Abstract
Type 2 diabetes mellitus (T2D) is a complex disease characterized by β-cell failure in the setting of insulin resistance. The current evidence suggests that genetic predisposition, and environmental factors can impair the capacity of the β-cells to respond to insulin resistance and ultimately lead to their failure. However, genetic studies have demonstrated that known variants account for less than 10% of the overall estimated T2D risk, suggesting that additional unidentified factors contribute to susceptibility of this disease. In this review, we will discuss the different stages that contribute to the development of β-cell failure in T2D. We divide the natural history of this process in three major stages: susceptibility, β-cell adaptation and β-cell failure and provide an overview of the molecular mechanisms involved. Further research into mechanisms will reveal key modulators of β-cell failure and thus identify possible novel therapeutic targets and potential interventions to protect against β-cell failure.
Keywords: β-cell failure, β-cell development, β-cell programming, glucolipotoxicity, islet biology, insulin resistance
Introduction
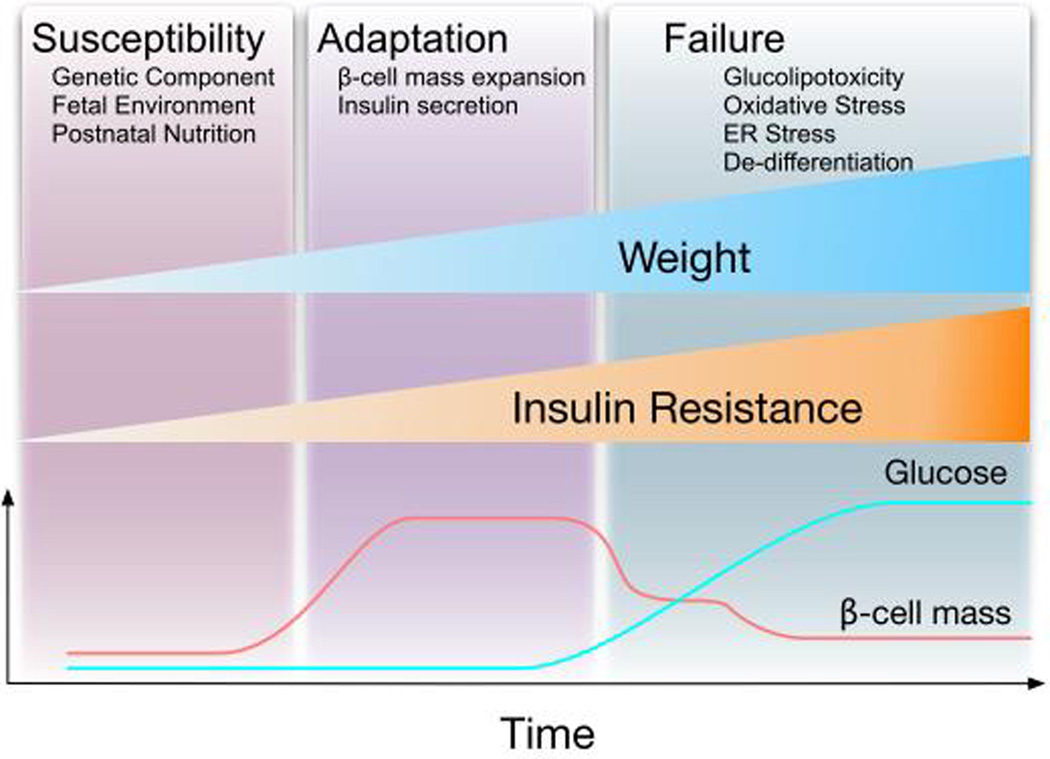
Type 2 diabetes (T2D) is characterized by relative insulin deficiency in response to increase in insulin demand induced by insulin resistance. Experiments in rodent models and human specimens suggest that the failure of β-cells to increase mass and function is a central event in the development of this disease. Multiple factors play a role in the adaption of β-cells during the natural history of T2D. Based on our current understanding of the disease, we would like to divide the adaptation of β-cells during the natural history of T2D in three phases: susceptibility, adaptation, and failure (Figure 1). The susceptibility of individuals to develop diabetes is determined by genetic components, the fetal environment, and the nutrient environment during the first few years of life (Figure 1). It is currently believed that these factors are crucial to control the functional β-cell mass before adulthood. Most individuals will not develop T2D unless they are exposed to conditions of increased insulin demand such as obesity induced insulin resistance. In fact, the majority of the obese population develops insulin resistance and β-cells compensate in response to increased insulin demand by expansion and increase in insulin secretion. Glucose homeostasis in these individuals is conserved at the expense of elevated insulin levels by enhancing insulin secretion and β-cell mass (adaptation phase, Figure 1). However, in a fraction of obese individuals β-cells fail to properly compensate and hyperglycemia occurs. Human epidemiologic studies using self-reported survey-based data place estimates that only a fraction adult males and females BMI>30 develop T2D 1–3. The chronic exposure of β-cells to hyperglycemia and other metabolic abnormalities triggered by obesity induces detrimental effects on β-cells manifested with progressive loss of β-cells, deterioration of function and possibly dedifferentiation (β-cell failure, Figure 1). In the current review, we provide an overview of some of the major established factors that regulate each of the different stages of β-cells during the pathogenesis of T2D.
Figure 1. Natural history of the adaptation of β-cells to obesity and diabetes.
It is clear that the majority of obese individuals develop insulin resistance. The β-cells adapt to insulin resistance by increasing mass and the function. The β-cells compensate appropriately in the majority of obese individuals and hyperinsulinemia is a common finding in obesity. However, in a fraction of obese subjects β-cells fail to compensate appropriately with development of hyperglycemia and diabetes. After the development of hyperglycemia, glucose acts synergistically with other factors to induce β-cell failure. The evolution of β-cells in this process can be divided in three major stages: 1. Individuals with high risk of diabetes are born with increase susceptibility by genetic component, the fetal environment and the nutrient environment during the first years of life. 2. As individuals gain weight there is a phase of adaptation. 3. Finally, individuals with increase susceptibility develop β-cell failure by the interaction of different process.
Susceptibility: Factors regulating the accrual of β-cell mass
The β-cell mass in adult humans and rodents is achieved during the first two decades or four weeks of postnatal life respectively4–6. Theoretically while there is no data to support this concept, it is plausible to believe that the β-cell mass at the end of these early stages can provide some measure of protection from or risk for T2D. Therefore, an understanding of what establishes β-cell mass at birth and early postnatal stages have been the focus of intense research. In animal models, rodents with higher β-cell mass in early life have some protection from developing glucose intolerance when challenged streptozotocin injection7. These models establish a link between alterations in β-cell mass at birth and the development of diabetes later in life. This section will review some of the major factors that determine how rodents and humans arrive at their final of β-cell mass. We will focus on genetic components, the fetal environment, and the nutrient status during the early years of life as critical components in defining β-cell mass in adults. These components contribute to the remodeling of β-cell mass during three distinct phases of the organism’s lifespan. These are embryonic development, the postnatal proliferative and remodeling phase, and the slow turnover that occurs once baseline β-cell mass is reached.
Genetic components altering β-cell development
Rodents
Most of our understanding about pancreas development comes from rodent experiments (extensively reviewed here8,9). The earliest stage of pancreas development begins in the mouse on embryonic day (E)8.5. Under the influence of a number of secreted factors from the adjacent primitive gut and vascularization, the presumptive pancreas is progressively defined within early endoderm. A dorsal anlage (quickly followed by a ventral one) expressing pancreas transcription factor 1a (Ptf1a) and indian hedgehog (Ihh), but not sonic hedgehog (Shh) progressively buds out of the primitive endoderm then grows and branches within its surrounding mesenchyme. Pancreatic duodenal homeobox 1 (Pdx1) expression follows, and the pancreatic epithelium then proliferates and differentiates under the influence of signaling from its surrounding mesenchyme (murine pancreatic development is extensively reviewed in9–11). Pdx1 and Ptf1a are central to pancreatic development and mice with a germline inactivating mutation of either gene display pancreatic agenesis12,13. In addition heterozygous Pdx1 mutant exhibit decreased β-cell mass and progressively develop glucose intolerance14 indicating that Pdx1 levels also influence postnatal β-cell mass and function. Several other transcription factors like SRY (sex determining region Y)-box 9 (SOX9), forkhead box (FOX) A1/2, hepatocyte nuclear factor (HNF) 1β, and GATA4/6 have also been found to play a critical role in forming the pool of multipotent pancreatic progenitor cells. Deletion of Sox9 in pancreatic progenitors leads to marked hypoplasia of the pancreas15. Likewise, the inactivation of both GATA4 and 6 in pancreatic progenitors also leads to pancreatic agenesis16,17. Examination of the role of Hnf1β is more difficult due to the mutation being embryonically lethal but studies using tetraploid aggregation showed pancreatic agenesis in these offspring18. A global knockout of Hnf6 also leads to pancreatic hypoplasia19. Similarly conditional deletion of both FoxA1 and 2 under the Pdx1 promoter led to severe pancreas hypoplasia20. A number of studies have demonstrated that the proper growth of the pancreas requires complex interactions from the surrounding mesenchyme (reviewed in21). Secreted factors like Follistatin regulate the proper balance between the endocrine and exocrine compartments22 while growth factors control its proliferation. In the absence of fibroblast growth factor (FGF) 10 for instance (secreted by the pancreatic mesenchyme), the initial formation of the pancreas seems normal, but all growth and differentiation quickly halt, leading to a drastic hypoplasia of the pancreatic anlages23.
From the multipotent progenitor stage some cells express Sox9 and then progress to a bipotent trunk cell, which is capable of further differentiating into a ductal or endocrine cell24. During the earliest stages of pancreatic organogenesis, Notch signaling leads to the activation of hairy and enhancer of split (Hes) 1 and promotes the acinar fate in most uncommitted pancreatic cells, while only a few escape Notch activation and express Neurogenin 3 (Neurog3) and then commit to the endocrine lineage25. Neurog3 activation results from a balance between a number of transcription factors outlined above (Pdx1, SOX9, FOXA2, HNF1β, Gli-similar (GLIS) 3 and HNF6), and its Notch-promoted inhibitor Hes1. Neurog3 knock out animals display no endocrine cells in the pancreas at birth26. These endocrine progenitors then require transient Notch activation27 before being directed by a coordinated cascade of transcription factor activation to further differentiate into single hormone producing cell fates. Important transcription factors in the development of β-cells include Nkx 6.1, NeuroD1, regulatory factor×(Rfx) 6, islet (Isl)1, NKX2,2, and Pax4. Nkx 6.1 knockout mice are born with a severe and selective deficiency in β-cells28. Mice with knockout of Rfx6 have a decrease in all islet cells, with the exception of PP cells29. Knock out of NeuroD1, Pax4, or Pax6 lead to decreased β-cell number or absence of β-cells at birth30–32. The first hormone-producing cells are detectable on embryonic day E9.5 but increase in number at E13.5, a period of pancreatic development known as the secondary transition33,34. By E14.5 each known type of hormone producing islet cell is detectable. The number and proliferation of Pdx1 and Neurog3 progenitors has been shown to correlate with β-cell mass at birth35,36. β-cell proliferation and differentiation occur in the latter part of the embryonic period, and the combination of these processes determines β-cell mass present at birth with β-cell neogenesis still playing the predominant role during the embryonic period.
Humans
Due to the limited tissue available for studies and the difficulty in determining exact embryonic dating fewer details are known about human embryonic pancreas development. Studies of early human pancreatic development have been limited, but this knowledge has recently been bolstered. In humans PDX1 expression is detected around embryonic day E3037,38. Humans with homozygous mutations in the PDX1 gene are born with pancreatic agenesis39. These individuals have permanent neonatal diabetes as well as exocrine pancreas insufficiency. Interestingly, patients with heterozygous PDX1 mutations have increased susceptibility to diabetes with diagnosis reported to occur as young as 2 years of age40,41. Around week 7 the expression NEUROG3 is initially detected and then rises sharply at weeks 8-1037. This rise in NEUROG3 expression corresponds with the detection of the first hormone-positive cells in the developing pancreas. Humans with heterozygous mutations in the NEUROG3 gene develop childhood onset diabetes while those with mutations in both alleles develop permanent neonatal diabetes42,43. Other key pancreatic transcription factors like PAX6 and NEUROD1 have also been found to harbor mutations in patients with syndromes of permanent neonatal diabetes44,45. The link between these transcription factor mutations and severe diabetes with neonatal onset establishes their importance in human β-cell development.
Genome wide association studies (GWAS) show that the majority of genes linked to T2D play a role in β-cell function or mass. The most consistently associated genes include transcription factors regulated by Wnt signaling (transcription factor 7-like 2 (TCF7L2), hematopoetically expressed homeobox (HHEX)), peroxisome proliferator-activated receptor (PPARγ), cell cycle regulators (CDK5 regulatory subunit-associated protein 1-like 1 (CDKAL1), cyclin-dependent kinase inhibitor (CDKN) 2A/B, cell division cycle protein (CDC 123), the potassium channel KCNJ11, and the zinc transporter solute carrier family 30 (SLC30)A8 (reviewed in46,47). Important pancreatic transcription factors have also been identified in T2D GWAS. Variants in HNF1β, HNF1α, Notch2, and GLIS3 have been associated to T2D48,49. While no single gene variant was found to be the cause of T2D in these studies, the combined effect of these and other β-cell genes may lead to defective compensatory β-cell responses to insulin resistance and obesity. Diabetes risk loci have also been found in genes that are not known to be transcription factors important in β-cell development. It is possible that these loci have a more significant role in β-cell function, which also contributes to T2D risk. In addition, the majority of monogenic diabetes (MODY) syndromes also result from mutations in key β-cell transcription factors. Mutations in one allele of HNF4α were found to be the cause of MODY150. MODY2 results from glucokinase (GCK) mutation 51. Mutations in HNF 1α were found to cause MODY352. As previously mentioned, MODY4 was found to be caused by mutations in PDX153. Mutations in HNF1β have been found to cause MODY554. MODY6 results from mutations in the NEUROD1 gene 55. Finally, MODYX is defined as a Maturity Onset Diabetes of the Young without mutations in the known MODY-gene. In summary, these results underscore the importance of β-cells in the pathogenesis of the disease. In addition, the discovery of genes from GWAS together with those responsible for monogenic forms of diabetes has provided critical information to understand different aspects of β-cell biology and pancreas development.
Environmental factors regulating β-cell development and neonatal β-cell mass
Humans
Human epidemiologic studies established a link between gestational or early life nutrient stressors and a risk for metabolic disease in adulthood, and this concept was termed developmental programming, Infants born to mothers exposed to famine during mid or late pregnancy were found to have a higher glucose response to oral glucose challenge when compared to controls56. Moreover, other studies showed that low birth weight was associated with abnormal glucose homeostasis and a higher risk for metabolic syndrome57–59. In these studies the propensity for impaired glucose tolerance was worsened if the adults were obese. Based on the results of animal studies one may assume that infants exposed to inadequate nutrition during gestation may be born with a decrease in β-cell mass. However the few human studies that exist have conflicting findings. A small autopsy study of pancreata from small for gestational age (SGA) infants showed a decreased percentage of endocrine tissue and number of β-cells per islet when compared to controls60. A more recent autopsy study, however, did not replicate this finding61. These studies are challenging to perform and limited in size, thus it is difficult to draw conclusions about alterations in β-cell mass at birth and risk of T2D in children exposed to adverse nutrient conditions during fetal life.
Less is known about the impact of overnutrition and obesity on the offspring risk for T2D. Understanding the contribution of these factors is especially important given the prevalence of obesity in women of reproductive age. Recent studies have explored the hypothesis that states of overnutrition and obesity during pregnancy will induce permanent changes in multiple tissues leading to the development of glucose intolerance and metabolic syndrome. One large study of T2D in youth found an increased odds ratio of T2D in children whose mothers were obese62. However, the impact on β-cell function in children born to obese pregnant women is unknown. Recent studies performed in offspring of mothers who had bariatric surgery showed that children born after their mothers had bariatric surgery had improved markers of metabolic syndrome than their siblings born prior to surgery and these changes were associated with methylation of genes that are thought to be related to insulin levels63. However, the impact of maternal overnutrition or obesity on offspring β-cell mass or function is unknown. In addition to obesity, a link between exposure to gestational diabetes and the eventual risk of T2D has been stablished64. These studies demonstrate that offspring born after exposure to gestational diabetes had a 3.7 odds ratio of developing T2D as compared to their siblings64. These studies demonstrate that maternal obesity or hyperglycemia also create an adverse nutrient exposure to the developing fetus that has long term consequences for T2D risk.
Rodents
As discussed in the section on genetic factors in β-cell development, a critical component of the β-cell mass at birth is the number of pancreatic progenitors present throughout development. These progenitors are exquisitely sensitive to stressors during development. If a stress is imposed within a critical window in β-cell development β-cell mass may be negatively impacted. Evidence of the importance of progenitor number was provided by a study in which pancreatic progenitors were ablated at different points during development, and the neonatal β-cell mass was unable to recover from the insult65. Therefore loss of pancreatic progenitors leads to a smaller pancreas overall and a decrease in β-cell mass.
In order to gain an understanding of the molecular mechanisms that underlie developmental programming of T2D, animal models of developmental stressors have been developed. Nutritional status during embryonic development exerts a strong influence on the number of pancreatic β-cells present at birth in animal models. The types of gestational stressors shown in animal models to impact neonatal β-cell mass include global calorie restriction, protein restriction, and decreased uterine blood flow. In the low-protein model animals are exposed to chow with limited protein during the entire duration of gestation. When their pancreata are later examined the animals exhibit decreased β-cell mass at birth and reduced insulin secretion later in life66. This was found to be the result of decreased β-cell replication both during development and at birth and increased rates of apoptosis during lactation67. In the calorie restriction model, a 50% reduction in food intake is imposed on dams during the latter part of gestation. This results in a decrease in β-cell mass at birth thought to be caused by an impairment of β-cell neogenesis, due to the normal rates of proliferation that were observed68. Indeed an embryonic study of rats fed a 50% calorie restricted diet from conception showed a decrease in Pdx1 and Ngn3 progenitors at E1569. Impairment of uterine blood flow is another model established to examine the effects of intrauterine growth restriction on the endocrine pancreas. In this model there is no apparent alteration in β-cell mass until the 15 weeks of age, at which point it is reduced by 50% compared to controls70. Thus an exposure limited to the late gestational period has an effect on the eventual adult β-cell mass that is achieved. These experimental systems highlight the sensitivity of β-cell development to nutrient stressors and demonstrate that abnormalities in the fetal nutrient environment result in long-term consequences in β-cell mass.
More recently investigations into developmental programming of T2D have focused on the effect of high fat diet during gestation on neonatal β-cell mass. Administration of high fat diet during gestation leads to compromised β-cell development and function, evident by altered expression of key factors that maintain the β-cell phenotype, and display a reduced β-cell mass in the adult71,72. Another study examined the impact of diet-induced obesity during gestation and lactation. They found that offspring had impaired glucose tolerance and a decrease in pancreatic insulin content at 6 months73. Interestingly in this and other models, the impaired glucose tolerance is preceded by increased pancreatic insulin content in the obesity-exposed offspring elevated over controls early in life. The long-term consequences of fetal overnutrition on β-cell mass are less understood but the current data suggest that fetal high fat administration during pregnancy induces adverse changes in β-cell development and function in neonatal offspring and these changes ultimately result in β-cell failure and eventual development of T2D.
Given the dramatic rise in diagnoses of T2D in recent decades and the lack of a significant contribution found in GWAS studies the focus of molecular diabetes research turned to exploring epigenetic mechanisms74. These include changes in DNA methylation and histone acetylation, impacting gene transcription as well as expression of non-coding microRNAs (miR). Indeed, animal studies have shown that changes in β-cell mass and function result from epigenetic alterations induced by developmental stressors. Using an experimental model of intrauterine growth restriction (IUGR) by placental artery ligation, the Pdx-1 promoter of adult offspring was found to have increased methylation of CpG islands as well as an increase in the silencing histone mark H3K9me2 and a decrease in the activating mark H3K4me375. In addition, Hnf4α P2 promoter is epigenetically silenced in islets of the offspring of dams exposed to low protein during gestation76. The importance of miR species in developmental programming of β-cell has been recently established77,78. Protein restriction during pregnancy caused a β-cell secretory defect in adult offspring by increase in the expression of non-coding RNA miR199a-3p and miR-342. Interestingly, these miRNAs were also shown to reduce mTOR levels in isolated β-cells from mice exposed to protein restriction during fetal life77. In terms of developmental programming by high fat diet, paternal high fat diet exposure prior to mating induces islet expression of Il13ra2, a protein related to the Jak-Stat signaling pathway, and this change was accompanied by changes in methylation in the transcription start site79. Maternal high fat diet administration during pregnancy led to hyperglycemia in the mother and subsequent decreased glucose responsiveness of offspring islets in vitro which was associated with decreased Pdx1 and glucokinase levels80. Thus periconceptional, intrauterine and early postnatal adverse nutrient exposures are sufficient to induce epigenetic changes in the offspring and lead to permanent alterations in β-cell mass or function.
Postnatal β-cell mass remodeling
Rodents
Another important influence on pancreatic β-cell mass is the expansion that occurs postnatally. At this point the animal is adapting to the postnatal nutrient environment and continuing to undergo significant pancreatic development. In rodents there is a high level of β-cell proliferation that has been reported to reach rates of 4% on day 2 of life81,82. The proliferation rate continues to be elevated throughout the lactation period, when compared to adult animals, but declines steadily to a rate ranging from 0.5% to 3% in various studies81,83,84. There is also an increase in β-cell apoptosis that occurs postnatally (reviewed in 85). In rats this rate is higher at birth than in adult animals but sharply increases to a rate of over 3% from postnatal day 3 to 24 with a peak on days 13 and 17. Correspondingly, β-cell mass does not increase until after day 20 when the rate of apoptosis begins to decline81. Thus the early postnatal rise in β-cell mass is less than would be estimated by the β-cell proliferation. This wave of apoptosis is considered to be part of a period of pancreas remodeling. The importance of this period and the factors that stimulate this proliferation are not fully understood. Parasympathetic nervous system stimulation, thyroid hormones and lactogens have been found to play a role86–88.
Humans
At the time of birth the pancreas has been estimated to contain 3-5% β-cells89 and the current data provide support for a postnatal wave of β-cell proliferation in the first year of life. Studies of the postmortem pancreata of normal infants that died of causes unrelated to the pancreas found a peak in β-cell replication in the early postnatal period of up to about 2-3%, which then fell to below 1% by age 6-12 months4,90,91. These studies all determined the number of proliferating β-cells using immunostaining for Ki67. Evidence in humans suggests that the largest increases in β-cell mass occur in the first 5 years of life and result largely from β-cell replication4. Thus, in humans the process of β-cell mass accrual is largely completed by age 5 years. In terms of β-cell apoptosis, one study found an increase in the rate of apoptosis of up to 1.5% in the period from 32 weeks of gestation to 2 months postnatally91. A different study of developing human pancreata revealed a consistently elevated apoptosis rate of up to 4% during embryonic life but no increase in this rate near the time of term birth89. Other human autopsy studies have not been able to substantiate a wave of postnatal apoptosis in humans4,90. These studies have established the importance of β-cell replication during embryonic development as a major contributor to β-cell mass accrual in humans. These results should be interpreted with caution, however, as the clinical condition of the patient, medications given prior to death, and the time to fixation of the tissues could all have an impact on the morphologic appearance of the pancreata.
Environmental factors regulating postnatal β-cell remodeling
Rodents
Animal models have also demonstrated an influence of nutritional interventions limited to the postnatal period on adult β-cell mass. High fat diet exposure during lactation only led to an increase in body fat and glucose intolerance but no change in β-cell mass92. Low protein diet administered only during lactation resulted in a decrease in β-cell mass at postnatal day 2193. Thus the lactation period is also a critical period of β-cell sensitivity to nutritional stressors. In another study low protein exposure from postnatal week 3-6 in rats also led to a permanent impairment in insulin secretion. This illustrates that the critical period of β-cell programming may extend throughout juvenile life in rodents94.
A common feature of these abnormal nutrient exposures in both embryonic and early postnatal life is an eventual failure of the β-cells and progression to impaired glucose tolerance. It is likely that the failure of the offspring β-cells occur by similar mechanisms. These β-cells may be more poorly equipped to tolerate stressors encountered in adult life including oxidative and ER stress. Premature senescence via telomere shortening has also been implicated95. Another major category of molecular modifications that have been found to play a role in developmental programming is epigenetic alterations, as mentioned above.
It is important to understand that these gestational and early life stressors also have effects on other organ systems important for glucose homeostasis. Nutritional stresses can impact the liver, adipose tissue, and hypothalamic appetite control among others. While these effects are beyond the scope of this review they contribute to the overall risk of T2D in the offspring. In addition, in cases of overnutrition there are multiple nutrients that may play a causative role in β-cell damage. The individual contributions of maternal glucose and insulin need to be understood using animal models.
Humans
After the first 5 years of life in humans, β-cell mass is maintained through a balance of a low level (<1%) of proliferation and apoptosis. The contribution of proliferation to adult β-cell mass is clear in rodent models but may be limited in humans after the third decade of life5. It is not yet clear whether other processes like transdifferentiation or neogenesis contribute to the maintenance of normal adult β-cell mass. Overall the adult complement of β-cell mass is set early in life and processes occurring in prenatal life and the first several postnatal years provide the most significant contribution to this process. The impact of nutritional stressors confined to the lactation period has not been examined in detail in humans. The work of Barker et al did show a correlation between a lower body weight at age 1 year and an elevated 2 hour glucose on oral glucose tolerance testing in adult males96. Other studies that have been conducted examine childhood obesity as an endpoint. Prospective studies are essential for lactation research, and the length of follow-up needed to examine T2D risk makes this type of study extremely difficult. There are interesting links between infants born small for gestational age who have exuberant catch-up growth and obesity and insulin resistance in childhood and adulthood 97,98. The relationship between catch-up growth and risk for T2D is not clear. In addition, the effect of these nutrient fluxes on the accrual of β-cell mass is not known.
The studies outlined above establish the importance of early developmental time points in the normal development of β-cell mass. They also highlight the sensitivity of the developing pancreas to nutritional levels. At the end of this period of early growth and development the small fraction of the pancreas that is comprised of β-cells is the organism’s primary defense against the development of T2D. When these β-cells are challenged as in the case of obesity or pregnancy (as discussed below), it is possible that humans with a decreased β-cell mass will not be able to compensate for this increased metabolic demand. Thus, a detailed understanding of the critical periods and influences on the accrual of β-cell mass will be key to decreasing the risk of T2D.
β-cell adaptation to insulin resistance states
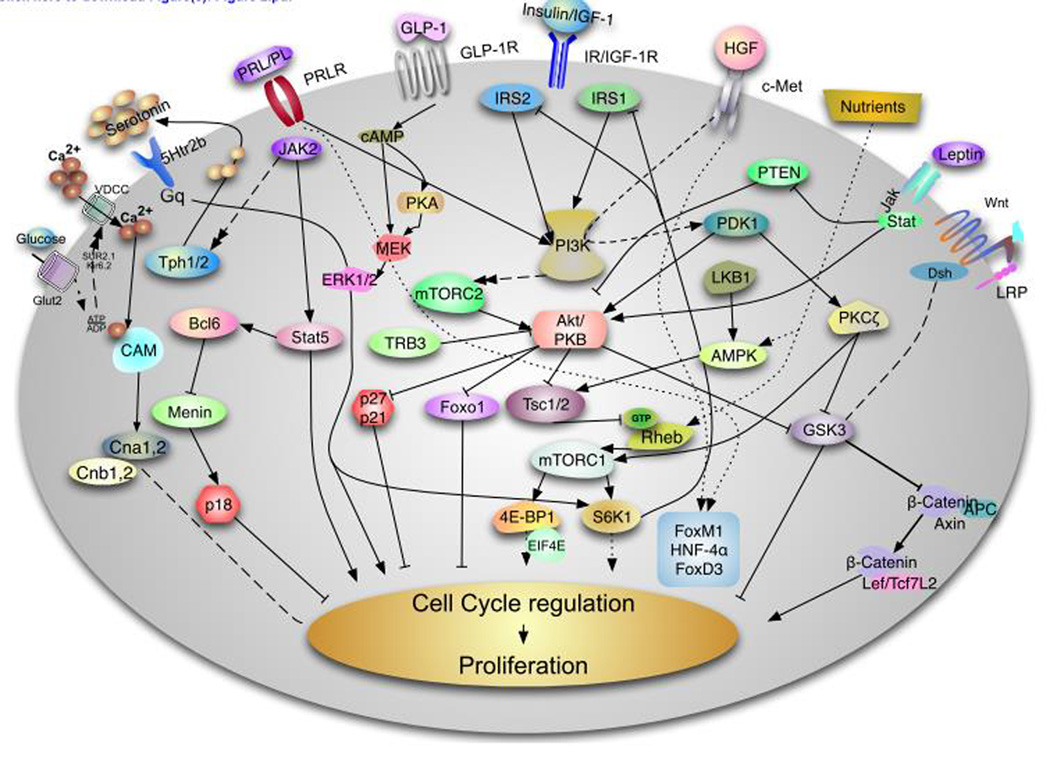
β-cell mass exhibits a slow turnover after the remodeling phase observed during the first four weeks (rodents) and five years (humans) of life. However, β-cells can expand during conditions of insulin resistance (pregnancy99obesity,100 and genetic models of insulin resistance101–103) and these responses determine the susceptibility to T2D. Proliferation of β-cells has been proposed as a major component for these adaptive responses in animal models84,104,105. However, β-cell hypertrophy (cell size) contributes to the expansion of functional β-cell mass in different proportions in young and older animals106,107. In rodents, β-cell mass increases throughout life in parallel with the increment in body weight, but the contribution of β-cell hypertrophy and β-cell hyperplasia to changes in β-cell mass is different depending on the age of the animals. Both β-cell number and size increase in the initial months of life; in contrast, only β-cell size increases in old animals106,107. How β-cells expand in response to insulin resistance is not entirely clear but extracellular signals including growth factors, such as insulin, and nutrients, such as glucose and amino acids, have been shown to play a role. The capacity of β-cells to expand in response to metabolic stress decreases with age in both humans and mice108,109. During the next few sections we will describe in more detail some of the mechanisms responsible for adaptation of β-cells during different stress conditions. Figure 2 attempts to provide an overview of the major signaling pathways involved in regulation of β-cell mass expansion.
Figure 2. Signaling pathways involved in Rodent β-cell proliferation.
This diagram describes some of the major pathways responsible for rodent β-cell proliferation. This field is evolving and we have attempted to provide a summary of the major pathways involve this process. The insulin/insulin-like growth factor I (IGF1) receptor, hepatocyte growth factor and glucagon like peptide 1 (GLP1) signal through the IRS/PI3 kinase pathway to regulate Akt by modulating PDK1. Complete activation of Akt requires the phosphorylation of this kinase by mTORC2. The serine threonine kinase has been demonstrate to play a major role in regulation of β-cell proliferation, survival and cell size by acting on multiple downstream targets including FoxO1, tuberous sclerosis complex (TSC) 1 and 2, Glycogen synthase kinase (GSK) 3 and the cell cycle inhibitors p27cip and p21 among others. Akt activity is negatively regulated directly and indirectly by TRB3 and PTEN. Downstream of Akt, the TSC/mTORC1 integrates signals from growth factors and nutrients. Nutrients can modulate this pathway directly by acting on the protein Rheb or indirectly by regulating the activity of the AMPK pathway, which is also regulated by LKB1. mTORC1 regulates protein translation and cell size by regulating S6K and 4E-BPs. HGF binding to its tyrosine kinase receptor, c-Met, results PKCζ activation which in turns inactivates GSK3β and increases phosphorylation and activation of mTORC1. Activation of the prolactin receptor induces Jak2/Stat5 signaling and/or Jak2/Bcl6/Menin to induce β-cell proliferation. Recent data shows that lactogens induce proliferation by activation of tryptophan hydroxylases (Tph1/2 with generation of serotonin. Serotonin is secreted and act in paracrine fashion to assumed to act via the HTR2b receptor to modulate intracellular calcium and PKC or PI3K members. Glucose is transported by the glucose transporter Glut2 and metabolizes to increase the ATP ratio, which subsequently inhibits the Kir6.2 channel resulting depolarization and calcium influx. Intracellular calcium signaling is a major driver of β-cell proliferation by regulating multiple signaling pathways including Calmodulin (Cam) Calcineurin (Cna1,2 and Cnb1,2) and NFAT transcription. In parallel, glucose activates ChREBP and cMyc to induce cell cycle progression (not shown). Upon binding to GLP-1 GLP-1 receptor results in elevation of cAMP, protein kinase A (PKA) activation and this ultimately results in which can phosphorylation of β-catenin by MEK/ERK1/2 pathway. Leptin acts via the JAK-STAT pathway induces Akt signaling by inhibiting PTEN. The Wnt/frizzled pathway acts by inhibiting GSK-3β and blocking phosphorylation of β-catenin modulate transcription of Lef/Tcf7L2 dependent genes such as cyclin D2 and potentially cyclin D1 and cMyc and activates cell cycle progression. It is important to note that our understanding of human β-cell proliferation is notably limited compared to that of rodents.
β-cell mass in response to pregnancy
During times of prolonged metabolic demand for insulin, including pregnancy, the endocrine pancreas can respond via maternal β-cell hyperplasia and increased insulin secretion to maintain normal blood glucose. Several experimental models in rodents have shown that when β-cell expansion fails to compensate during pregnancy, diabetes occurs, suggesting that defective maternal β-cell adaptation can lead to gestational diabetes mellitus110–113. In human gestational diabetes (GDM) markedly increases the risk of developing T2D after delivery114. Pregnancy could thus be seen as a “stress-test” that reveals a maternal predisposition to T2D. Although the risk of T2D after GDM varies according to the population being studied and the length of follow-up, about 70% of GDM developed T2D when examined at 28 years postpartum (reviewed in115).
The mechanisms that control the adaptive expansion of β-cell mass, focusing on the islet's response to pregnancy, include an increase in β-cell size and an alteration in the balance between β-cell proliferation and apoptosis (reviewed in111). Whether β-cell neogenesis from non-β-cell progenitors is one of the processes involved in the maternal β-cell expansion during pregnancy has not yet been determined. A recent study showed that following a labeling pulse before pregnancy, there is a decrease in the labeling index of β-cells during gestation, suggesting that conversion of non-β-cell progenitors into β-cells might also contribute to maternal β-cell expansion in pregnancy116,117.
Prolactin
Studies in genetic mouse models of GDM have provided insights into the molecular mechanisms of β-cell expansion during pregnancy. These experiments highlight the role of prolactin during pregnancy. Elevation of prolactin is one of many physiological adaptations during pregnancy. Increased prolactin (PRL) induces β-cell proliferation by activation of PRL receptors and activation of Jak2/STAT5 signaling (Figure 2). Transgenic overexpression of prolactin in β-cells resulted in an increase in proliferation and β-cell mass, while global deletion of prolactin receptor reduced β-cell mass and led to impaired insulin secretion118,119. In addition to this mechanism, PRL induces MAPK/ERK, AKT/mTOR and IRS1/2 signaling as well as inhibits p18 and p27120–122. Signaling pathways activated by these kinases are important for regulation of β-cell proliferation and survival.
c-Met/HGF
In addition to prolactin, other receptors have also been implicated in the expansion of β-cells during pregnancy. One of these receptors is the c-Met receptor, which is stimulated by hepatocyte growth factor (HGF, Figure 2). Expression of c-Met and HGF is upregulated in the rat islet endothelium on gestational day 15 when β-cell proliferation and islet vascularization is increased 123. Indeed, mice with conditional deletion of c-Met in the β-cell exhibit impaired glucose tolerance and defective β-cell expansion by day 15 of pregnancy. c-Met signals through production of phosphatidylinositol trisphosphate (PIP3) and activation of PDK-1. PDK-1 then phosphorylates and activates PKCζ, leading to inactivation of GSK3β and increased phosphorylation and activation of mechanistic Target of Rapamycin Complex 1 (mTORC1, Figure 2).
Serotonin
Recent studies have demonstrated that lactogens can induce β-cell expansion during pregnancy by regulation of serotonin receptor signaling. Prolactin and placental lactogen induce expression of the enzymes required for serotonin (5-hydroxytryptamine) synthesis (tryptophan hydroxylase 1 and tryptophan hydroxylase 2) in a phospho-Stat5-dependent manner124,125 (Figure 2). The increase in Tph1 induces serotonin synthesis and secretion, acting on 5Htr2b in an autocrine/paracrine manner to increase β-cell mass. Inhibition of this pathway can cause insufficient β-cell mass expansion during pregnancy and gestational diabetes.
Other maternal hormones
Steroids hormones have been to shown to inhibit the lactogen-induced β-cell proliferation, survival, and function in vivo126,127. Experimental models of administration of dexamethasone during pregnancy have shown that upregulation of steroid hormone levels are responsible for the normalization of the β-cell mass at the end of the pregnancy (decreased proliferation and increased β-cell death). Progesterone and estradiol could also play a role affecting the proliferation during pregnancy, although the mechanisms of how β-cell mass is increased and regulated are still unclear128–130.
Transcriptional regulators
Regulation of transcription has been demonstrated to play an important role in β-cell expansion during pregnancy. Experiments using genetic models demonstrate that the tumor suppressor Menin expression is attenuated during pregnancy (Figure 2). Menin associates with myeloid/lymphoid or mixed-lineage leukemia 2 (MLL2) to induce histone methylation and expression of target genes such as p27Kip1 and p18INK4C112. Therefore, a decrease in Menin expression regulates β-cell cycle in pregnancy by decreasing expression of the cell cycle inhibitor p27 and p18INK4C.
Transcription factors such as FoxM1, HNF-4α and Forkhead box D3 (FoxD3) are required for the expansion of β-cell mass and the proliferative response of the β-cells during pregnancy in mice. FoxM1 is induced by both PrlR and c-Met mediated signaling. Conditional FoxM1 deletion in the pancreas impairs β-cell proliferation and expansion during pregnancy in part by alterations in the Menin/p27 pathway113,131,132. FoxM1 has also been shown to activate the transcription of Birc5, an anti-apoptotic gene upregulated during the peak of β-cell proliferation in pregnancy133. Similar to FoxM1pregnant females with conditional deletion of HNF-4α display a reduction in β-cell mass and proliferation, suggesting that this transcription factor is required for pregnancy-induced hyperplasia, a critical adaptation for maintaining glucose homeostasis during pregnancy134. FoxD3 is another transcription factor suppressed during pregnancy in β-cells. FoxD3 deletion in the pancreas induces glucose intolerance due to abnormal β-cell mass expansion and proliferation during pregnancy by regulation of Foxm1, Skp2, Ezh2, Akt2, and Cdkn1a135. Together, these data suggest that the ability of β-cells to compensate for increased insulin resistance during pregnancy requires the simultaneous induction of both proliferative and survival pathways.
In addition to increased β-cell mass, β-cells adapt to pregnancy by increasing insulin synthesis, enhanced glucose metabolism99elevated levels of glucokinase136 and glucose transporters leading to increased glucose-stimulated insulin secretion. Importantly, a return to normal β-cell homeostasis is achieved during the first week postpartum by means of a decrease in β-cell proliferation and size, and increased β-cell death 99; 81; 111. It has been demonstrated that the normal low rates of apoptosis in β-cells are upregulated postpartum in the mother and in the neonate81,137. In addition, steroid hormones are known inhibitors of lactogen-induced β-cell proliferation, survival and function126,127. As such, some studies have led to the hypothesis that upregulation of steroid hormone levels might be responsible for the normalization of β-cell mass and insulin secretion in the early postpartum period 138.
β-cell mass in response to obesity
Obesity is a persistent state of hyperinsulinemia and is a known risk factor for T2D. However, most obese individuals do not develop T2D because β-cells adapt to insulin resistance by increasing β-cell mass and insulin secretion139. The current view is consistent with the concept that genetic and environmental factors contribute to one’s susceptibility to T2D. In rodents, β-cell mass increases throughout the post-weaning lifespan, closely matching the increment in body weight. Clearly obesity is a major determinant of insulin sensitivity, as studies in human and animal models indicate that weight loss and gain correlate with increasing and decreasing insulin sensitivity respectively140,141. With the stipulation that data in humans are limited, it is currently accepted that in nondiabetic subjects obesity is associated with a modest expansion of β-cell mass, possibly amounting to 10-30% for each 10 kg of weight increment142,143.
In the face of insulin resistance, the normal response of the pancreatic islet to an excessive fuel load is to enhance insulin secretion by both increasing function and β-cell mass expansion. This compensatory hyperinsulinemia maintains blood glucose levels within the normal range until the β-cells can no longer produce sufficient insulin, resulting initially in glucose intolerance and eventually T2D144. Moreover, the β-cell is also susceptible to glucolipotoxic effects, as well as to the detrimental effects of proinflammatory cytokines arising from visceral obesity145 or derived from the islets themselves and from recruited immune cells including macrophages146–149. The combined actions of these metabolic stressors contribute to progressive β-cell dysfunction (discussed in the next section). In fact, β-cell failure has been described as the primary determinant of whether an insulin-resistant individual will progress to diabetes139. A comprehensive description of the mechanisms responsible for β-cell mass in normal or diabetogenic conditions is beyond the scope of this review but we will highlight some of the major concepts in this process.
Rodents
A physiological adaptive expansion of β-cell mass can occur via a net increase in the generation of new β-cells, a net decrease in β-cell death, or an increase in β-cell size. Rodents receiving a high-fat diet for an extended period (3–6 months) have an increase in β-cell mass via increased β-cell proliferation104,150,151. Animal models of insulin resistance have also demonstrated the plasticity of β-cell mass, providing results with significant expansion of β-cell mass in vivo101,103,152. It has long been appreciated that glucose can stimulate β-cell proliferation in experimental models of glucose infusion 153,154. This effect could be due either to hyperglycemia-induced depolarization and Ca2+ influx or secondary insulin secretion in an autocrine/paracrine fashion. Glucose metabolism can ultimately induce proliferation by regulation of the calcium-calmodulin calcineurin signaling or induction of transcription factors155,156. Overall, recent results demonstrate a role for insulin signaling in β-cell growth and suggest that the increased circulating insulin that normally accompanies elevated glucose levels may be primarily responsible for the expansion of β-cell mass in response to hyperglycemia157–160. However, it is clear that the combination of glucose and insulin is a strong stimulus for β-cell growth in the setting of an increased insulin demand.
In addition to insulin and glucose, other circulating factors can trigger β-cell growth in insulin resistant states. Factors from others organs such as leptin or hepatic growth factor (adipose tissue), osteocalcin (bones), gastrin, GLP-1 and GIP (gut), growth hormone (pituitary), among others, are potential regulators of the adaptive expansion of β-cell mass (reviewed in161). Several studies have demonstrated how these extrinsic factors can activate certain molecular mechanisms in the β-cells (Figure 2). The major signaling events linking these membrane processes to β-cell expansion are described in Figure 2. A discussion of the molecular mechanisms and pathways responsible for β-cell expansion are beyond the scope of this review, and we would like to direct attention to recent comprehensive reviews162,163. There is ample evidence that the IGF1/PI3K/AKT/MTOR signaling cascade is involved in β-cell compensation in animals with genetic or high-fat diet-induced insulin resistance164–166 (Figure 2). Cell cycle regulators such as CDK4, cyclin D2, p27, and p21 are implicated as potential mediators of the increased proliferation in states of insulin resistance167–170. For example, in pregnancy, the pathways that are activated not only increase neogenesis or proliferation, but such survival pathways also seem to be important to maintain the increased β-cell mass. To this end, various animal models have substantiated the critical role of maintaining or even enhancing β-cell survival in the setting of insulin resistance171,172. In summary, our current knowledge suggests that β-cell expansion is a complex process regulated by multiple signaling pathways that ultimately converge to regulate proliferation, survival, cell size, and insulin secretion. The individual contribution of these components during the adaptation of β-cells to insulin resistance is complex, but proliferation and hypertrophy appear to be important during the phase of expansion of β-cells and apoptosis could play a role during the final stages of β-cell failure and hyperglycemia.
Humans
Evidence suggests that adult human β-cells proliferation in response to obesity is more limited. In humans, a recent study showed a 50% increase in β-cell mass induced by obesity but with a wide variance not explained by obesity or age173. In this study and in agreement with Rahier et al174the investigators show the increment in βcell mass with obesity is due to increased numbers of cells rather than an increase in cell size. An increased β-cell nuclear diameter was also reported and associated with a prior report of increased-insulin secretion in obesity175. Likewise, the same studies showed that this increase was without a detectable increase in β-cell replication. These findings could reflect that the rate of replication is too small to be detected or the β-cell formation is from different sources (neogenesis)176.
As in pregnancy, the mass expansion increased by obesity could be reversible. Weight loss is generally associated with an improvement in whole-body insulin sensitivity. It seems that the modality of weight loss, the degree of caloric restriction and how the insulin secretion falls in proportion to the rise in insulin sensitivity with weight loss may play a role of its own in the coordinated changes in insulin sensitivity and β-cell function (reviewed in177). Also, weight loss is followed by an increase in insulin sensitivity and an increase in β-cell function178 probably due to a decrease in the glucolipotoxicity effects179. A reduction in islet mass through increased β-cell death occurs in several physiological and pathological situations, and these are also increased after glucose deprivation81,137.
In contrast to rodents, less is known about the mechanisms responsible for β-cell mass regulation in humans (reviewed in162,163). Our current knowledge indicates that there are some differences between the contribution of proliferation and hypertrophy during the adaptation of β-cells to insulin resistance in humans and rodents. However, it is important to mention that limitations in organ preservation could impair some of the measurements used for β-cell proliferation and thus this process could be an underappreciated contributor for human β-cell expansion.
In summary, we have significant knowledge about how rodent β-cells expand in conditions of increased insulin demand. Published evidence suggests that β-cell mass expansion in response to physiological (i.e. pregnancy) and pathological states of insulin resistance is a complex process orchestrated by multiple pathways. It is possible that some of these pathways such as lactogen signaling and serotonin signaling could be specific to pregnancy. However, which are the most critical pathways and how they synergize to induce β-cell expansion during specific conditions is not completely understood. It is likely that β-cell mass expansion during insulin resistance states results from a synergistic effect of multiple pathways and the importance of some of the pathways can be specific for a particular phase of the adaptation of β-cells to insulin resistance. In addition, the signaling pathways that govern the expansion of β-cells in early postnatal life could be different from those required to respond to insulin resistance that occurs later in life. Future research is required to identify how these pathways could be manipulated for therapeutic purposes and to discover novel approaches for the treatment of diabetes.
β-cell Failure
The failure of β-cells to adapt to insulin resistance is necessary for the development of T2D. Therefore, the molecular mechanisms responsible for β-cell failure (loss of both function and mass) have been the focus of study for multiple laboratories around the world in an attempt to prevent or slow the progression of this disease. In addition to all the components mentioned in previous sections, it is well accepted that multiple pathways acting synergistically ultimately result in β-cell dysfunction. As discussed previously, an important feature of T2D is the physiological response of the β-cells to adapt to stressors by enhancing both function and mass. However, as the disease progresses, increased and incessant β-cell workload in remaining β-cell results in T2D.
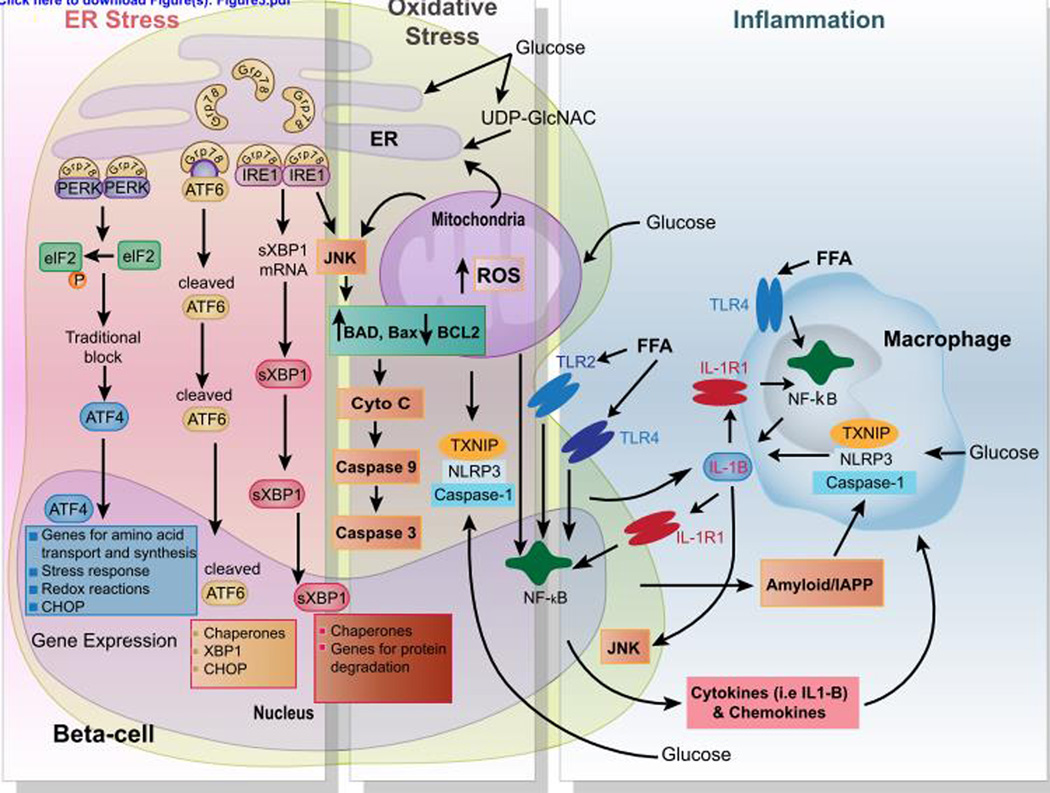
While physiological glucose stimulation is essential for the maintenance of the β-cell physiology, chronic hyperglycemia (glucotoxicity) plays a central role in β-cell failure by inducing deleterious effects on both β-cell mass and function and creates a vicious cycle that contributes to the progressive loss of functional β-cell mass (Figure 3). The presence of chronic and elevated free fatty acids (FFA) and lipid intermediates (lipotoxicity) in the islet microenvironment further overwhelms the existing population of β-cells working assiduously to meet the metabolic demand imposed by insulin resistance in peripheral tissues. The current evidence suggests that the deleterious effects of lipids are observed predominantly in the presence of high glucose, thus, glucolipotoxicity is more appropriate to describe deleterious effects of lipids amplified by glucose on β-cell mass and function. In this section, we will discuss some of the major mechanisms for how the concomitant effects of nutrients and the diabetic milieu negatively impact β-cell survival and function. Based on multiple observations it is believed that glucolipotoxicity (reviewed in180) during latter stages of the natural history of diabetes is a major factor in inducing β-cell failure.
Figure 3. Multifactorial signaling events in β-cell failure.
Chronic glucolipotoxic conditions induce prolonged ER and oxidative stress, and islet inflammation that cause β-cell death. Once β-cells are committed to death as a result of unresolved ER stress under glucolipotoxic conditions (high levels of glucose, free fatty acids (FFA), and OGlcNac) several well-characterized mechanisms that involve the PERK, ATF6, and IRE1 branches of the UPR act to mediate β-cell apoptosis. These involve association of Bax/Bad with IRE1 leading to the activation of JNK and an ATF6 and PERK-mediated increase in CHOP levels and activity. CHOP can then activate expression of proteins involve in pro-apoptotic pathways. High concentrations of glucose promote the activation of the inflammasome complex consisting of NLRP3, TXNIP, and Caspase1 in both β-cells and macrophages. FFA and islet-derived islet amyloid polypeptide (IAPP) also directly trigger the NLRP3/TXNIP/Caspase1 inflammasome complex. Subsequently, pro-IL-1β is processed by the inflammasome complex and secreted in the microenvironment. In turn, IL-1β sustains autocrine and paracrine activation of both β-cells and macrophages, exacerbating the chronic inflammatory responses in the islets. Furthermore, FFA binds to their cognate receptors (i.e., Toll-like receptor 2 and 4 (TLR2-4)), leading to NF-κB activation and the production of various proinflammatory chemokines and cytokines, including the proform of IL-1β.
During the pathogenesis of T2D, β-cells are continually exposed to an overabundant supply of nutrients (glucose, free fatty acids, and amino acids). The metabolic abnormalities leading to the early events that trigger β-cell failure are not completely uncovered. Recent data suggests that in addition to hyperlipidemia, plasma concentrations of amino acids (particularly leucine, isoleucine, valine, proline, phenylalanine, tyrosine, and glutamine) and related metabolites (byproducts of branched-chain amino acids (BCAA) catabolism) are elevated in insulin resistant states 181. Thus, in addition to glucolipotoxic conditions, it is possible that high concentrations of BCAA (reviewed in182) may play a pathogenic role in β-cell failure in obese individuals. Experiments in animal models support this concept by showing that chronic exposure to elevated levels of leucine in mice may have detrimental effects on both β-cell function and insulin sensitivity183. How the increase in BCAA could impact β-cell adaptation or failure in conditions of insulin resistance is unclear, but it is likely that nutrient-regulated pathways such as mTOR are involved. In skeletal muscle, the presence of elevated BCAA and insulin resistance in obesity is associated with chronic activation of mTOR, p70-S6kinase 1 (S6K-1), c-Jun N-terminal kinase (JNK), and phosphorylation of insulin receptor substrate-1 (IRS1) at Ser307. These effects were reversed by Rapamycin, an mTORC1 inhibitor181. In addition, various models of obesity such as wild-type mice on a high fat diet or genetically mutated mice, K/K A(y) and ob/ob, show chronic S6K1 activity in fat cells184. Moreover, S6K1-deficient mice are protected from high fat diet induced-obesity by enhancing insulin sensitivity. Unlike wild type mice on a high fat diet, S6K1-deficient mice show unaltered phosphorylation of IRS1 at Ser307, suggesting that under conditions where there are high levels of nutrients, S6K1 negatively regulates insulin signaling in peripheral tissues185. These and other studies established the concept that the nutrient-regulated mTOR/S6K-1 signaling pathway exerts negative feedback on insulin signaling by acting on IRS proteins185–188. In vivo evidence supporting the negative feedback on insulin signaling comes from studies in mice over-expressing S6K in β-cells. These mice demonstrate impaired cell cycle and survival because of impaired IRS/Akt signaling 187. The biological and physiological effects of this feedback regulation during adaptation to obesity and insulin resistance are not quite clear, but modulation of IRS signaling by mTOR/S6K could contribute and partly explain some of the abnormalities that ultimately result in β-cell failure and diabetes in conditions of nutrient overload. In contrast to classic feedback loops, where a threshold is reached before inhibition occurs, mTORC1 seems to suppress growth factor signaling in a more gradual and continual fashion. Therefore, it is conceivable that mTORC1 activation could initially play a physiologic role in the adaptation to nutrient excess and obesity, but chronic and persistent hyperactivation could lead to reduced IRS signaling by a negative feedback loop. Thus, chronic elevated levels of BCAA in insulin resistant state may promote mTOR/S6K signaling and play a pathogenic role in the development of T2D in addition to hyperglycemia and dyslipidemia. A key unresolved question in the field is to determine how an increase in BCAA synergizes with hyperglycemia and hyperlipidemia to modulate β-cell expansion and/or failure in response to obesity and insulin resistance.
A strong body of evidence, both from in vitro and in vivo studies, supports the concept of glucolipotoxicity in β-cell failure and has provided insights into the mechanisms that contribute to failure under these conditions. Although the order of events have not been fully mapped out and the exact mechanisms involved in β-cell failure are not known, several mechanisms including the generation of reactive oxygen species (ROS), activation of endoplasmic reticulum (ER) stress, upregulation of chronic hexosamine signaling pathway/O-GlcNAcylation, and induction of proinflammatory cytokines in islets are thought to adversely affect β-cells by reducing their capacity to proliferate, impairing insulin secretion, decreasing insulin gene expression, and ultimately promoting uncontrolled β-cell death. This section of the review focuses on these mechanisms in the context of glucolipotoxic and high BCAA conditions.
ER Stress
The ER is responsible for the biosynthesis and folding of newly synthesized insulin destined for secretion in response to high metabolic demand (reviewed in189). A functional ER requires several factors such as adequate levels of ATP and Ca,2+ as well as an optimal oxidizing environment to allow for disulfide-bond formation and proper protein folding190. Because of this specialized environment, the ER is highly sensitive to stresses that perturb ATP and Ca2+ levels, as well as the redox state of this organelle. Glucotoxic, lipotoxic, and inflammatory conditions reduce the protein folding capacity of the ER, resulting in the accumulation and aggregation of unfolded proteins, a condition referred to as ER stress191. Myriad pathological factors in a diabetic milieu perturb ER function and cause ER stress192. Thus, β-cells have evolved to elicit a multi-faceted and complex signaling cascade to mitigate stress. This cascade is collectively termed the unfolded protein response (UPR).
The concerted and complex signaling involved in the UPR is mediated through three ER transmembrane receptors: pancreatic ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6)193. Under normal conditions, all three ER stress receptors are maintained in an inactive state through their association with the ER chaperone, GRP78 (also known as BiP). However, under accumulation of unfolded proteins, GRP78 dissociates from the three receptors, which leads to their activation and triggers the UPR (Figure 3)194. Initially, the UPR is a pro-survival response to reduce the accumulation of unfolded proteins and restore normal ER function195. As long as the UPR can relieve stress, β-cells can produce the proper amount of insulin and maintain ER homeostasis. However, if protein aggregation is persistent and the UPR fails to maintain ER homeostasis, ER stress signaling switches from a pro-survival to a pro-apoptotic signal. The exact molecular mechanisms that facilitate this switch are not fully elucidated. However, it is known that the initiation phase involves the activation of the UPR. As mentioned above, chronic UPR signaling leads to the commitment phase of ER stress-induced apoptosis, where PERK, ATF6, and IRE1 can trigger pro-apoptotic signals through C/EBP homologous protein (CHOP) or JNK. Subsequently, these signals activate the final execution phase involving the Bcl-2 family members and ultimately lead to caspase-3 activation, which results in β-cell death196 (Figure 3).
In rodent models, increased ER stress markers have been demonstrated in mouse islets from db/db mice197. Insulin-2 mutations in Akita mice induce accumulation of misfolded insulin and progressive β-cell loss caused by ER stress, demonstrating the importance of this pathway for β-cell survival198. Similarly, mutation of the wolfram syndrome 1 (wfs1) gene 199,200, which has a protective function against ER stress201, causes Wolfram syndrome. This disease is characterized by insulin-dependent diabetes caused by nonautoimmune loss of β-cell mass and impaired insulin secretion198,202,203. Another genetic model of ER stress induced diabetes is observed in PERK deficient mice204. Similar diabetes phenotype is observed in humans with PERK-null mutations also known as Wolcott-Rallison syndrome205. While T2D is the focus of this review, it is interesting to point out that ER stress is also emerging as a common factor in the etiology of both T1D and T2D192. Islets from cadaveric donors with T1D and T2D exhibited positive staining for activating transcription factor 3 (ATF3)206. A recent study showed enhanced immunostaining for BiP, XBP-1, and CHOP in pancreatic sections from human donors with T1D, suggesting that ER stress contributes to the pathogenesis of T1D207. Taken together, these data suggest that ER stress is present in human β-cells and that this could be a common mechanism for both insulin resistance and β-cell failure.
Current evidence suggests that ER stress is a critical contributor to β-cell failure in T2D. Because of the challenges involved in the assessment of ER stress in living individuals, the use of primary human islets in vitro confirmed the central role of ER stress in diabetes pathophysiology. Elevated levels of circulating proinflammatory cytokines reduce ER Ca2+ storage and induce ER stress responses in human islets208. Prolonged culture of human islets in standard conditions (10% FBS and 5mM Glucose) induces ER stress209. Evidence of ER stress has also been detected in transplanted human islets210. Lipotoxic conditions have shown to induce ER stress in human islets211. Multiple studies demonstrate ER stress in human from cadaveric donors with T2D ex vivo. Huang et al tested whether β-cells in T2D are characterized by ER stress. Indeed, perinuclear and nuclear CHOP staining were more frequent in obese diabetic when compared to lean or obese nondiabetic individuals212. Islets from patients with T2D show higher expression of BiP and XBP-1 when exposed to high glucose, suggesting a higher susceptibility to ER stress compared to non-diabetic individuals213. Enhanced staining for activating transcription factor 3 (ATF3) was observed in T2D cadaveric donors206. In line with these observations, ER density volume was significantly increased in T2D β-cells213.
These previous data underscore the importance of ER stress as one of the mechanisms responsible for alterations in insulin secretion and β-cell failure in later stages of the pathogenesis of diabetes. How the insulin resistant state and hyperglycemia ultimately result in ER stress is unclear, but it is likely that β-cell mass and function are negatively affected by chronic ER stress that is caused by nutrient overload in the presence of chronic hyperglycemia, and elevated levels FFA and BCAA. Because β-cells are "fuel sensors", they monitor and react to elevated nutrient load by increasing the production and release of insulin. While glucose is the strongest insulin secretagogue, fatty acids also potentiate glucose-stimulated insulin secretion acutely (GSIS) (reviewed in214). However, chronic exposure of islets to elevated concentrations of fatty acids causes impairment of GSIS. In vitro experiments have demonstrated that islets exposed to high glucose and palmitate exhibit increased ER stress markers and β-cell apoptosis suggesting that this mechanism could play a role in the β-cell loss observed in diabetes. However, the role of amino acids, particularly BCAA, in modulating ER function is unclear. Perhaps a combination of glucose and FFA with chronic elevations in BCAA may synergize and drives a state of chronic hyperinsulinemia182. Therefore, constant secretory pressure on the β-cell may ultimately contribute to β-cell dysfunction by causing insulin protein misfolding in the ER. It is possible that BCAA could modulate the effects of hyperglycemia and FFA on ER stress by modulating nutrient sensing pathways such as mTOR. Indeed, recent data suggest that inhibition of mTOR complex 1 by Rapamycin treatment ameliorates the effects of glucolipotoxicity on ER stress and apoptosis215,216. Rapamycin treatment also improves diabetes in Akita mice217. These studies suggest that mTORC1 is an important modulator of ER stress and contributes to β-cell failure in glucolipotoxic conditions.
Oxidative Stress
Chronic hyperglycemia causes increased glucose metabolism through oxidative phosphorylation. This induces mitochondrial dysfunction and the production of reactive oxygen species (ROS)218. β-cells are highly susceptible to oxidative stress due to the overabundance of ROS in the islet microenvironment in response to high concentrations of glucose and intrinsically low expression of anti-oxidant enzyme defense mechanisms. For example, the principal antioxidant enzymes superoxide dismutase 1 and 2 (SOD1-2), glutathione peroxidase 1 (GPX1), and catalase (CAT) show lower expression in islets compared to other tissues219. Moreover, islets have been shown to have poor DNA repair capacity against oxidative damage220. Markers of oxidative stress are significantly higher in the islets of patients with T2D compared to controls, and the levels of these markers positively correlate with the degree of impairment in insulin secretion221–223. As such, oxidative stress is an important mechanism responsible for β-cell dysfunction caused by chronic hyperglycemia.
Several studies suggest that oxidative stress is critical for the induction of β-cell dysfunction. Exposure of isolated human islets and pancreatic β-cell line HIT-T15 to high glucose concentrations causes an increase in intracellular peroxide levels, which in turn decreases insulin content and secretion221. In contrast, increasing antioxidant intrinsic defense mechanisms in β-cells in vitro or animal models of T2D by overexpression of antioxidant proteins or treatment with exogenous antioxidants ameliorates β-cell damage caused by prolonged exposure to supraphysiologic glucose concentrations222,223. For example, improved β-cell function in db/db mice and ZDF rats were observed when treated with antioxidant agents. A similar improvement in β-cell function was observed in isolated islets from diabetic individuals treated with antioxidant agents223. These results implicate that ROS play an important role in the deleterious effects of chronic hyperglycemia and hyperlipidemia on β-cells.
The mechanisms by which ROS decrease β-cell mass and function are not completely understood. Increased in oxidative stress leads to decreased transcription of the insulin gene by decreasing Pdx1 and MafA binding activity to the insulin gene (reviewed in180). Oxidative stress can further affect the localization of these transcription factors. For example, MafA is translocated to the cytoplasm under oxidative stress conditions224. ROS is also known to activate stress-induced pathways, including nuclear factor κB (NF-κB), c-Jun N-terminal kinase (JNK), and hexosamine pathway (see below). These pathways individually or collectively affect insulin gene expression. Over-expression of a dominant-negative mutant JNK preserved Pdx1 DNA binding to the insulin gene in islets exposed to H2O2. Moreover, rat islets overexpressing this JNK mutant maintained insulin gene expression under STZ treatment225. The activation of the JNK signaling pathway after induction of oxidative stress may further inhibit IRS1 and IRS2, as demonstrated in various metabolic cells (i.e. primary hepatocytes and 3T3-L1 adipocytes)226–228, thereby obstructing insulin signaling. Oxidative stress can further disrupt β-cell mass and function by inducing ER stress208 and trigger the formation of the inflammasome complex (described below) that initiates the production and secretion of pro-apoptotic concentrations of interleukin-1β in islets (Figure 3)229.
It is important to note that physiological expression of ROS may be required for normal β-cell function. Low concentrations of H2O2 in INS-1 cells and mouse islets increase insulin secretion in response to glucose213. Thus ROS at low levels are required for β-cell function but chronic elevation is harmful and may ultimately cause β-cell dysfunction230.
Islet Inflammation
Obesity and T2D are associated with chronic inflammation characterized by the presence of cytokines and immune cell infiltration in tissues involved in energy homeostasis, including fat, liver, muscle, and islets. Although inflammation can be triggered by metabolic signals, how over-nutrition and obesity (high concentration of glucose, lipids, and BCAA) initiate and sustain inflammation in metabolically active tissues including the β-cells is not fully characterized. In response to a glucolipotoxic microenvironment, β-cells are most likely affected by the contribution of pro-inflammatory factors derived from the islets themselves and from recruited immune cells including macrophages146–149. β-cells are capable of producing cytokines (i.e. interleukin 1 (IL1)-β) in response to high levels of glucose, FFA, and islet-derived islet amyloid polypeptide (IAPP, Figure 3)149,231,232. In the early stages of T2D, islets respond to glucolipotoxicity by generating inflammatory factors such as IL1-β and IL6,233 possibly as a pro-survival mechanism. Indeed, low concentrations of IL-1-β promote β-cell survival and function 234. However, prolonged exposure and higher concentrations of IL-1-β inhibit β-cell insulin secretion and promote Fas-dependent apoptosis in part by activating nuclear factor (NF)-B233,235. The mechanisms of IL-1-β-induced apoptosis are expansive. In both human and rodent islets, pro-apoptotic effects of this cytokine require Ca2+ influx and the activation of both the p38 extracellular signal-regulated kinase (ERK)1/2 mitogen-activated protein kinases (MAPK)236–238. The effects of IL-1β on blocking glucose oxidation and causing apoptosis are partly mediated by Nitric oxide (NO). When exposed to IL-1β, β-cells express an inducible form of nitric oxide synthase (iNOS)239,240 which catalyzes the conversion of L-arginine to citrulline and NO. Overproduction of NO contributes to the IL-1β-induced apoptosis by activating JNK and p38 MAPK and inhibiting Akt241. Moreover, IL-1β via NO can induce ER stress by down-regulating sarcoendoplasmic reticulum pump Ca2+ ATPase 2b (SERCA2b) and by depleting ER Ca2+ 242,243. The toxic effects of IL-1β in β-cells is further exacerbated by high glucose-induced ROS formation, which at high levels can initiate the formation of the inflammasome complex, consisting of NLRP3 (Nucleotide-binding oligomerization domain, Leucine-rich Repeat and Pyrin domain containing 3), thioredoxin (TRX)-interacting protein (TXNIP), and caspase-1 (Figure 3). Activation of the inflammasome complex stimulates the production and secretion of IL-1β229. Because β-cells are more prone to ROS production (due to having enhanced mitochondrial activity and other factors mentioned above) they are likely more susceptible to IL-1-β-induced apoptosis233.
Other chronically elevated cytokines induced by obesity and T2D, such as IL-6, may also play a role in islet failure (reviewed in244). Unlike IL-1-β, the role of IL-6 in β-cells remains controversial245–247. In support of the deleterious effects of IL-6, obese individuals and patients with T2D show elevated levels of IL-6. Furthermore, high levels of IL-6 predict T2D development248. In a prediabetic animal model, circulating IL-1-β and IL-6 levels cause ER stress and islet dysfunction,208 suggesting that inflammation is present early in the development of T2D and can contribute to the initiation of ER stress-induced islet dysfunction.
Reduced pancreatic β-cell mass in patients with T2D are known to be accompanied by amyloid plaque deposits associated with inflammatory response249. Deposition of IAPP is a typical feature of islets in patients with T2D. Human IAPP forms aggregates and fibrils resulting in amyloid plaque deposition. In contrast to human IAPP, rodent IAPP does not aggregate. However, overexpression of human IAPP in rodents results in β-cell dysfunction (reviewed in250). High concentrations of IAPP capable of aggregating are toxic to β-cells through the induction of apoptosis via activation of caspase-3251the JNK pathway252oxidative253 and ER stress254as well as induction of Fas255. In addition to the pro-apoptotic effect in β-cells, IAPP contributes to islet inflammation by recruiting and activating macrophages231. For example, human IAPP aggregates activate NLRP3-dependent inflammasome in bone-marrow derived dendritic cells causing IL-1-β processing and production231,232. β-cells are also induced by human IAPP to produce chemokines such MCP1-1 to promote monocyte recruitment in β-cells231. IAPP aggregates therefore recruit macrophages to the islet or act on resident islet macrophages to promote a pro-inflammatory milieu in T2D. The role of the Toll-like receptors 2 and 4 and the NLRP3 inflammasome in triggering islet inflammation by promoting macrophage-mediated β-cell dysfunction is reviewed by Westwell-Roper et al256. Finally, the concept that islet inflammation contributes to β-cell failure in T2D is further supported with studies with anti-inflammatory medications. For example, IL-1 receptor antagonist protects human islets from the deleterious effects of glucotoxicity257. A clinical trial of IL-1 receptor antagonist showed improved insulin secretion and a reduction in the proportion of proinsulin to insulin secreted in patients with T2D compared to placebo-treated individuals258. These studies show that modulation of the immune system can improve glucose homeostasis and perhaps prevent or delay the development of T2D.
Hexosamine Biosynthetic Pathway and O-GlcNAcylation
Among the different mechanisms involved in the deleterious effects of glucose, less attention has been given to the hexosamine biosynthetic pathway (HBP) and O-GlcNac Glycosylation (O-GlcNAcylation). O-GlcNAcylation, a reversible post-translational protein modification, consists of the attachment of N-acetylglucosamine (GlcNAc) N-acetylglucosamine (GlcNAc) to the serine or threonine residues of cytosolic or nuclear proteins. This process is controlled by two enzymes; O-GlcNAc transferase (OGT) and O-linked β-N-acetylglucosaminidase (OGA), which catalyze the addition and removal of O-GlcNAc, respectively. Approximately 2–3% of glucose entering the β-cell is shunted to the HBP for the synthesis of uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc), the substrate of OGT. Therefore, O-GlcNAcylation regulates protein functions according to glucose availability. Not surprisingly, hyperglycemia enhances glucose flux through the hexosamine pathway converting fructose-6-phosphate and glutamine into glucosamine-6-phosphate and glutamate by the rate-limiting enzyme of the pathway, glutamine:fructose-6-phosphate amidotransferase (GFAT). Then, glucosamine-6-phosphate is further metabolized to UDP-GlcNAc. Known OGT target proteins include Pdx1, FoxO1, NeuroD1, Akt, and IRS2. Enhanced O-GlcNac posttranslational modifications lead to alteration of protein function and subsequently, important changes in gene expression that have been proposed to contribute to β-cell glucotoxicity259. Prolonged activation of the HBP has been reported to be deleterious for β-cells. Transgenic mice overexpressing GFAT show a β-cell insulin secretion dysfunction and glucose desensitization phenotype, which mimics glucose toxicity260. Studies from isolated rat islets and insulinoma cell lines (INS-1 or βTC-6) indicate that the HBP can promote β-cell apoptosis261,262. Moreover, GFAT overexpression in rat islets or glucosamine treatment impaired GSIS in parallel to increased H2O2 production, reduced DNA binding activity of Pdx1, and reduced expression of insulin, Glut2, and Glucokinase261,263. O-GlcNAcylation of Pdx1 has been demonstrated in hyperglycemic Goto-Kakizaki and in Streptozotocin-induced diabetes models in rats264,265. Infusion of high levels of glucosamine in rats markedly impaired GSIS266 and increased β-cell apoptosis264,266,267. Excessive activation of the hexosamine pathway has also been proposed to play a role in high glucose-induced human β-cell apoptosis, and recent data suggest that O-GlcNAcylation of FoxO1 in β-cells promotes Akt inhibition and apoptosis under glucotoxic conditions268,269. While elevated O-GlcNAcylation is associated with deterioration of GSIS and apoptosis in these models, they show an association but not a causal effect.
Although excessive O-GlcNac modification of proteins in β-cells may be deleterious, the hexosamine pathway also plays an important physiological role. Pharmacological inhibition of GFAT by azaserine or OGT knockdown in MIN6 cells reduced insulin secretion. Mice overexpressing OGA demonstrated a defect in insulin secretion, suggesting that reduced O-GlcNAcylation causes defects in insulin synthesis and secretion264,270. Furthermore, in vitro knockdown of OGT using shRNA impairs GSIS in MIN6 cells264. These seemingly opposite results highlight the need for knockout models in β-cells to directly assess the role of GFAT, OGA, and OGT on β-cell mass and function in vivo. What is clear is that these data suggest that O-GlcNAcylation is important in β-cells, but chronic overactivation of this process can have deleterious effects.
β-cell dedifferentiation
β-cell dedifferentiation in an emerging concept that has been the focus of a number of recent studies. In the past few years, new evidence accumulated to illustrate that the pancreas was more “plastic” than we originally thought and that dedifferentiation and transdifferentiation were taking place with increased physiological demand, or inflammation (reviewed in271,272). The process and its implications was described in more details in different models of over-stimulated β-cells in rodents273 or with insulin secretion defects274. The work of Talchai et al. was based on the notion that pancreatic β-cells can be forced to display some degree of plasticity under “genetically extreme conditions”. Expanding on this concept, the authors used lineage-tracing to follow the fate of mature β-cells in multipare mice, after β-cells have been forced to adapt to increased demands in Insulin, as well as with aging. In both models, a number of β-cells progressively loose their β-cell characteristics. With dedifferentiation, C-peptide, Pcsk1, and Pcsk2, Glut2 and Gck decrease in expression as the mRNAs encoding Pdx1, Nkx6.1, NeuroD1, MafA are progressively down-regulated. These results were further reinforced when Gao et al. later demonstrated that dedifferentiation was controlled by Pdx1 itself275. In a recent publication, Zhiyu et al. demonstrated that the same process could be observed in a model of insulin secretory deficiency, and that it was (to a certain degree) reversible274. The potential for the treatment of Type 1 and Type 2 diabetes could be quite significant but the process remains to be demonstrated in human (commented in271).
Signaling pathways activated by glucolipotoxic conditions but not limited to the induction of ER and oxidative stress, cytokines pathways, and O-GlcNAcylation can also affect the expression of genes required to maintain β-cell function and fate. Indeed, failure of the adaptive UPR and ER stress in islets can contributes to β-cell dedifferentiation and is linked with abnormalities in the expression of key β-cell genes, such as Pdx1276. Similarly, chronic ROS could contribute to dedifferentiation by down regulation of differentiated β-cell markers such as Glut2, Pdx1, and MafA277. In support of this concept, Guo et al also reported loss of MafA, NKX6.1, and Pdx1 in response to ROS in the setting of hyperglycemia278. Nevertheless, the reduction of differentiation markers such as MafA and NKX6.1 in db/db mice 278,279 upon the development of hyperglycemia and a decrease in MafA, NKX6.1, and Pdx1 expression in human islets from T2D individuals underscore the effects of the glucolipotoxic milieu in the differentiation identity of β-cells 278,280. Finally, it is important to mention that it remains unclear if β-cell dedifferentiation occurs in human T2D patients. Interestingly, Guo et al reported that T2D islets present unaltered levels of endocrine progenitor-like markers278, which might suggest that dedifferentiation may not occur during the progression of T2D in humans.
Summary: Crosstalk among ER and oxidative stress, islet inflammation, and the hexosamine pathway leads to β-cell exhaustion
β-cell failure is driven by β-cell “hyper-stimulation” and subsequent “exhaustion” in the presence of insulin resistance, glucolipotoxic and aminoacidotoxic conditions, and insufficient functional β-cell mass. Crosstalk among different signaling systems and cellular responses such as ER and oxidative stress and pathways activating pro-inflammatory cascades sets a vicious feed-forward cycle that worsens β-cell dysfunction and possibly promotes dedifferentiation. The complexity of the natural history of T2D poses many challenges in terms of designing therapeutics or strategies for modulating this disease. Therefore a multiple targeted intervention instead of single and uniform therapy must be applied to ameliorate the course of T2D and improve glucose homeostasis. Limited data from few clinical trials have indirectly explored the effects of antidiabetic medications on preservation of β-cell function. A major drawback of these studies is the lack of direct measurements of β-cell function as a major endpoint in the analysis. In addition, the effects of antidiabettic agents can vary depending on the stage of the disease. To answer some of these questions, the Restoring Insulin Secretion (RISE) Consortium will assess different strategies to improve β-cell function, using glucose-lowering therapies such as metformin, insulin, GLP-1 agonists or obesity-reducing therapies such as gastric banding surgery in adult and pediatric populations281.
As we move forward, it is imperative to obtain a clearer picture of the mechanisms responsible for β-cell failure and to understand the crosstalk between signaling systems and different mediators and cell types that contributes to β-cell dysfunction. It possible that a subset of β-cells exposed to sustained stress, such as hyperglycemia, deconstruct their mature state (i.e. dedifferentiate) instead of dying. If this scenario is correct and if it is a common event, then this presents an opportunity to reverse this state and regain fully functional β-cells. For the time being, critical questions remain unanswered. For example, how critical is β-cell dedifferentiation to human T2D? What are the requirement conditions (genetic and environmental factors) for this to occur? What is clear is that more studies are needed to obtain a complete picture of the molecular biology of β-cell dysfunction and the sequence of events of how the T2D develop and how we can prevent its progression
Acknowledgments
The authors apologize to the many authors whose important publications were not cited because of lack of space. The authors wish to acknowledge funding resources for this essential contribution to this work. E.B.M. is supported by the National Institutes of Health (NIH) Grant RO1-DK073716, DK084236, and MERIT award IBX002728A and Juvenile Diabetes Research Foundation (JDRF) grant 17-2013-416. E.U.A was supported by an NIH training grant (2T32DK071212-06), Post-Doctoral Fellowship from the Hartwell Foundation, and a Career Development Award from NIH (K01-DK-103823). C.C-M was supported by a Junior Faculty Award from the American Diabetes Foundation (N013797). We thank Ronald Mark Ygona for assistance in preparing Figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA : the journal of the American Medical Association. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Must A, et al. The disease burden associated with overweight and obesity. JAMA : the journal of the American Medical Association. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 4.Meier JJ, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perl S, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. The Journal of clinical endocrinology and metabolism. 2010;95:E234–E239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 7.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. The Journal of clinical investigation. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mechanisms of development. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 9.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes & development. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Current opinion in genetics & development. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 11.Cano DA, Soria B, Martin F, Rojas A. Transcriptional control of mammalian pancreas organogenesis. Cellular and molecular life sciences : CMLS. 2013 doi: 10.1007/s00018-013-1510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offield MF, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi Y, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nature genetics. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JD, et al. Increased islet apoptosis in Pdx1+/− mice. The Journal of clinical investigation. 2003;111:1147–1160. doi: 10.1172/JCI16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour PA, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasco M, Delgado I, Soria B, Martin F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122:3504–3515. doi: 10.1172/JCI63240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xuan S, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122:3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haumaitre C, et al. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Developmental biology. 2003;258:105–116. doi: 10.1016/s0012-1606(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serup P. Signaling pathways regulating murine pancreatic development. Seminars in cell & developmental biology. 2012;23:663–672. doi: 10.1016/j.semcdb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development (Cambridge, England) 1998;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- 23.Bhushan A, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development (Cambridge, England) 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- 24.Lynn, et al. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2007 doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apelqvist Å, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 26.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cras-Méneur C, Li L, Kopan R, Permutt MA. Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window. Genes to cells : devoted to molecular & cellular mechanisms. 2009;23:2088–2101. doi: 10.1101/gad.1800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander M, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 29.Smith SB, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 31.Sander M, et al. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 32.Naya FJ, et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gittes GK, Rutter WJ. Onset of cell-specific gene expression in the developing mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemp JD, Walther BT, Rutter WJ. Protein synthesis during the secondary developmental transition of the embryonic rat pancreas. J Biol Chem. 1972;247:3941–3952. [PubMed] [Google Scholar]
- 35.Attali M, et al. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–1258. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- 36.Haumaitre C, Lenoir O, Scharfmann R. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Molecular and cellular biology. 2008;28:6373–6383. doi: 10.1128/MCB.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennings RE, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514–3522. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper K, et al. Beta cell differentiation during early human pancreas development. The Journal of endocrinology. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 39.Wright NM, Metzger DL, Borowitz SM, Clarke WL. Permanent neonatal diabetes mellitus and pancreatic exocrine insufficiency resulting from congenital pancreatic agenesis. American journal of diseases of children. 1993;147:607–609. doi: 10.1001/archpedi.1993.02160300013005. [DOI] [PubMed] [Google Scholar]
- 40.Stoffers DA, Ferrer J, Clarke W, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nature Genetics. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 41.Fajans SS, et al. Obesity and hyperinsulinemia in a family with pancreatic agenesis and MODY caused by the IPF1 mutation Pro63fsX60. Translational research : the journal of laboratory and clinical medicine. 2010;156:7–14. doi: 10.1016/j.trsl.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-beta/ALK5-mediated Smad3 activation. Embo J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio-Cabezas O, et al. Permanent Neonatal Diabetes and Enteric Anendocrinosis Associated With Biallelic Mutations in NEUROG3. Diabetes. 2011;60:1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon BD, et al. Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. American journal of medical genetics. Part A. 2009;149A:2543–2546. doi: 10.1002/ajmg.a.33081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubio-Cabezas O, et al. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59:2326–2331. doi: 10.2337/db10-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nature reviews. Genetics. 2007;8:657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 47.Grarup N, Sandholt CH, Hansen T, Pedersen O. Genetic susceptibility to type 2 diabetes and obesity: from genome-wide association studies to rare variants and beyond. Diabetologia. 2014;57:1528–1541. doi: 10.1007/s00125-014-3270-4. [DOI] [PubMed] [Google Scholar]
- 48.Zeggini E, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nature genetics. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature genetics. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamagata K, et al. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 51.Velho G, Hattersley AT, Froguel P. Maternal diabetes alters birth weight in glucokinase-deficient (MODY2) kindred but has no influence on adult weight, height, insulin secretion or insulin sensitivity. Diabetologia. 2000;43:1060–1063. doi: 10.1007/s001250051490. [DOI] [PubMed] [Google Scholar]
- 52.Yamagata K, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3) Nature. 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]
- 53.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nature genetics. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 54.Horikawa Y, et al. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nature genetics. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 55.Sagen JV, et al. Diagnostic screening of NEUROD1 (MODY6) in subjects with MODY or gestational diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2005;22:1012–1015. doi: 10.1111/j.1464-5491.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 56.Ravelli AC, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 57.Barker DJ, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 58.Brufani C, et al. Obese children with low birth weight demonstrate impaired beta-cell function during oral glucose tolerance test. The Journal of clinical endocrinology and metabolism. 2009;94:4448–4452. doi: 10.1210/jc.2009-1079. [DOI] [PubMed] [Google Scholar]
- 59.Veening MA, Van Weissenbruch MM, Delemarre-Van De Waal HA. Glucose tolerance, insulin sensitivity, and insulin secretion in children born small for gestational age. The Journal of clinical endocrinology and metabolism. 2002;87:4657–4661. doi: 10.1210/jc.2001-011940. [DOI] [PubMed] [Google Scholar]
- 60.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. British journal of obstetrics and gynaecology. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 61.Beringue F, et al. Endocrine pancreas development in growth-retarded human fetuses. Diabetes. 2002;51:385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- 62.Dabelea D, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guenard F, et al. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11439–11444. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabelea D, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 65.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 66.Petrik J, et al. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861–4873. doi: 10.1210/endo.140.10.7042. [DOI] [PubMed] [Google Scholar]
- 67.Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107–118. doi: 10.1159/000243170. [DOI] [PubMed] [Google Scholar]
- 68.Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 69.Dumortier O, et al. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia. 2007;50:2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- 70.Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- 71.Cerf ME, et al. Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. American journal of physiology. Regulatory, integrative and comparative physiology. 2005;288:R1122–R1128. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- 72.Cerf ME, Williams K, Chapman CS, Louw J. Compromised beta-cell development and beta-cell dysfunction in weanling offspring from dams maintained on a high-fat diet during gestation. Pancreas. 2007;34:347–353. doi: 10.1097/MPA.0b013e31802ee9ae. [DOI] [PubMed] [Google Scholar]
- 73.Samuelsson AM, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 74.Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456:18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 75.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. The Journal of clinical investigation. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandovici I, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alejandro EU, et al. Maternal diet-induced microRNAs and mTOR underlie beta cell dysfunction in offspring. The Journal of clinical investigation. 2014 doi: 10.1172/JCI74237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dumortier O, et al. Maternal Protein Restriction Leads to Pancreatic Failure in Offspring: Role of Misexpressed MicroRNA-375. Diabetes. 2014;63:3416–3427. doi: 10.2337/db13-1431. [DOI] [PubMed] [Google Scholar]
- 79.Ng SF, et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 80.Cerf ME, Chapman CS, Muller CJ, Louw J. Gestational high-fat programming impairs insulin release and reduces Pdx-1 and glucokinase immunoreactivity in neonatal Wistar rats. Metabolism: clinical and experimental. 2009;58:1787–1792. doi: 10.1016/j.metabol.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 82.Miller K, et al. Islet formation during the neonatal development in mice. PLoS One. 2009;4:e7739. doi: 10.1371/journal.pone.0007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 85.Trudeau JD, et al. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 86.Aguayo-Mazzucato C, et al. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569–1580. doi: 10.2337/db12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarussio D, et al. Nervous glucose sensing regulates postnatal beta cell proliferation and glucose homeostasis. J Clin Invest. 2014;124:413–424. doi: 10.1172/JCI69154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Auffret J, et al. Defective prolactin signaling impairs pancreatic beta-cell development during the perinatal period. Am J Physiol Endocrinol Metab. 2013;305:E1309–E1318. doi: 10.1152/ajpendo.00636.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meier JJ, et al. Beta-cell development and turnover during prenatal life in humans. European journal of endocrinology / European Federation of Endocrine Societies. 2010;162:559–568. doi: 10.1530/EJE-09-1053. [DOI] [PubMed] [Google Scholar]
- 90.Gregg BE, et al. Formation of a human beta-cell population within pancreatic islets is set early in life. The Journal of clinical endocrinology and metabolism. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- 92.Vogt MC, et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156:495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez-Trejo A, et al. Developmental programming of neonatal pancreatic beta-cells by a maternal low-protein diet in rats involves a switch from proliferation to differentiation. Am J Physiol Endocrinol Metab. 2012;302:E1431–E1439. doi: 10.1152/ajpendo.00619.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Swenne I, Crace CJ, Milner RD. Persistent impairment of insulin secretory response to glucose in adult rats after limited period of protein-calorie malnutrition early in life. Diabetes. 1987;36:454–458. doi: 10.2337/diab.36.4.454. [DOI] [PubMed] [Google Scholar]
- 95.Tarry-Adkins JL, et al. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1521–1528. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 96.Hales CN, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Bmj. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mericq V, et al. Differences in body composition and energy expenditure in prepubertal children born term or preterm appropriate or small for gestational age. Journal of pediatric endocrinology & metabolism : JPEM. 2009;22:1041–1050. doi: 10.1515/jpem.2009.22.11.1041. [DOI] [PubMed] [Google Scholar]
- 98.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatric endocrinology reviews : PER. 2008;6:241–247. [PubMed] [Google Scholar]
- 99.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1997;29:301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 100.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 101.Bruning JC, et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 102.Hull RL, et al. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 103.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 104.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kushner JA. Beta-cell growth: an unusual paradigm of organogenesis that is cyclin D2/Cdk4 dependent. Cell Cycle. 2006;5:234–237. doi: 10.4161/cc.5.3.2399. [DOI] [PubMed] [Google Scholar]
- 106.Matveyenko AV, Veldhuis JD, Butler PC. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and beta-cell mass to age-related insulin resistance in rats. Am J Physiol Endocrinol Metab. 2008;295:E832–E841. doi: 10.1152/ajpendo.90451.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes. 2000;49:1341–1346. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- 108.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Assche FA, Gepts W, Aerts L. Immunocytochemical study of the endocrine pancreas in the rat during normal pregnancy and during experimental diabetic pregnancy. Diabetologia. 1980;18:487–491. doi: 10.1007/BF00261705. [DOI] [PubMed] [Google Scholar]
- 111.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karnik SK, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 113.Zhang H, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59:143–152. doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 115.Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes care. 2007;30:1314–1319. doi: 10.2337/dc06-2517. [DOI] [PubMed] [Google Scholar]
- 116.Butler AE, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abouna S, et al. Non-beta-cell progenitors of beta-cells in pregnant mice. Organogenesis. 2010;6:125–133. doi: 10.4161/org.6.2.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vasavada RC, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 119.Freemark M, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 120.Amaral ME, et al. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. The Journal of endocrinology. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- 121.Amaral ME, et al. Prolactin-signal transduction in neonatal rat pancreatic islets and interaction with the insulin-signaling pathway. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2003;35:282–289. doi: 10.1055/s-2003-41303. [DOI] [PubMed] [Google Scholar]
- 122.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology. 2004;145:4162–4175. doi: 10.1210/en.2004-0201. [DOI] [PubMed] [Google Scholar]
- 123.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 124.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature medicine. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schraenen A, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia. 2010;53:2589–2599. doi: 10.1007/s00125-010-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fujinaka Y, Takane K, Yamashita H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- 127.Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology. 1993;133:2227–2234. doi: 10.1210/endo.133.5.8404674. [DOI] [PubMed] [Google Scholar]
- 128.Picard F, et al. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta-cell proliferation. Proc Natl Acad Sci U S A. 2002;99:15644–15648. doi: 10.1073/pnas.202612199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nieuwenhuizen AG, Schuiling GA, Hilbrands LG, Bisschop EM, Koiter TR. Proliferation of pancreatic islet-cells in cyclic and pregnant rats after treatment with progesterone. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1998;30:649–655. doi: 10.1055/s-2007-978952. [DOI] [PubMed] [Google Scholar]
- 130.Nieuwenhuizen AG, et al. Progesterone stimulates pancreatic cell proliferation in vivo. European journal of endocrinology / European Federation of Endocrine Societies. 1999;140:256–263. doi: 10.1530/eje.0.1400256. [DOI] [PubMed] [Google Scholar]
- 131.Zhang H, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Molecular endocrinology. 2006;20:1853–1866. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
- 132.Demirci C, et al. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61:1143–1152. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rieck S, et al. The transcriptional response of the islet to pregnancy in mice. Molecular endocrinology. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta RK, et al. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plank JL, Frist AY, LeGrone AW, Magnuson MA, Labosky PA. Loss of Foxd3 results in decreased beta-cell proliferation and glucose intolerance during pregnancy. Endocrinology. 2011;152:4589–4600. doi: 10.1210/en.2010-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. The Journal of endocrinology. 2007;193:367–381. doi: 10.1677/JOE-07-0043. [DOI] [PubMed] [Google Scholar]
- 137.Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461–5468. doi: 10.1210/endo.136.12.7588296. [DOI] [PubMed] [Google Scholar]
- 138.Holness MJ, Sugden MC. Dexamethasone during late gestation exacerbates peripheral insulin resistance and selectively targets glucose-sensitive functions in beta cell and liver. Endocrinology. 2001;142:3742–3748. doi: 10.1210/endo.142.9.8379. [DOI] [PubMed] [Google Scholar]
- 139.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. The Journal of clinical investigation. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sims EA, et al. Endocrine and metabolic effects of experimental obesity in man. Recent progress in hormone research. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 141.Freidenberg GR, Reichart D, Olefsky JM, Henry RR. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988;82:1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iozzo P, et al. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. The Journal of clinical endocrinology and metabolism. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 143.Yoon KH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. The Journal of clinical endocrinology and metabolism. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 144.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Archives of medical research. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 145.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 147.Tang C, et al. Susceptibility to fatty acid-induced beta-cell dysfunction is enhanced in prediabetic diabetes-prone biobreeding rats: a potential link between beta-cell lipotoxicity and islet inflammation. Endocrinology. 2013;154:89–101. doi: 10.1210/en.2012-1720. [DOI] [PubMed] [Google Scholar]
- 148.Deopurkar R, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes care. 2010;33:991–997. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boni-Schnetzler M, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 150.Sachdeva MM, Stoffers DA. Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol. 2009;23:747–758. doi: 10.1210/me.2008-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finegood DT, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 152.Kahn MB, et al. Influence of serum cholesterol on atherogenesis and intimal hyperplasia after angioplasty: inhibition by amlodipine. American journal of physiology. Heart and circulatory physiology. 2005;288:H591–H600. doi: 10.1152/ajpheart.00617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 154.Alonso LC, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–1801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Molecular endocrinology. 2001;15:1758–1767. doi: 10.1210/mend.15.10.0702. [DOI] [PubMed] [Google Scholar]
- 156.Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. The Journal of biological chemistry. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 157.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 158.Ohsugi M, et al. Reduced expression of the insulin receptor in mouse insulinoma (MIN6) cells reveals multiple roles of insulin signaling in gene expression, proliferation, insulin content, and secretion. The Journal of biological chemistry. 2005;280:4992–5003. doi: 10.1074/jbc.M411727200. [DOI] [PubMed] [Google Scholar]
- 159.Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary beta-cell proliferation via Raf-1 kinase. Endocrinology. 2008;149:2251–2260. doi: 10.1210/en.2007-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Johnson JD, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci U S A. 2006;103:19575–19580. doi: 10.1073/pnas.0604208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sachdeva MM, Stoffers DA. Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Molecular endocrinology. 2009;23:747–758. doi: 10.1210/me.2008-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bernal-Mizrachi E, et al. Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jetton TL, et al. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54:2294–2304. doi: 10.2337/diabetes.54.8.2294. [DOI] [PubMed] [Google Scholar]
- 165.Okada T, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rachdi L, et al. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhong L, et al. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest. 2007;117:2869–2876. doi: 10.1172/JCI32198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cozar-Castellano I, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 169.Miyawaki K, et al. Transgenic expression of a mutated cyclin-dependent kinase 4 (CDK4/R24C) in pancreatic beta-cells prevents progression of diabetes in db/db mice. Diabetes research and clinical practice. 2008;82:33–41. doi: 10.1016/j.diabres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 170.Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes. 2004;53:149–159. doi: 10.2337/diabetes.53.1.149. [DOI] [PubMed] [Google Scholar]
- 171.Liadis N, et al. Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes. 2007;56:2302–2311. doi: 10.2337/db06-1771. [DOI] [PubMed] [Google Scholar]
- 172.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saisho Y, et al. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, obesity & metabolism. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 175.Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased beta-cell turnover. Diabetes care. 2006;29:1554–1559. doi: 10.2337/dc06-0392. [DOI] [PubMed] [Google Scholar]
- 176.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 177.Ferrannini E, et al. beta-cell function in obesity: effects of weight loss. Diabetes. 2004;53(Suppl 3):S26–S33. doi: 10.2337/diabetes.53.suppl_3.s26. [DOI] [PubMed] [Google Scholar]
- 178.Kelley DE, et al. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. The Journal of clinical endocrinology and metabolism. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 179.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 180.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu Z, Jeppesen PB, Gregersen S, Larsen LB, Hermansen K. Chronic exposure to leucine in vitro induces beta-cell dysfunction in INS-1E cells and mouse islets. The Journal of endocrinology. 2012;215:79–88. doi: 10.1530/JOE-12-0148. [DOI] [PubMed] [Google Scholar]
- 184.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 185.Pende M, et al. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Molecular and cellular biology. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 187.Elghazi L, et al. Decreased IRS signaling impairs {beta}-cell cycle progression and survival in transgenic mice overexpressing S6K in {beta}-cells. Diabetes. 2010 doi: 10.2337/db09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Current biology : CB. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 189.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annual review of biochemistry. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gaut JR, Hendershot LM. The modification and assembly of proteins in the endoplasmic reticulum. Current opinion in cell biology. 1993;5:589–595. doi: 10.1016/0955-0674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 191.Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes, obesity & metabolism. 2010;12(Suppl 2):76–82. doi: 10.1111/j.1463-1326.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 192.Evans-Molina C, Hatanaka M, Mirmira RG. Lost in translation: endoplasmic reticulum stress and the decline of beta-cell health in diabetes mellitus. Diabetes, obesity & metabolism. 2013;15(Suppl 3):159–169. doi: 10.1111/dom.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kaufman RJ, et al. The unfolded protein response in nutrient sensing and differentiation. Nature reviews. Molecular cell biology. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 194.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature cell biology. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 195.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual review of biochemistry. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 196.Wali JA, et al. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell death & disease. 2014;5:e1124. doi: 10.1038/cddis.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Laybutt DR, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 198.Wang J, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. The Journal of clinical investigation. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Strom TM, et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Human molecular genetics. 1998;7:2021–2028. doi: 10.1093/hmg/7.13.2021. [DOI] [PubMed] [Google Scholar]
- 200.Inoue H, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nature genetics. 1998;20:143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 201.Fonseca SG, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. The Journal of clinical investigation. 2010;120:744–755. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 203.Shang L, et al. beta-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes. 2014;63:923–933. doi: 10.2337/db13-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 205.Delepine M, et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature genetics. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 206.Hartman MG, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marhfour I, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 208.O'Neill CM, et al. Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology. 2013;154:3077–3088. doi: 10.1210/en.2012-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Erbel S, Reers C, Nawroth PP, Ritzel RA. Prolonged culture of human islets induces ER stress. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2010;118:81–86. doi: 10.1055/s-0029-1238318. [DOI] [PubMed] [Google Scholar]
- 210.Negi S, et al. Evidence of endoplasmic reticulum stress mediating cell death in transplanted human islets. Cell transplantation. 2012;21:889–900. doi: 10.3727/096368911X603639. [DOI] [PubMed] [Google Scholar]
- 211.Cunha DA, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. Journal of cell science. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang CJ, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 213.Marchetti P, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 214.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 215.Bachar E, et al. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PloS one. 2009;4:e4954. doi: 10.1371/journal.pone.0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kato H, et al. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell death and differentiation. 2012;19:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bachar-Wikstrom E, et al. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013;62:1227–1237. doi: 10.2337/db12-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free radical biology & medicine. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 220.Modak MA, Parab PB, Ghaskadbi SS. Pancreatic islets are very poor in rectifying oxidative DNA damage. Pancreas. 2009;38:23–29. doi: 10.1097/MPA.0b013e318181da4e. [DOI] [PubMed] [Google Scholar]
- 221.Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Del Guerra S, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 223.Lupi R, et al. Insulin secretion defects of human type 2 diabetic islets are corrected in vitro by a new reactive oxygen species scavenger. Diabetes & metabolism. 2007;33:340–345. doi: 10.1016/j.diabet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 224.Guo S, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. The Journal of clinical investigation. 2013 doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kaneto H, et al. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 226.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 227.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 228.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature immunology. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 230.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, abnd the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 231.Westwell-Roper C, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. Journal of immunology. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 232.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Maedler K, et al. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes. 2006;55:2713–2722. doi: 10.2337/db05-1430. [DOI] [PubMed] [Google Scholar]
- 235.Petersen LG, et al. IL-1beta-induced pro-apoptotic signalling is facilitated by NCAM/FGF receptor signalling and inhibited by the C3d ligand in the INS-1E rat beta cell line. Diabetologia. 2006;49:1864–1875. doi: 10.1007/s00125-006-0296-2. [DOI] [PubMed] [Google Scholar]
- 236.Larsen CM, et al. Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem. 1998;273:15294–15300. doi: 10.1074/jbc.273.24.15294. [DOI] [PubMed] [Google Scholar]
- 237.Maedler K, et al. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- 238.Fei H, Zhao B, Zhao S, Wang Q. Requirements of calcium fluxes and ERK kinase activation for glucose- and interleukin-1beta-induced beta-cell apoptosis. Molecular and cellular biochemistry. 2008;315:75–84. doi: 10.1007/s11010-008-9791-8. [DOI] [PubMed] [Google Scholar]
- 239.Eizirik DL, Cagliero E, Bjorklund A, Welsh N. Interleukin-1 beta induces the expression of an isoform of nitric oxide synthase in insulin-producing cells, which is similar to that observed in activated macrophages. FEBS letters. 1992;308:249–252. doi: 10.1016/0014-5793(92)81285-t. [DOI] [PubMed] [Google Scholar]
- 240.Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6:399–406. doi: 10.1016/1043-4666(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 241.Storling J, et al. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia. 2005;48:2039–2050. doi: 10.1007/s00125-005-1912-2. [DOI] [PubMed] [Google Scholar]
- 242.Cardozo AK, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 243.Chan JY, Cooney GJ, Biden TJ, Laybutt DR. Differential regulation of adaptive and apoptotic unfolded protein response signalling by cytokine-induced nitric oxide production in mouse pancreatic beta cells. Diabetologia. 2011;54:1766–1776. doi: 10.1007/s00125-011-2139-z. [DOI] [PubMed] [Google Scholar]
- 244.Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17:860–872. doi: 10.1016/j.cmet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 245.Suzuki T, et al. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes. 2011;60:537–547. doi: 10.2337/db10-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.da Silva Krause M, et al. Physiological concentrations of interleukin-6 directly promote insulin secretion, signal transduction, nitric oxide release, and redox status in a clonal pancreatic beta-cell line and mouse islets. The Journal of endocrinology. 2012;214:301–311. doi: 10.1530/JOE-12-0223. [DOI] [PubMed] [Google Scholar]
- 247.Oh YS, Lee YJ, Park EY, Jun HS. Interleukin-6 treatment induces beta-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes/metabolism research and reviews. 2011;27:813–819. doi: 10.1002/dmrr.1233. [DOI] [PubMed] [Google Scholar]
- 248.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 249.Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology. 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 250.Montane J, Klimek-Abercrombie A, Potter KJ, Westwell-Roper C, Bruce Verchere C. Metabolic stress, IAPP and islet amyloid. Diabetes, obesity & metabolism. 2012;14(Suppl 3):68–77. doi: 10.1111/j.1463-1326.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 251.Park YJ, et al. The role of caspase-8 in amyloid-induced beta cell death in human and mouse islets. Diabetologia. 2014;57:765–775. doi: 10.1007/s00125-013-3152-1. [DOI] [PubMed] [Google Scholar]
- 252.Subramanian SL, et al. cJUN N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55:166–174. doi: 10.1007/s00125-011-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zraika S, et al. Oxidative stress is induced by islet amyloid formation and timedependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Casas S, et al. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes. 2007;56:2284–2294. doi: 10.2337/db07-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Park YJ, et al. Deletion of Fas protects islet beta cells from cytotoxic effects of human islet amyloid polypeptide. Diabetologia. 2012 doi: 10.1007/s00125-012-2451-2. [DOI] [PubMed] [Google Scholar]
- 256.Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunology and cell biology. 2014;92:314–323. doi: 10.1038/icb.2014.4. [DOI] [PubMed] [Google Scholar]
- 257.Ehses JA, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 259.Copeland RJ, Han G, Hart GW. O-GlcNAcomics - Revealing Roles of OGlcNAcylation in Disease Mechanisms and Development of Potential Diagnostics. Proteomics Clin Appl. 2013 doi: 10.1002/prca.201300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cooksey RC, Pusuluri S, Hazel M, McClain DA. Hexosamines regulate sensitivity of glucose-stimulated insulin secretion in beta-cells. Am J Physiol Endocrinol Metab. 2006;290:E334–E340. doi: 10.1152/ajpendo.00265.2005. [DOI] [PubMed] [Google Scholar]
- 261.Kaneto H, et al. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- 262.Kang ES, et al. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Experimental cell research. 2008;314:2238–2248. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 263.Yoshikawa H, et al. Glucosamine-induced beta-cell dysfunction: a possible involvement of glucokinase or glucose-transporter type 2. Pancreas. 2002;24:228–234. doi: 10.1097/00006676-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 264.Akimoto Y, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 265.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Increased O-GlcNAc transferase in pancreas of rats with streptozotocin-induced diabetes. Diabetologia. 2000;43:1239–1247. doi: 10.1007/s001250051519. [DOI] [PubMed] [Google Scholar]
- 266.Shankar RR, Zhu JS, Baron AD. Glucosamine infusion in rats mimics the beta-cell dysfunction of non-insulin-dependent diabetes mellitus. Metabolism: clinical and experimental. 1998;47:573–577. doi: 10.1016/s0026-0495(98)90242-6. [DOI] [PubMed] [Google Scholar]
- 267.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–2825. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.D'Alessandris C, et al. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:959–961. doi: 10.1096/fj.03-0725fje. [DOI] [PubMed] [Google Scholar]
- 269.Fardini Y, et al. O-GlcNAcylation of FoxO1 in pancreatic beta cells promotes Akt inhibition through an IGFBP1-mediated autocrine mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:1010–1021. doi: 10.1096/fj.13-238378. [DOI] [PubMed] [Google Scholar]
- 270.Soesanto Y, et al. Pleiotropic and age-dependent effects of decreased protein modification by O-linked N-acetylglucosamine on pancreatic beta-cell function and vascularization. J Biol Chem. 2011;286:26118–26126. doi: 10.1074/jbc.M111.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ziv O, Glaser B, Dor Y. The Plastic Pancreas. Developmental cell. 2013;26:3–7. doi: 10.1016/j.devcel.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 272.Weir GC, Aguayo-Mazzucato C, Bonner-Weir S. β-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5 doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β Cell Dedifferentiation in Diabetes and Redifferentiation following Insulin Therapy. Cell metabolism. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gao T, et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell metabolism. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lombardi A, et al. Increased hexosamine biosynthetic pathway flux dedifferentiates INS-1E cells and murine islets by an extracellular signal-regulated kinase (ERK)1/2-mediated signal transmission pathway. Diabetologia. 2012;55:141–153. doi: 10.1007/s00125-011-2315-1. [DOI] [PubMed] [Google Scholar]
- 277.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 278.Wei F, et al. miR-99b-targeted mTOR induction contributes to irradiation resistance in pancreatic cancer. Molecular cancer. 2013;12:81. doi: 10.1186/1476-4598-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Matsuoka TA, et al. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes. 2010;59:1709–1720. doi: 10.2337/db08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yang BT, et al. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Molecular endocrinology. 2012;26:1203–1212. doi: 10.1210/me.2012-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Consortium R. Restoring Insulin Secretion (RISE): design of studies of beta-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes care. 2014;37:780–788. doi: 10.2337/dc13-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]