp.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The "Challenge Hypothesis": Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. The American Naturalist. 1990;136(6):829–846. [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert Visual Attention Modulates Face-Specific Activity in the Human Fusiform Gyrus: fMRI Study. Journal of Neurophysiology. 1998;79(3):1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4(2):139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48(5):522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Jacoris S, Snowdon CT. Sexual communication between breeding male and female cotton-top tamarins (Saguinus oedipus), and its relationship to infant care. American Journal of Primatology. 2004;64(1):57–69. doi: 10.1002/ajp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.