Abstract
Background & Aims
The efficacy of treatment of Helicobacter pylori infection has decreased steadily due to increasing resistance to clarithromycin, metronidazole, and levofloxacin. Resistance to amoxicillin is generally low, and high intragastric pH increases the efficacy of amoxicillin, so we investigated whether a combination of a high-dose proton-pump inhibitor and amoxicillin (dual therapy) was more effective than standard first-line or rescue therapies in eradicating H pylori.
Methods
We performed a large-scale, multi-hospital trial to compare the efficacy of a high-dose dual therapy (HDDT) with that of standard therapies in treatment-naïve (n=450) or treatment-experienced (n=168) patients with H pylori infection. Treatment-naïve patients were randomly assigned to groups given HDDT (rabeprazole 20 mg and amoxicillin 750 mg, 4 times/day for 14 days; group A1), sequential therapy for 10 days (group B1), or clarithromycin-containing triple therapy for 7 days (group C1). Treatment-experienced patients were randomly assigned to groups given HDDT for 14 days (group A2), sequential therapy for 10 days (B2), or levofloxacin-containing triple therapy for 7 days (C2). H pylori infection was detected using the 13C–urea breath test. We evaluated factors associated with treatment outcomes.
Results
In the intention-to-treat treat analysis, H pylori was eradicated in 95.3% of patients in group A1 (95% confidence interval [CI], 91.9%–98.8%), 85.3% in B1 (95% CI, 79.6%–91.1%), and 80.7% in group C1 (95% CI, 74.3%–87.1%). Infection was eradicated in 89.3% of patients in group A2 (95% CI, 80.9%–97.6%), 51.8% in group B2 (95% CI, 38.3%–65.3%), and 78.6% (95% CI, 67.5%–89.7%). The efficacy of HDDT was significantly higher than that of currently recommended regimens, irrespective of CYP2C19 genotype. Bacterial resistance to drugs was associated with treatment failure. There were no significant differences between groups in adverse events or patient adherence.
Conclusions
HDDT is superior to standard regimens as empiric first-line or rescue therapy for H pylori infection, with similar safety profiles and tolerability. ClinicalTrials.gov no: NCT01163435.
Keywords: PPI, proton pump inhibitor, bacterial infection, stomach, microbe
Introduction
Helicobacter pylori (H. pylori) infection is common worldwide and is strongly associated with gastrointestinal diseases including peptic ulcer and gastric cancer.1 Clarithromycin-containing triple therapy has been recommended in many guidelines as the first-line therapy for the treatment of H. pylori infection.2–4 However, due to increasing antibiotic resistance, higher treatment failure rate for H. pylori is a growing global concern.2,5 Sequential therapy, quadruple therapy with or without bismuth compounds, and levofloxacin-containing triple therapy have all been recommended as the first-line, alternative, or rescue therapies2,5 but their eradication rates vary among studies and are usually below 90% by intention-to-treat analysis.6 Meanwhile, the failure of first-line therapy for H. pylori can significantly increase the development of secondary antibiotic resistance,7,8 further limiting the efficacy of subsequent rescue therapies. Although antimicrobial susceptibility testing is recommended in regions of high antibiotic resistance and after treatment failure,2,5 it is technique-dependent and not readily available in most areas. Therefore, it is important to design a treatment regimen of substantially high efficacy and can be used empirically without the need for susceptibility testing.
Unlike H. pylori resistance rates to clarithromycin (CLA), metronidazole (MTZ) or levofloxacin (LEV), H. pylori resistance to amoxicillin (AMO), both primary and acquired, have been reported to be uncommon.7,9,10 AMO is also unique in that its bactericidal effect against H. pylori is time-and pH-dependent because AMO is more stable at a higher intragastric pH.11–13 Thus, an optimized dual therapy consisting of high-dose PPI and AMO may have a selective advantage over currently recommended sequential, CLA-or LEV-containing therapy.
Except that quadruple therapy with bismuth compound is hardly used in Taiwan because bismuth subcitrate was not readily available, in this large-scale multi-hospital and randomized trial, we compared the efficacy and tolerability of high-dose dual therapy with those of currently recommended therapies (sequential therapy, CLA-containing triple therapy, and LEV-containing triple therapy) in treatment-naïve or treatment-experienced H. pylori-infected subjects. Potential factors influencing treatment outcomes were also examined.
Materials and Methods
Study design, settings, and participants
This prospective, randomized study was conducted at one medical center (National Taiwan University Hospital) and four community hospitals in the northern Taiwan region. The study was approved by the Research Ethics Committee of National Taiwan University Hospital and registered at ClinicalTrials.gov (number NCT01163435). All authors had access to the study data and had reviewed and approved the final manuscript. Patients (aged ≥ 20) having H. pylori-positive chronic gastritis with/without peptic ulcers (duodenal or gastric ulcers) were recruited. Exclusion criteria included pregnancy or nursing, serious concomitant illness, malignant tumors, history of hypersensitivity to study drugs, severe ulcer bleeding, previous gastric surgeries, taking PPIs or antibiotics in the previous month. Patients without previous anti-H. pylori treatment were invited to receive the first-line regimens, whereas patients who had previously received anti-H. pylori therapies were invited to receive rescue regimens.
A computer-generated random number sequence was blocked (1:1:1, block size: 6) into three subgroups: A1, B1 and C1 (or A2, B2, and C2). The assignment was recorded on a group assignment card and sealed in opaque envelops by an independent statistician. All investigators were masked to the randomization sequence. After giving their written informed consent, each patient was assigned a number by enrolling order and randomly allocated, according to group assignment card, to one of three treatment groups for first-line or rescue therapies. For the first-line therapy, group A1 (high-dose dual therapy; HDDT) consisted of rabeprazole (20 mg, QID) and AMO (750 mg, QID) for 14 days; group B1 (sequential therapy; ST) consisted of rabeprazole (20 mg, BID) and AMO (1000 mg, BID) for 5 days, followed by rabeprazole (20 mg, BID), MTZ (500 mg, BID), and CLA (500 mg, BID) for 5 additional days; group C1 (CLA-containing triple therapy; CLA-TT) consisted of rabeprazole (20 mg, BID), AMO (1000 mg, BID), and CLA (500 mg, BID) for 7 days. For the rescue regimens, group A2 (HDDT) was the same as group A1; group B2 (ST) was the same as group B1; group C2 (LEV-containing triple therapy; LEV-TT) consisted of rabeprazole (20 mg, BID), AMO (1000 mg, BID), and LEV (250 mg, BID) for 7 days.
Procedures
All subjects underwent an upper endoscopy with gastric biopsy before the initiation of assigned treatment regimen. H. pylori status was determined by histology, culture, 13C-urea breath test (13C-UBT). During the treatment period, patients were instructed to avoid acidic foods (e.g., citrus fruits or juices) to minimize the impact of ingested foods on increasing intragastric acidity which can alter drug activity. Subjects also completed a standardized questionnaire and recorded symptoms and daily drug consumption during the treatment period in a diary card. After completing the course of the treatment, the patients were followed in outpatients clinic to investigate patient adherence and adverse effects of treatments. Patients taking rescue therapies were requested to obtain their previous medical records pertaining to H. pylori treatment. CYP2C19 and IL-1β-511 genetic polymorphism were identified by polymerase chain reaction-based restriction fragment length polymorphism.
Histology, bacterial culture, 13C-urea breath test and genotyping of CYP2C19 and IL-1β-511
H. pylori status and susceptibility testing
The initial H. pylori status was considered positive based on (1) positive bacterial cultures or (2) positive histological examination with confirmation by 13C-UBT testing.14 Four to eight weeks after treatment completion, H. pylori eradication was determined by 13C-UBT. A result of ≥ 5 units was considered a positive breath test. The E-test (AB Biodisk, Solna, Sweden) was used to evaluate the resistance to antibiotics according to MIC (minimum inhibitory concentration) values of > 0.5, ≥ 1, ≥ 8, and > 1 mg/L for AMO, CLA, MTZ, and LEV, respectively.14
Statistical analysis
The sample size in the first-line therapy was designed based on the assumption that there were about 93% and 81% cure rates in HDDT and CLA-TT groups, respectively.6,13 Accordingly, 140 to 150 patients were required for each group to give the study a power of at least 80% at 5% statistical significance level with about 10% drop-out rate. For the rescue therapy, based on an assumption of about 90% and 75% cure rates in HDDT and LEV-TT groups, respectively,13,15 100 patients were required for each group. However, the trial for rescue treatment was prematurely terminated with 56 patients in each group because the cure rate in ST group was significantly worse than the expected outcome. Nevertheless, this patient number provided the rescue regimens a power of 85% at 5% significant level.
Categorical variables are described by percentages and continuous variables by mean with standard deviation. The eradication rates and the 95% confidence intervals (95% CI) were calculated by intention-to-treat (ITT) and per-protocol (PP) analyses. Differences in demographic information, eradication rates, and adverse events among different groups were determined by the χ2 test or one-way ANOVA, followed by both multiple comparisons with Bonferroni correction and the Tukey’s method for all-pairwise comparisons. Univariate analysis was performed using χ2 test, two-sample t-test, one-way ANOVA, or simple logistic regression to explore significant predictive variables, which were listed in Table 1, followed by a multiple logistic regression analysis. A backward/forward strategy and the Wald statistic were used for model comparisons. The impacts of factors were described by odds ratios and 95% CI. Two-side P values less than 0.05 were considered statistically significant. The Mann-Whitney test was used to compare the distributions of MIC. The SPSS statistical software, version 17 was used for analyses.
Table 1.
Demographics of patients receiving first-line and rescue regimens
| First-line regimens | HDDT group | ST group | CLA-TT group | P-value |
|---|---|---|---|---|
| No. of patients | 150 | 150 | 150 | |
| Male | 46.0 (69/150) | 39.3 (59/150) | 39.3 (59/150) | 0.405 |
| Age, years | 53.4±10.4 | 53.4±13.0 | 54.3±12.3 | 0.798 |
| BMI, kg/m2 | 23.8±3.5 | 24.1±3.6 | 23.7±3.6 | 0.832 |
| Life Style | ||||
| Regular Alcohol use | 15.0 (22/147) | 8.8 (13/148) | 13.5 (20/148) | 0.247 |
| Smoking | 25.9 (35/135) | 22.7 (31/136) | 25.9 (36/136) | 0.984 |
| Peptic ulcer disease | 68.7 (103/150) | 65.3 (98/150) | 66.7 (100/150) | 0.841 |
| Bacterial density | ||||
| Mild | 30.0 (45/150) | 28.0 (42/150) | 29.3 (44/150) | 0.948 |
| Moderate | 32.7 (49/150) | 34.0 (51/150) | 40.7 (61/150) | 0.317 |
| Severe | 37.3 (56/150) | 38.0 (57/150) | 30.0 (45/150) | 0.285 |
| CYP2C19 genotype | ||||
| EM | 43.6 (65/150) | 44.0 (66/150) | 45.3 (68/150) | 0.956 |
| IM | 43.6 (65/150) | 42.7 (64/150) | 42.0 (63/150) | 0.993 |
| PM | 12.8 (19/150) | 12.7 (19/150) | 12.7 (19/150) | 1.000 |
| IL-1β-511 genotype | ||||
| C/C | 28.7 (43/150) | 25.3 (38/150) | 24.0 (36/150) | 0.665 |
| C/T | 52.0 (78/150) | 54.0 (81/150) | 63.3 (95/150) | 0.112 |
| T/T | 18.7 (28/150) | 20.0 (30/150) | 12.7 (19/150) | 0.204 |
| Antibiotic sensitivity | ||||
| AMO-R | 0.0 (0/150) | 0.7 (1/150) | 0.7 (1/150) | 1.000 |
| MTZ-R | 34.7 (52/150) | 36.7 (55/150) | 33.3 (50/150) | 0.844 |
| CLA-R | 15.3 (23/150) | 16.7 (25/150) | 17.3 (26/150) | 0.924 |
| CLA-S/MTZ-S | 57.3 (86/150) | 56.0 (84/150) | 56.7 (85/150) | 0.993 |
| CLA-S/MTZ-R | 27.3 (41/150) | 27.3 (41/150) | 26.0 (39/150) | 0.972 |
| CLA-R/MTZ-S | 8.0 (12/150) | 7.3 (11/150) | 10.0 (15/150) | 0.754 |
| CLA-R/MTZ-R | 7.3 (11/150) | 9.3 (14/150) | 7.3 (11/150) | 0.841 |
| LEV-R | 16.0 (24/150) | 16.7 (25/150) | 16.0 (24/150) | 1.000 |
| Rescue regimens | HDDT group | ST group | LEV-TT group | P-value |
|---|---|---|---|---|
| No. of patients | 56 | 56 | 56 | |
| Male | 41.1 (23/56) | 37.5 (21/56) | 37.5 (21/56) | 0.940 |
| Age, years | 53.4±12.3 | 55.8±12.3 | 50.4±13.0 | 0.084 |
| BMI, kg/m2 | 24.2±3.4 | 23.7±4.5 | 23.9±4.0 | 0.850 |
| Life Style | ||||
| Regular Alcohol use | 14.3 (8/56) | 10.9 (6/55) | 10.7 (6/56) | 0.871 |
| Smoking | 20.4 (11/54) | 23.2 (13/56) | 25.0 (14/56) | 0.870 |
| Peptic ulcer disease | 57.1 (32/56) | 66.1 (37/56) | 66.1 (37/56) | 0.567 |
| Bacterial density | ||||
| Mild | 44.6 (25/56) | 41.1 (23/56) | 41.1 (23/56) | 0.942 |
| Moderate | 26.8 (15/56) | 25.0 (14/56) | 37.5 (21/56) | 0.324 |
| Severe | 28.6 (16/56) | 32.1 (18/56) | 21.4 (12/56) | 0.480 |
| CYP2C19 genotype | ||||
| EM | 46.4 (26/56) | 41.1 (23/56) | 44.6 (25/56) | 0.888 |
| IM | 35.7 (20/56) | 44.6 (25/56) | 41.1 (23/56) | 0.654 |
| PM | 17.9 (10/56) | 14.3 (8/56) | 14.3 (8/56) | 0.895 |
| IL-1β-511 genotype | ||||
| C/C | 32.1 (18/56) | 21.4 (12/56) | 23.2 (13/56) | 0.401 |
| C/T | 46.4 (26/56) | 58.9 (33/56) | 50.0 (28/56) | 0.434 |
| T/T | 21.4 (12/56) | 19.6 (11/56) | 26.8 (15/56) | 0.716 |
| Antibiotic sensitivity | ||||
| AMO-R | 3.6 (2/56) | 1.8 (1/56) | 3.6 (2/56) | 1.000 |
| MTZ-R | 50.0 (28/56) | 52.7 (29/56) | 53.6 (30/56) | 0.943 |
| CLA-R | 85.7 (48/56) | 80.0 (44/56) | 80.4 (45/56) | 0.721 |
| CLA-S/MTZ-S | 7.1 (4/56) | 9.1 (5/56) | 12.5 (7/56) | 0.647 |
| CLA-S/MTZ-R | 7.1 (4/56) | 10.9 (6/56) | 7.1 (4/56) | 0.734 |
| CLA-R/MTZ-S | 42.9 (24/56) | 38.2 (21/56) | 33.9 (19/56) | 0.634 |
| CLA-R/MTZ-R | 42.9 (24/56) | 41.8 (23/56) | 46.4 (26/56) | 0.888 |
| LEV-R | 21.4 (12/56) | 23.6 (13/56) | 17.9 (10/56) | 0.746 |
| Treatment failure | ||||
| One failure | 53.6 (30/56) | 53.6 (30/56) | 51.8 (29/56) | 1.000 |
| Two failure | 30.4 (17/56) | 32.1 (18/56) | 30.4 (17/56) | 1.000 |
| Three failure | 5.5 (3/56) | 5.5 (3/56) | 8.9 (5/56) | 0.792 |
| ≥ four failure | 10.7 (6/56) | 8.9 (5/56) | 8.9 (5/56) | 1.000 |
Data are mean ± SD or % (n/N); HDDT: high-dose dual therapy; ST: sequential therapy; CLA-TT: clarithromycin-containing triple therapy; LEV-TT: levofloxacin-containing triple therapy; BMI: body mass index; AMO-R: amoxicillin resistant; CLA-S: clarithromycin susceptible; CLA-R: clarithromycin resistant; MTZ-S: metronidazole susceptible; MTZ-R: metronidazole resistant. LEV-R: levofloxacin resistant; EM: homozygous extensive metabolizer; IM: heterozygous extensive metabolizer; PM: poor metabolizer.
Results
From Aug 2010 to Jul 2013, 1567 patients were assessed for eligibility and 450 and 168 patients were enrolled and randomly allocated to the first-line and rescue therapies, respectively. Five patients in the first-line groups (2, 2, and 1 patients in HDDT, ST, and CLA-TT groups, respectively) and 2 patients in the rescue ST group were excluded due to consent withdrawal, loss to follow-up, or poor adherence (Supplementary Figures 1A and B). A total of 617 H. pylori strains (99.8%) were successfully cultured. The demographic data, including gender, age, body mass index, life-style, peptic ulcer disease, bacterial density, CYP2C19 and IL-1β-511 genotypes, and antibiotic susceptibility were similar among patients in different groups of first-line and rescue therapies (Table 1).
For the first-line therapies, the ITT eradication rates were 95.3% (95% C.I. 91.9–98.8), 85.3% (79.6–91.1), and 80.7% (74.3–87.1) for HDDT, ST, and CLA-TT groups, respectively. For the rescue treatments, the ITT eradication rates were 89.3% (80.9–97.6), 51.8% (38.3–65.3), and 78.6% (67.5–89.7) for HDDT, ST, and LEV-TT groups, respectively (Table 2). These data indicate that the efficacy of HDDT was superior to the efficacy of ST or CLA-TT in treatment-naïve patients and of ST in treatment-experienced patients. HDDT also appeared to be more efficacious than LEV-TT. For both first-line and rescue treatments, the occurrence of overall adverse events and protocol adherence were similar when compared across all groups except bad taste which was observed more frequently in ST and CLA-TT groups than in HDDT group (Table 3).
Table 2.
Eradication rates by ITT analysis and PP analysis in the first-line and rescue regimens
| First-line regimens | HDDT group | ST group | CLA-TT group | P-value |
|---|---|---|---|---|
| ITT analysis | ||||
| Eradication rate | 95.3 (143/150) | 85.3 (128/150) | 80.7 (121/150) | <0.001 |
| 95% C.I. | 91.9–98.8 | 79.6–91.1 | 74.3–87.1 | |
| P-value* | ||||
| HDDT group | - | 0.025 | <0.001 | |
| ST group | 0.025 | - | 0.440 | |
| CLA-TT group | <0.001 | 0.440 | - | |
| PP analysis | ||||
| Eradication rate | 96.6 (143/148) | 86.5 (128/148) | 81.2 (121/149) | <0.001 |
| 95% C.I. | 93.7–99.6 | 80.9–92.1 | 74.9–87.6 | |
| P-value* | ||||
| HDDT group | - | 0.018 | <0.001 | |
| ST group | 0.018 | - | 0.328 | |
| CLA-TT group | <0.001 | 0.328 | - | |
| Rescue regimens | HDDT group | ST group | LEV-TT group | P-value |
|---|---|---|---|---|
| ITT analysis | ||||
| Eradication rate | 89.3 (50/56) | 51.8 (29/56) | 78.6 (44/56) | <0.001 |
| 95% C.I. | 80.9–97.6 | 38.3–65.3 | 67.5–89.7 | |
| P-value* | ||||
| HDDT group | - | <0.001 | 0.363 | |
| ST group | <0.001 | - | 0.002 | |
| LEV-TT group | 0.366 | 0.002 | - | |
| PP analysis | ||||
| Eradication rate | 89.3 (50/56) | 53.7 (29/54) | 78.6 (44/56) | <0.001 |
| 95% C.I. | 80.9–97.6 | 40.0–67.4 | 67.8–89.7 | |
| P-value* | ||||
| HDDT group | - | <0.001 | 0.363 | |
| ST group | <0.001 | - | 0.006 | |
| LEV-TT group | 0.363 | 0.006 | - | |
Data are % (n/N); HDDT: high-dose dual therapy; ST: sequential therapy; CLA-TT: clarithromycin-containing triple therapy; LEV-TT: levofloxacin-containing triple therapy; ITT: intention-to-treat; PP: per-protocol; C.I.: confidence interval.
P values from pairwise comparison made by Tukey’s all-pairwise test.
Table 3.
Adverse events and protocol adherence in patients receiving first-line or rescue regimens
| First-line regimens | HDDT group (N=148) | ST group (N=148) | CLA-TT group (N=149) | P-value |
|---|---|---|---|---|
| Adverse event | ||||
| No. of patients | 23.0 (34/148) | 33.2 (49/148) | 26.8 (40/149) | 0.149 |
| Total No. of events | 39 | 78 | 53 | |
| Abdominal distress | 4.7 (7/148) | 6.1 (9/148) | 3.4 (5/149) | 0.503 |
| Dysgeusia (bad taste) | 0.7 (1/148) | 10.8 (16/148) | 10.1 (15/149) | 0.001 |
| Nausea | 2.0 (3/148) | 6.1 (9/148) | 2.1 (4/149) | 0.136 |
| Diarrhea | 4.7 (7/148) | 8.8 (13/148) | 8.1 (12/149) | 0.351 |
| Dizziness | 7.4 (11/148) | 10.8 (16/148) | 4.7 (7/149) | 0.145 |
| Pruritus (itching) | 2.7 (4/148) | 2.0 (3/148) | 2.0 (3/149) | 0.927 |
| Others | 4.1 (6/148) | 6.8 (10/148) | 3.4 (5/149) | 0.339 |
| Adherence | 95.3 (142/149) | 98.0 (146/149) | 98.7 (147/149) | 0.258 |
| Rescue regimens | HDDT group (N=56) | ST group (N=54) | LEV-TT group (N=56) | P-value |
|---|---|---|---|---|
| Adverse event | ||||
| No. of patients | 28.6 (16/56) | 35.2 (19/54) | 32.1 (18/56) | 0.798 |
| Total No. of events | 20 | 25 | 22 | |
| Abdominal distress | 1.8 (1/56) | 9.3 (5/54) | 5.4 (3/56) | 0.197 |
| Dysgeusia (bad taste) | 1.8 (1/56) | 7.4 (4/54) | 1.8 (1/56) | 0.205 |
| Nausea | 7.1 (4/56) | 3.7 (2/54) | 3.6 (2/56) | 0.733 |
| Diarrhea | 8.9 (5/56) | 11.1 (6/54) | 8.9 (5/56) | 0.896 |
| Dizziness | 5.4 (3/56) | 3.7 (2/54) | 5.4 (3/56) | 1.000 |
| Pruritus (itching) | 5.4 (3/56) | 1.9 (1/54) | 1.8 (1/56) | 0.621 |
| Others | 3.6 (2/56) | 5.6 (3/54) | 10.7 (6/56) | 0.205 |
| Adherence | 96.4 (53/55) | 96.3 (52/54) | 100.0 (56/56) | 0.397 |
Data are % (n/N). HDDT: high-dose dual therapy; ST: sequential therapy; CLA-TT: clarithromycin-containing triple therapy; LEV-TT: levofloxacin-containing triple therapy. Adherence: took at least 80% of drugs.
For factors that influence treatment outcomes, antibiotic resistance was the most important determinant for treatment failure in both treatment-naïve group (Table 4) and treatment-experienced group (Table 5), based on univariate and multiple logistic regression analyses. Resistance to CLA significantly reduced the eradication rates in treatment-naïve ST (by 40%) and CLA-TT groups (by 74%). MTZ resistance significantly reduced the eradication rates in both treatment-naïve (by 20%) and treatment-experienced ST groups (by 53%). CLA/MTZ dual resistance significantly reduced the H. pylori eradication rates in treatment-naïve patients receiving ST (by 65%) or CLA-TT (by 84%) and in treatment-experienced patients receiving ST (by 63%). The resistance to LEV or AMO significantly reduced the eradication rate in LEV-TT group (71% and 82% drop, respectively). In addition to antibiotic resistance, age and the presence of peptic ulcer significantly reduced the eradication rates of CLA-TT and LEV-TT, respectively. Moreover, the frequency of previous treatment failure significantly affected the eradication rate of all rescue regimens. However, CYP2C19 genotype of patients did not affect the treatment outcome of these groups. Other factors that did not have a significant impact on eradication rates were listed in Supplementary Table 1.
Table 4.
Univariate and multiple logistic regression analyses of factors that may influence H. pylori eradication in patients receiving first-line regimens.
| First-line regimens | HDDT group | ST group | CLA-TT group |
|---|---|---|---|
| Univariate analysis | |||
| Amoxicillin Resistance | |||
| Yes | - | 100.0 (1/1) | 0.0 (0/1) |
| No | 95.3 (143/150) | 85.2 (127/149) | 81.2 (121/149) |
| P-value | - | 1 | 0.193 |
| Clarithromycin Resistance | |||
| Yes | 95.7 (22/23) | 52.0 (13/25) | 19.2 (5/26) |
| No | 95.3 (121/127) | 92.0 (115/125) | 93.5 (116/124) |
| P-value | 1 | <0.001 | <0.001 |
| Metronidazole Resistance | |||
| Yes | 92.3 (48/52) | 72.7 (40/55) | 76.0 (38/50) |
| No | 96.9 (95/98) | 92.6 (88/95) | 83.0 (83/100) |
| P-value | 0.236 | 0.001 | 0.381 |
| Clarithromycin and Metronidazole Resistance | |||
| CLA-S/MTZ-S | 97.7 (84/86) | 94.0 (79/84) | 92.9 (79/85) |
| CLA-S/MTZ-R | 90.2 (37/41) | 87.8 (36/41) | 94.9 (37/39) |
| CLA-R/MTZ-S | 91.7 (11/12) | 81.8 (9/11) | 26.7 (4/15) |
| CLA-R/MTZ-R | 100.0 (11/11) | 28.6 (4/14) | 9.1 (1/11) |
| P-value | 0.245 | <0.001 | <0.001 |
| Levofloxacin Resistance | |||
| Yes | 95.8 (23/24) | 72.0 (18/25) | 66.7 (16/24) |
| No | 95.2 (120/126) | 88.0 (110/125) | 83.3 (105/126) |
| P-value | 1 | 0.059 | 0.087 |
| CYP2C19 genotype | |||
| EM | 92.3 (60/65) | 89.4 (59/66) | 76.5 (52/68) |
| IM | 96.9 (63/65) | 87.5 (56/64) | 85.7 (54/63) |
| PM | 100.0 (19/19) | 84.2 (16/19) | 78.9 (15/19) |
| P-value | 0.358 | 0.949 | 0.441 |
| Age, years | |||
| not eradicated | 58.29 ± 8.361 (7) | 57.09 ± 12.405 (22) | 49.34 ± 12.941(29) |
| eradicated | 53.17 ± 10.413 (143) | 53.38 ± 13.09 (128) | 55.53 ± 11.929 (121) |
| P-value | 0.203 | 0.217 | 0.015 |
| Multiple logistic regression | |||
| Dual resistance | 0.015(0.003–0.085) | ||
| P-value | <0.001 | ||
| Clarithromycin resistance | 0.008(0.002–0.039) | ||
| P-value | <0.001 | ||
| Age, years | 1.086(1.029–1.145) | ||
| P-value | 0.003 | ||
Data for univariate analysis are % (n/N) or mean ± S.D. (n) and data for multiple logistic regression are odds ratio (95% C.I.); HDDT: high-dose dual therapy; ST: sequential therapy; CLA-TT: clarithromycin-containing triple therapy; CLA: clarithromycin; MTZ: metronidazole; S: susceptible; R: resistant. Dual resistance: clarithromycin and metronidazole resistance; EM: homozygous extensive metabolizer; IM: heterozygous extensive metabolizer; PM: poor metabolizer.
Table 5.
Univariate and multiple logistic regression analyses of factors that may influence H. pylori eradication in patients receiving rescue regimens.
| Rescue regimens | HDDT group | ST group | LEV-TT group |
|---|---|---|---|
| Univariate analysis | |||
| Amoxicillin Resistance | |||
| Yes | 50.0 (1/2) | 0.0 (0/1) | 0.0 (0/2) |
| No | 90.7 (49/54) | 53.7 (29/54) | 81.5 (44/54) |
| P-value | 0.205 | 0.473 | 0.043 |
| Clarithromycin Resistance | |||
| Yes | 89.6 (43/48) | 47.7 (21/44) | 77.8 (35/45) |
| No | 87.5 (7/8) | 72.7 (8/11) | 81.8 (9/11) |
| P-value | 1 | 0.185 | 1 |
| Metronidazole Resistance | |||
| Yes | 82.1 (23/28) | 27.6 (8/29) | 70.0 (21/30) |
| No | 96.4 (27/28) | 80.8 (21/26) | 88.5 (23/26) |
| P-value | 0.193 | <0.001 | 0.114 |
| Clarithromycin and Metronidazole Resistance | |||
| CLA-S/MTZ-S | 75.0 (3/4) | 80.0 (4/5) | 85.7 (6/7) |
| CLA-S/MTZ-R | 100.0 (4/4) | 66.7 (4/6) | 75.0 (3/4) |
| CLA-R/MTZ-S | 100.0 (24/24) | 81.0 (17/21) | 89.5 (17/19) |
| CLA-R/MTZ-R | 79.2 (19/24) | 17.4 (4/23) | 69.2 (18/26) |
| P-value | 0.133 | <0.001 | 0.433 |
| Levofloxacin Resistance | |||
| Yes | 83.3 (10/12) | 38.5 (5/13) | 20.0 (2/10) |
| No | 90.9 (40/44) | 57.1 (24/42) | 91.3 (42/46) |
| P-value | 0.599 | 0.343 | <0.001 |
| CYP2C19 genotype | |||
| EM | 84.6 (22/26) | 34.8 (8/23) | 76.0 (19/25) |
| IM | 90.0 (18/20) | 60.0 (15/25) | 82.6 (19/23) |
| PM | 100.0 (10/10) | 75.0 (6/8) | 75.0 (6/8) |
| P-value | 0.393 | 0.084 | 0.910 |
| Peptic ulcer disease | |||
| Yes | 90.6 (29/32) | 45.9 (17/37) | 89.2 (33/37) |
| No | 87.5 (21/24) | 63.2 (12/19) | 57.9 (11/19) |
| P-value | 1 | 0.267 | 0.014 |
| Previous treatment failure | |||
| not eradicated | 3.500 ± 2.588 (6) | 2.185 ± 1.178 (27) | 3.000 ± 1.954 (12) |
| eradicated | 1.640 ± 0.964 (50) | 1.276 ± 0.455 (29) | 1.546 ± 0.875 (44) |
| P-value | 0.139 | 0.001 | 0.027 |
| Multiple logistic regression | |||
| Dual resistance | 0.033(0.005–0.204) | ||
| P-value | <0.001 | ||
| levofloxacin resistance | 0.005(0.000–0.115) | ||
| P-value | 0.001 | ||
| Peptic ulcer disease | 11.084(1.171–104.919) | ||
| P-value | 0.036 | ||
| Previous treatment failure | 0.482(0.266–0.874) | 0.158(0.029–0.852) | 0.302(0.118–0.773) |
| P-value | 0.016 | 0.032 | 0.013 |
Data for univariate analysis are % (n/N) or mean ± S.D. (n) and data for multiple logistic regression are odds ratio (95% C.I.); HDDT: high-dose dual therapy; ST: sequential therapy; LEV-TT: levofloxacin-containing triple therapy; CLA: clarithromycin; MTZ: metronidazole; S: susceptible; R: resistant. Dual resistance: clarithromycin and metronidazole resistance; EM: homozygous extensive metabolizer; IM: heterozygous extensive metabolizer; PM: poor metabolizer.
For the previous regimens patients received in treatment-experienced groups, CLA-TT (97.6%) was the most commonly prescribed first-line therapy. MTZ-TT and LEV-TT were chosen only after one and two treatment failures, respectively (Supplementary Figure 2). Among the 617 strains that were successfully cultured, the resistant rates of H. pylori to AMO, MTZ, CLA, CLA/MTZ dual, and LEV were 0.4%, 34.9%, 16.4%, 7.6%, and 16.2%, respectively, in the first-line treatment groups, and were 3%, 52.1%, 82%, 43.7%, and 21%, respectively, in the rescue treatment groups. It is worth noting that CLA and CLA/MTZ dual resistance significantly increased from the first-line treatment groups to the rescue treatment groups (Supplementary Table 2).
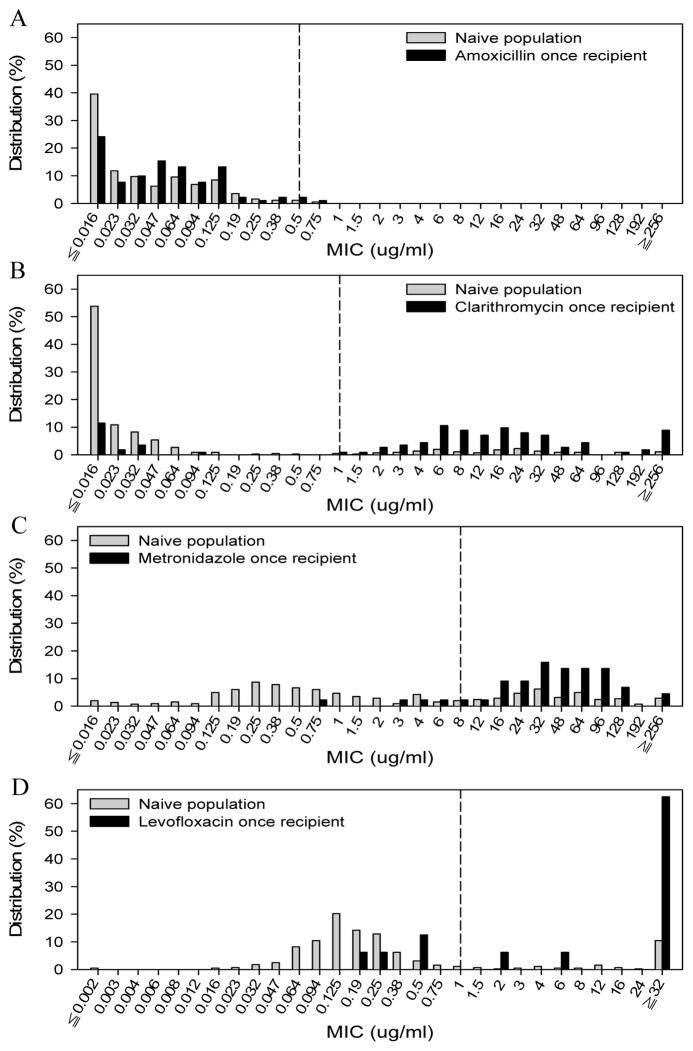
A comparison of the MIC distribution between these treatment-naïve patients and patients who had exposed only once to AMO, CLA, MTZ or LEV for anti-H. pylori therapies in these treatment-experienced groups revealed a dramatic shift towards increased resistance in CLA, MTZ, and LEV recipient but not in AMO recipient (Figure 1). The resistance rates changed from 16.4%, 34.9% and 16.2% in treatment-naïve groups to 82.3%, 90.9% and 75.0% in CLA-recipient, MTZ-recipient, and LEV-recipient, respectively.
Figure 1. Distribution of MIC (minimum inhibitory concentrations) values of isolated H. pylori in response to (A) amoxicillin, (B) clarithromycin, (C) metronidazole, and (D) levofloxacin.
H. pylori was isolated from patients enrolled for first-line therapies (naïve group) and, among rescue groups, patients who had previously received amoxicillin, clarithromycin, metronidazole or levofloxacin only once on anti-H. pylori therapies (antibiotic once recipient group). The breakpoint for resistance to each antibiotic was indicated by the vertical dash line.
Discussion
To our best knowledge, this is the first large-scale prospective, randomized trial comparing the efficacy of HDDT to that of the currently recommended treatment for first-line or rescue therapies for H. pylori infection. We also successfully cultured almost all strains (617/618) of enrolled patients, which provided adequate sample size to analyze the impact of resistance patterns. Our results showed that HDDT cured more than 95% of treatment-naïve patients and about 90% of treatment-experienced patients, and was superior to standard first-line or rescue therapies, irrespective of the CYP2C19 genotype and antibiotic resistance patterns.
Current guidelines recommend CLA-TT, ST and (bismuth or non-bismuth) quadruple therapies for first-line treatment of H. pylori infection.2,16 However, the high reported resistance rates of CLA and MTZ significantly reduces the efficacy of these regimens.5,6,17 To improve the treatment outcome, it is therefore important to use antibiotics, such as AMO, that have low resistance rates to H. pylori.7,9,10 AMO is usually used twice daily to compromise with other concentration-dependent antibiotics (e.g., CLA, MTZ and LEV) in the anti-H. pylori therapies.2 However, dual therapies given PPI and AMO twice daily did not achieve satisfactory treatment outcomes.18 Instead, its effectiveness can be improved by giving both drugs at higher doses/frequencies.19 It was observed that the eradication rate was generally higher when dual therapy was given four times daily compared to three times daily.16 This is because it is critical to maintain steady plasma concentration of AMO above the MIC with more frequent dosing for its bactericidal effect against H. pylori.11,12 Furthermore, to optimize AMO therapeutic efficacy, it depends on an intragastric pH of 5.5 or higher, achievable by higher doses and frequency of PPIs,13 and avoidance of acidic foods.20 High-dose PPIs may also exert direct antimicrobial activities against H. pylori.21 Although PPIs are metabolized by CYP2C19 which is an important factor for suboptimal-dose dual therapy,22 a regimen of four times daily dosing maintained the intragastric pH at a value higher than 6.5 regardless of CYP2C19 genotype.23 This is consistent with our finding that HDDT was able to achieve high therapeutic efficacy in CYP2C19 extensive metabolizers (Tables 4 and 5) and can be corroborated by the finding of Furuta et al.,24 showing the eradication rate of H. pylori were 100% in extensive metabolizers. Thus, an optimized high-dose PPI and AMO dual therapy is likely to be superior to suboptimally dosed dual therapy and has the selective advantage over standard CLA-, MTZ-, or LEV-containing therapies in most regions of the world reporting increasing H. pylori resistance to CLA, MTZ, and/or LEV.
The prevalence of primary resistance to AMO, MTZ, and CLA were about 2%, 44%, and 29%, respectively, in America; 0.7%, 35%, and 18%, respectively, in Europe; 2%, 38%, and 21%, respectively, in Asia.16,25 In Europe, the H. pylori resistance to CLA has increased from 9.8% to 17.5% over the past 10 years and a rapid emergence of LEV resistance (to 14.1%) is noted.25 Comparable to these reports from different geographic regions, our results showed that the primary resistance to AMO, MTZ, CLA, and LEV were 0.4%, 34.9%, 16.4%, and 16.2%, respectively. In this regard, the treatment outcome shown by the present study may be also applicable to other geographic areas. Our findings also showed that the application of CLA-TT resulted in a significant increase of not only CLA resistance but also CLA/MTZ dual resistance.
Among the factors reported to affect the treatment outcome of H. pylori infection, our results showed that antibiotic resistance is the major determinant of treatment failure against H. pylori. In our study, the medical history of previous H. pylori therapies for each patient was documented. We found a rapid acquisition of H. pylori antibiotic resistance to CLA, MTZ, or LEV, but not AMO (Figure 1). The possible explanation for the difference in acquired antibiotic resistance is that single point mutation can lead to resistance to CLA, MTZ and LEV, while multiple site mutations are required to induce AMO resistance.26 In this regard, the application of therapies containing CLA, MTZ, or LEV should be cautious although the resistant rates of H. pylori to these antibiotics vary among geographic areas.16 HDDT may prove to be the empiric therapy of choice irrespective of local antibiotic resistance pattern.
Although prior meta-analysis showed limited impact of CLA-resistance on therapeutic efficacy of ST,27 leading to recommending ST as the alternative first-line treatment in high CLA resistance area,5 we found that the eradication rate of first-line ST was only 29% in patients with CLA/MTZ dual resistance. Likewise, while the use of ST as the second-line treatment has never been previously verified, we showed that the increase of dual resistant rate from 8% in treatment-naïve patients to 44% in treatment-experienced patients causing a decrease of eradication rate from 85% in naïve ST to 52% in rescue ST. These findings show the significant impact of CLA/MTZ dual resistance on the treatment outcome of ST, and indicate that first-line regimens should be carefully chosen as it may significantly impact the efficacy of available rescue therapy. In addition, patient education regarding close adherence to prescribed dose of treatment is imperative to avoid the development of multi-resistant H. pylori strains.
The strengths of this study include large sample size, parallel comparisons of first-line and rescue regimens, clear records of previous regimens for H. pylori therapies, high successful culture rate of H. pylori strains, and extensive analyses of factors that may influence the success of H. pylori eradication. However, some limitations exist. First, varying treatment durations were adopted in different groups. However, a recent meta-analysis study shows that the extending duration of CLA-TT from 7 days to 14 days would only slightly increase its efficacy (5%),28 and would provide only marginal clinical benefit.1,29 This may be related to the fact that the CLA-resistance cannot be overcome by increasing the dose and duration.5,30 Likewise, there was no significant difference in the efficacy between 14-day and 10-day ST.31,32 For LEV-TT, one meta-analysis showed the 10-day regimen was more effective than the 7-day regimen.15 However, our study along with a recent report indicate that the resistance rate of LEV may have already been high, limiting its use in the front-line treatment.5 Second, HDDT is a regimen with higher dosing frequency (4 times daily). Although the present study showed high adherence in the enrolled patients, this needs to be confirmed by studies conducted in different regions of the world.
In summary, this study demonstrated that HDDT consisting of high-dose PPI and AMO given four times daily was superior to standard first-line or rescue therapy for H. pylori infection, irrespective of CYP2C19 genotype. We found H. pylori rapidly acquired antibiotic resistance to CLA, MTZ, and LEV but not to AMO following a single course of anti-H. pylori therapy containing the respective antibiotics. Under these circumstances, empiric treatment with optimized HDDT, especially in regions with high rates of antibiotic resistance or when antimicrobial susceptibility testing is not readily available, would potentially achieve higher eradication rates, curb the emergence of multi-antibiotic resistant H. pylori strains, and fills a gap in clinical management of patients failing multiple courses of anti-H. pylori therapy given the overall low rate of amoxicillin resistance worldwide.
Supplementary Material
Acknowledgments
Supported by grants from the National Science Council of Taiwan (NSC-99-2314-B-002-095, NSC-100-2314-B-002-070) and the National Institute of Health (R01 DK087708). The sponsors of the study had no role in the study design, data collection, analysis, data interpretation, or writing of the manuscript.
The study was funded by the National Science Council of Taiwan (grant number, NSC-99-2314-B-002-095, NSC-100-2314-B-002-070) and the National Institute of Health (R01 DK087708). The sponsors of the study had no role in the study design, data collection, analysis, data interpretation, or writing of the manuscript. C-J Lin and H-L Wang contributed equally to this article. We acknowledge the contribution of the study patients, their families, and study-site personnel.
Abbreviations
- AMO
amoxicillin
- 13C-UBT
13C-urea breath test
- CLA-TT
clarithromycin-containing triple therapy
- HDDT
high-dose dual therapy
- ITT
intention-to-treat
- LEV-TT
levofloxacin-containing triple therapy
- MTZ
metronidazole
- PP
per-protocol
- PPI
proton pump inhibitor
- ST
sequential therapy
Footnotes
Disclosures
All authors have no conflicts of interest to disclose
Author Contributions
J-C Yang and C-J Lin had the study concept. J-C Yang, C-J Lin, and H-L Wang contributed to the study design and wrote the protocol and the draft of paper. H-L Wang, J-C Yang, and C-W Lu did the statistical analyses. C-T Shun and J-C Yang provided central histological and microbiological assessment. JY Kao revised the draft critically for important intellectual content. J-C Yang and JY Kao obtained funding. J-C Yang, J-D Chen, B-R Lin, M-J Shieh, M-C Chang, Y-T Chang, S-C Wei, L-C Lin, W-C Yeh, J-S Kuo, C-C Tung, Y-L Leong, T-H Wang, and J-M Wong enrolled and treated the patients and collected data. All authors contributed to the comment and interpretation of results and approved the final version of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McColl KEL. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Wong BCY. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 4.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 6.Vakil N, Vaira D. Treatment for H. pylori infection: New challenges with antimicrobial resistance. J Clin Gastroenterol. 2013;47:383–388. doi: 10.1097/MCG.0b013e318277577b. [DOI] [PubMed] [Google Scholar]
- 7.Heep M, Kist M, Strobel S, Beck D, Lehn N. Secondary resistance among 554 isolates of Helicobacter pylori after failure of therapy. Eur J Clin Microbiol Infect Dis. 2000;19:538–541. doi: 10.1007/s100960000288. [DOI] [PubMed] [Google Scholar]
- 8.Peitz U, Sulliga M, Wolle K, et al. High rate of post-therapeutic resistance after failure of macrolide-nitroimidazole triple therapy to cure Helicobacter pylori infection: impact of two second-line therapies in a randomized study. Aliment Pharmacol Ther. 2002;16:315–324. doi: 10.1046/j.1365-2036.2002.01173.x. [DOI] [PubMed] [Google Scholar]
- 9.Glupczynski Y, Megraud F, Lopez-Brea M, et al. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820–823. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JM, Silliman NP, Wang W, et al. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993–1999. Ann Intern Med. 2002;136:13–24. doi: 10.7326/0003-4819-136-1-200201010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859–1861. doi: 10.1128/aac.39.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 13.Yang JC, Lin CJ. CYP2C19 genotypes in the pharmacokinetics/pharmacodynamics of proton pump inhibitor-based therapy of Helicobacter pylori infection. Expert Opin Drug Metab Toxicol. 2010;6:29–41. doi: 10.1517/17425250903386251. [DOI] [PubMed] [Google Scholar]
- 14.Yang JC, Yang KC, Hsu CT, et al. A multicenter study on eradication of Helicobacter pylori infection in patients with duodenal ulcer by lansoprazole-antibiotics combined therapy. J Microbiol Immunol Infect. 1999;32:1–8. [PubMed] [Google Scholar]
- 15.Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: Current status and future concepts. World J Gastroenterol. 2014;20:5283–5293. doi: 10.3748/wjg.v20.i18.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507–514. doi: 10.1016/S0140-6736(11)60825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt RH. pH and Hp--gastric acid secretion and Helicobacter pylori: implications for ulcer healing and eradication of the organism. Am J Gastroenterol. 1993;88:481–483. [PubMed] [Google Scholar]
- 19.Bayerdorffer E, Miehlke S, Mannes GA, et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–1417. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Tutuian R, Hila A, et al. Ingestion of acidic foods minics gastroesophageal reflux during pH monitoring. Dig Dis Sci. 2005;50:1916–1920. doi: 10.1007/s10620-005-2961-6. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami Y, Akahane T, Yamaguchi M, et al. In vitro activities of rabeprazole, a novel proton pump inhibitor, and its thioether derivative alone and in combination with other antimicrobials against recent clinical isolates of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:458–461. doi: 10.1128/aac.44.2.458-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JC, Wang HL, Chern HD, et al. Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy. 2011;31:227–238. doi: 10.1592/phco.31.3.227. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290–301. doi: 10.1016/j.clpt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Furuta T, Shirai N, Takashima M, et al. Effects of genotypic differences in CYP2C19 status on cure rates for Helicobacter pylori infection by dual therapy with rabeprazole plus amoxicillin. Pharmacogenetics. 2001;11:341–348. doi: 10.1097/00008571-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Megraud F, Coenen S, Versporten A, et al. Helicobacer pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 26.Rimbara E, Noguchi N, Kawai T, et al. Mutations in penicillin-binding proteins 1, 2 and 3 are responsible for amoxicillin resistance in Helicobacter pylori. J Antimicrob Chemother. 2008;61:995–998. doi: 10.1093/jac/dkn051. [DOI] [PubMed] [Google Scholar]
- 27.Jafri NS, Homung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicoacter pylori infection in patients naïve to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 28.Fuccio L, Minardi ME, Zagari RM, et al. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 29.Zagari RM, Bianchi-Porro G, Fiocca R, et al. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: the HYPER Study. Gut. 2007;56:475–479. doi: 10.1136/gut.2006.102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Megraud F, Lehours P. Helicobacter pylori delection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 32.Hsu PI, Wu DC, Wu JY, et al. Is there a benefit to extending the duration of Helicobacer pylori sequential therapy to 14 days? Helicobacter. 2011;16:146–152. doi: 10.1111/j.1523-5378.2011.00829.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.