Abstract
Glutamatergic signaling abnormalities in cortico-striatal circuits are hypothesized to lead to the repetitive thoughts and behaviors of obsessive-compulsive disorder (OCD). To test this hypothesis, studies have used proton magnetic resonance spectroscopy (1H MRS) to measure glutamatergic compounds in the striatum of individuals with OCD. However, none has used methods that could measure glutamate minimally contaminated by glutamine and γ-aminobutyric acid (GABA) in striatal subregions. Therefore, in this study, a proton MRS imaging (1H MRSI) technique with relatively high spatial resolution at 3.0 T was used to measure minimally contaminated glutamate levels in three striatal subregions (i.e., dorsal caudate, dorsal putamen, and ventral striatum) in 15 unmedicated adults with OCD and 16 matched healthy control subjects. No significant group differences in glutamate levels were found in any of the three striatal subregions. In contrast, a study in unmedicated pediatric OCD patients that measured glutamatergic compounds in the dorsal caudate by MRS at 1.5T found significant elevations. Further studies are warranted to assess whether these discrepant MRS findings are due to differences in subject age or MRS methodology, or potentially are associated with glutamatergic gene variants implicated in OCD.
Keywords: magnetic resonance spectroscopy, OCD, striatum, caudate
1. Introduction
Structural and functional imaging studies indicate that obsessive-compulsive disorder (OCD) is associated with abnormal functioning of brain circuits that include the orbito-frontal cortex (OFC), anterior cingulate cortex (ACC), striatum, and thalamus (Milad and Rauch, 2012). Preclinical and clinical evidence suggests a role for glutamatergic abnormalities in OCD, including reports of associations between OCD and genes related to the glutamate system, of OCD-like behavior in mice following disruption of glutamatergic transmission in cortico-striatal circuits, and of the ability of glutamate-modulating drugs to ameliorate OCD symptoms (reviewed in Insel, 2012; Pittenger et al., 2011). Together, these observations have led to the hypothesis that abnormalities in glutamatergic signaling in OFC-striatal circuits may be implicated in the repetitive thoughts and behaviors that characterize OCD.
Several studies have tested this hypothesis by using proton magnetic resonance spectroscopy (1H MRS) to measure levels of glutamatergic compounds in the striatum of individuals with OCD and healthy control subjects (reviewed in Brennan et al., 2012). The results have been variable, with one study reporting elevations of glutamatergic compounds in the head of the left caudate in unmedicated pediatric patients that decreased with successful treatment (Rosenberg et al., 2000), another study reporting elevated glutamatergic compounds in the left caudate in medicated adult patients (Shekhar et al., 2008), and the majority of studies in both pediatric and adult patients finding no striatal glutamatergic abnormalities (Bartha et al., 1998a; Ebert et al., 1997; Lazaro et al., 2012; O’Neill et al., 2012; Starck et al., 2008; Whiteside et al., 2012; Whiteside et al., 2006). Multiple factors differed across these studies and may explain the discrepant results, including whether OCD subjects were receiving medication at the time of scanning, the magnetic field strength (ranging from 1.5T to 4.0T), the specific MRS methods used to derive the “glutamatergic compound” levels (and thus more or less contaminated with glutamine and γ-aminobutyric acid [GABA]), and the size and location of the striatal voxel of interest.
In the present study, we aimed to minimize or eliminate some of these potential confounds by: (a) studying only unmedicated patients; and (b) taking advantage of the relatively higher detection sensitivity at 3T to implement a MRS imaging (MRSI) method that enables detection of glutamate with minimal glutamine and GABA contamination – as opposed to glutamate that is obtained with approaches that do not attempt to minimize the glutamine and/or GABA contribution, commonly referred to as “Glx” -- from multiple striatal voxels. Based on the glutamatergic theory of OCD and a study at 1.5T that found increased glutamatergic compounds in the left dorsal caudate in unmedicated pediatric OCD patients (Rosenberg et al., 2000), we hypothesized that unmedicated adults with OCD will have increased glutamate in the left dorsal caudate compared to matched healthy control subjects. We simultaneously measured glutamate levels in the left dorsal putamen and ventral striatum to establish the specificity of the caudate finding, since these striatal subregions receive glutamatergic projections from different cortical regions (Haber and McFarland, 1999).
2. Methods
2.1. Subjects
The institutional review board of the New York State Psychiatric Institute/Columbia University approved the study. Subjects were recruited by advertisements and word of mouth and provided written informed consent.
Subjects had to be between the ages of 18 and 55 and free of any significant medical problems, current or past neurological disorders (other than Tic Disorder), and history of substance or alcohol abuse or dependence (including nicotine). Pregnant, nursing, and postmenopausal women and those using hormonal contraceptives were excluded. OCD subjects had to fulfill the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for OCD for at least one year as their primary psychiatric disorder and not be receiving psychotropic medications (for a minimum of six weeks) or psychotherapy at the time of the study. OCD subjects with other current Axis I disorders (except specific or social phobia) were excluded. Healthy controls had to have no current or past DSM-IV Axis I disorder, no exposure to psychoactive medications, and no family history of OCD as assessed by the Family History Screen (Weissman et al., 2000). Healthy controls were matched to the OCD subjects on age, sex, and ethno-racial categories. All subjects but two (n=1 OCD; n=1 healthy controls) were right-handed.
Diagnoses were determined during a psychiatric evaluation by expert clinicians and confirmed by trained clinical raters using the Structured Clinical Interview for DSM-IV (First et al., 1996). Medical health was established by history and physical examination; a urine drug test and pregnancy test for females were conducted on the day of scanning. OCD and depressive severity were assessed in OCD subjects by trained clinician raters using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS; Goodman et al., 1989a; Goodman et al., 1989b) and the 17-item Hamilton Depression Rating Scale (HAM-D, Hamilton, 1960). The Y-BOCS checklist provided current severity scores for each OCD subject along five symptom dimensions (contamination and cleaning, taboo thoughts, doubt and checking, symmetry and ordering, and hoarding) using the approach recommended by Pinto and colleagues (2009). A neuroradiologist confirmed that all MRI scans were free of gross structural abnormalities.
2.2. MR data acquisition procedures
The neuroimaging consisted of a single scanning session lasting approximately 60 min and included structural MRI and MRS acquisitions. It was conducted on a research-dedicated General Electric 3.0T EXCITE MR system at the New York State Psychiatric Institute using a standard quadrature single-channel head coil.
2.2.1. Structural MRI
A three-plane, low-resolution, high-speed scout imaging series was obtained, followed by a series of high-resolution scans, consisting of standardized axial, coronal and sagittal T1-, T2-, and spin density-weighted scans that were appropriately obliqued for prescribing the 1H MRS voxels. A T1-weighted Spoiled Gradient-Recalled echo (SPGR) volumetric scan (TR/TE = 30/8 ms, flip angle 45°, field of view 24 cm, 256 × 256 matrix, 124 coronal slices and a slice thickness of 1.0 mm) was acquired for brain tissue segmentation.
2.2.2 1H MRSI data acquisition
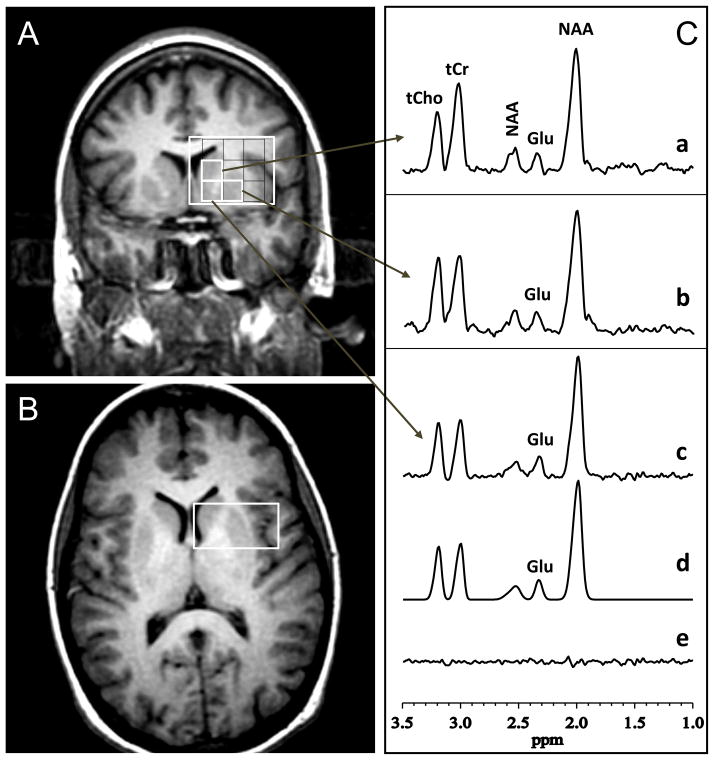
To obtain the levels of glutamate minimally contaminated by glutamine and GABA from subregions of the striatum, the multi-voxel or 1H MRS imaging (1H MRSI) version of the standard PRESS was implemented with TR 1500 ms, 20×20 phase-encoding steps, a spectroscopic imaging (SI) slice thickness of 2.0 cm, a field-of-view of 16 cm, and TE 80 ms, which was previously established to yield spectra in which the glutamate C-4 resonance at 2.35 ppm is minimally contaminated by the co-resonant glutamine C-4 peak (Mullins et al., 2008). The minimally contaminated glutamate levels thus derived will be referred to herein as simply glutamate. Within the SI slice (positioned coronally anterior to the anterior commissure), three individual voxels (each 0.8×0.8×2.0 cm3) were then positioned to contain the striatal subregions of interest (i.e., the left dorsal caudate, dorsal putamen, and ventral striatum), with positioning optimized for the dorsal caudate given our hypothesis. The positioning of these MRSI voxels within the PRESS-preselected volume of interest (VOI) is depicted in Figure 1 (panels A&B). The static magnetic field homogeneity within the VOI, which was automatically optimized by the scanner’s host computer, was typically 10–15 Hz as measured by the full-width at half maximum (FWHM) of the water resonance.
Figure 1.
Striatal Subregions and Associated Sample 1H MR Spectral Data. [A] Coronal and [B] axial localizer images showing the size and location of the volume of interest (large white box) pre-localized with the PRESS method. The spectroscopic imaging (SI) slice thickness was 20 mm, field of view 16 cm, with the slice positioned coronally anterior to the anterior commissure. The coronal view, [A], is overlaid with a grid of voxels that cover the striatum and its subregions. Within this grid, three individual voxels (0.8 × 0.8 × 2.0 cm3) were positioned to contain the dorsal caudate, dorsal putamen, and ventral striatum. The multi-voxel PRESS data set was acquired in 20 min using TE/TR 80/1500 ms, 20×20 phase-encoding (PE) steps, 2 excitations per PE. The arrows point to sample 1H spectra recorded from these three striatal voxels, labeled as follows in panel [C]: (a) dorsal caudate; (b) dorsal putamen; and (c) ventral striatum; Shown left-to-right in each spectrum are the resonances for total choline (tCho), total creatine (tCr), N-acetyl-L-aspartate (NAA, methylene), uncontaminated glutamate (Glu), and NAA (methyl); (d) A nonlinear least-squares best-fit spectrum obtained by fitting the experimental spectrum in (c) to a sum of pseudo-Voigt lineshape functions in the frequency domain; (e) The residuals of the difference between (d) and (e). The fitting procedure yields areas under the metabolite resonances, which are proportional to concentrations.
2.3. 1H MRS data processing and quantification
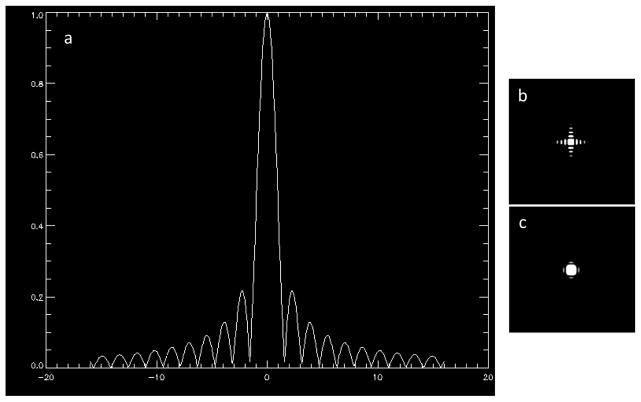
One objective of this study was to use the multivoxel or MRSI technique with a relatively high spatial resolution to measure glutamate levels simultaneously in striatal subregions (i.e., dorsal caudate, dorsal putamen, and ventral striatum). Using 20 × 20 phase-encoding steps, a field of view 16 cm and a slice thickness of 2 cm yielded 2D MRSI data in a “nominal” voxel size of 0.8 × 0.8 × 2.0 or 1.28 cm3, which was sufficiently small to fit within each of the striatal subregions as depicted in Figure 1 (panel A). There is a caveat, however. Rather than being perfectly rectangular, voxels obtained by phase-encoding have the shape of the point-spread function (PSF) illustrated in Figure 2 (Barker and Lin, 2006). Due to the limited or truncated number of phase-encoding steps, necessary to minimize scan time, the PSF is a sin(x)/x or sinc(x) function, with decaying sidebands (Figure 2a, b) that lead to contamination of signals inside the voxels with those from outside the voxel. While applying a smoothing filter (e.g., a Gaussian or Hamming function) to the PSF greatly reduces the sidebands (Figure 2c), this leads to a broadening of the voxel by ~40% (Barker and Lin, 2006). In this study, we opted to process all of our raw MRSI data without spatial filtering or PSF smoothing because levels of glutamate, the metabolite of interest, were relatively low and comparable across the three striatal subregions (Figure 1, panel C), and, under these conditions, cross-contamination would be negligible compared to what it would be if we broadened the voxels by ~40% with a spatial filter. To ensure optimal extraction of spectra from the three voxels of interest for post-processing, the MRSI grid was spatially shifted (Derby et al., 1989) as necessary.
Figure 2.
[a] Point spread function (PSF) for the spatial response of 16×16 linearly applied phase-encoding, showing the characteristic sin(x)/x or sinc(x) function with decaying sidebands due to the use of a limited or truncated number of phase-encoding steps.
[b] The PSF in [a] displayed as a 2D image scaled to 25% of maximum pixel intensity.
[c] The PSF in [b] shown after application of a Hamming filter. Note that the sidebands have been greatly reduced but at the expense of a ~40% increase in voxel size.
Details of the MRS data quality assessment criteria and procedures used in this study to retain or reject spectra for inclusion in group analyses are provided in the online supplement. For all the cases that fulfilled our quality assessment criteria, metabolite peak areas in each voxel were obtained using a robust and highly optimized public-domain Levenberg-Marquardt nonlinear least-squares IDL minimization routine (Markwardt, 2009) to model the resonances in the frequency domain as a linear combination of pseudo-Voigt functions, which enables more precise analysis of lineshapes that consist of mixtures of Lorentzian and Gaussian functions. The IDL fitting procedure automatically reports the covariance matrix for the estimated set of MRS parameters, from which the parameter correlation matrix, statistical errors at one standard deviation, and uncertainties are computed as measures of goodness of fit (Markwardt, 2009); we also assessed visually for each fit by examining the residual of the difference between the measured and best-fit spectra (Figure 1, panel C).
The spectral data were automatically corrected for susceptibility shifts due to slight variations in magnetic field strength across the brain, and then fitted in the frequency domain as described to obtain the peak area for glutamate. For normalization of levels across subjects, the glutamate peak areas were expressed in institutional units (i.u.) as ratios relative to the root-mean-square (rms) of the background noise in each voxel (i.e., as signal-to-noise ratios) – an approach that we have used previously in a number of publications (Kaufmann et al., 2004; Mathew et al., 2009; Weiduschat et al., 2014; Weiduschat et al., 2013).
2.4. Assessment of voxel tissue heterogeneity
Volumetric MRI-based tissue segmentation was performed to compare the proportions of gray matter (GM), white matter (WM), or CSF contained in the VOI. Segmentation and classification was based on the signal-intensity histogram obtained using the commercial software MEDx (Medical Numerics, Sterling, VA) from each subject’s volumetric (SPGR) MRI. From the histogram, a segmentation mask of each voxel was generated and the proportions of GM, WM, and CSF computed.
2.5. Statistical analyses
Demographic and clinical characteristics and proportion of tissue composition were compared across diagnostic groups using Fisher’s Exact or χ2 tests for categorical and independent sample t-tests for continuous variables. Independent t-tests were used to test for differences in total tissue glutamate levels between diagnostic groups. Based on findings from a study in unmedicated pediatric OCD patients (Rosenberg et al., 2000), we hypothesized that glutamate levels in the left dorsal caudate would be increased in adults with OCD compared to controls. Exploratory analyses examined group differences in glutamate levels in the left dorsal putamen and ventral striatum in subjects with viable MRS data in those regions as well. Within the OCD group, the relationship between glutamate levels in the striatal subregions (dorsal caudate, dorsal putamen, and ventral striatum) and OCD severity (measured by the Y-BOCS), age of OCD onset, and each of five OCD symptom dimensions (current contamination and cleaning, taboo thoughts, doubt and checking, symmetry and ordering, and hoarding) was examined using Pearson’s product-moment correlation. All statistical tests were 2-tailed with level of significance α= .05.
3. Results
3.1. Sample characteristics
The final sample consisted of 15 OCD and 16 healthy control subjects; an additional three subjects (1 healthy control and 2 OCD subjects) signed consent for the study but did not complete the MRI imaging. The demographic and clinical characteristics of subjects with viable MRS data are provided in Table 1. There were no significant group differences in age (t = −.24, df = 29, p = .81), sex (Fisher’s Exact Test, p =1.00), race (Caucasian versus non-Caucasian: χ2 = 0.06, df = 1, p =.81), or days since last menstrual period in females (t=0.54, df=19, p = .59).
Table 1.
Demographic and clinical characteristics
| Characteristics | OCD n=15 | Healthy control n=16 |
|---|---|---|
|
| ||
| Mean age in years (SD) | 30 (9) | 30 (8) |
|
| ||
| Number of males/females | 10/5 | 11/5 |
|
| ||
| Race-ethnicity | 6AA/5Cau/3His/1O | 6AA/6Cau/3His/1O |
|
| ||
| Mean age of OCD onset in years (SD) | 16 (10) | - |
|
| ||
| OCD in first degree relative (by subject self-report) | 4 | 0 |
|
| ||
| Mean Y-BOCS (SD) | 26 (3) | - |
|
| ||
| Mean HAM-D (SD) | 3 (4) | - |
|
| ||
| DSM-IV axis I psychiatric comorbidity: | ||
| None | 10 | 16 |
| Current specific or social phobia only | 2 | 0 |
| Current specific or social phobia with past | 2 | 0 |
| MDD | 0 | 0 |
| Past MDD only | 1 | 0 |
| History of substance or alcohol abuse/dependence | 0 | 0 |
|
| ||
| Current or past tic disorder | 0 | 0 |
|
| ||
| Treatment history: | ||
| Any prior exposure to psychotropic medications | 4 | 0 |
| SRIs | 3 | 0 |
| Mean wks (SD, range) since last SRI dose | 86 (61, 46–156) | 0 |
| Other medications (wks since last dose) | 1 (18) | 0 |
| Prior exposure therapy | 1 | 0 |
Abbreviations: AA, African-American; Cau, Caucasian; HAM-D, Hamilton Depression Rating Scale (17-item); HIS, Hispanic; MDD, major depressive disorder; O, other; OCD, obsessive-compulsive disorder; SD, standard deviation; SRI, serotonin reuptake inhibitor; wks, weeks; Y-BOCS, Yale-Brown Obsessive Compulsive Scale
The OCD subjects had clinically significant symptoms, with a mean Y-BOCS score of 26. All five OCD symptom dimensions were represented, with most subjects exhibiting symptoms in more than one domain. Three had symptoms primarily in one domain (contamination and cleaning symptoms, n=2; symmetry and ordering, n=1). As shown in Table 1, 11 of the 15 OCD subjects had never taken any psychotropic medications. Of the four ever exposed to psychotropic medications, all were free of them for at least 18 weeks. Only one OCD subject (one of those exposed to medication) had ever received CBT consisting of exposure and response prevention. None were receiving treatment at the time of MRS scanning.
Measures of tissue segmentation are provided in Table 2 for the three voxels. There were no significant group differences in tissue composition in these three voxels (dorsal caudate: all p-values > .46; dorsal putamen: all p-values > .25; ventral striatum: all p-values > .35).
Table 2.
Tissue segmentation in striatal subregions
| OCD (n=15) | Healthy control (n=16) | 95% CI for difference in means | |
|---|---|---|---|
|
| |||
| Tissue composition | Mean percent (SD) | Mean percent (SD) | |
|
| |||
| Dorsal caudate | |||
| CSF | 6.04 (4.28) | 6.36 (5.77) | −3.43, 4.08 |
| Gray matter | 62.24 (7.39) | 63.99 (5.83) | −3.12, 6.62 |
| White matter | 31.70 (9.95) | 29.63 (8.62) | −8.91, 4.75 |
|
| |||
| Dorsal putamen | |||
| CSF | 0.05 (0.05) | 0.15 (0.33) | −0.08, 0.28 |
| Gray matter | 62.74 (6.35) | 61.84 (5.96) | −5.42, 3.63 |
| White matter | 37.20 (6.34) | 38.01 (6.02) | −3.74, 5.34 |
|
| |||
| Ventral striatum | |||
| CSF | 0.03 (0.04) | 0.07 (0.15) | −0.04, 0.12 |
| Gray matter | 42.67 (8.29) | 42.91 (6.47) | −5.20, 5.68 |
| White matter | 57.30 (8.29) | 57.02 (6.49) | −5.72, 5.18 |
Abbreviations: CI, confidence interval; CSF, cerebral spinal fluid; OCD, obsessive-compulsive disorder; SD, standard deviation
3.2. Glutamate levels in striatal subregions
In the left dorsal caudate, glutamate levels did not significantly differ by group (t=−.80, df=29, p=.43). There also were no significant group differences in the left dorsal putamen (t=.93, df=23, p=.36) or the left ventral striatum (t=.70, df=25, p=.49). The observed mean values and 95% confidence intervals of the difference in the means by diagnostic group are provided in Table 3. Glutamate levels across the three striatal regions were not significantly correlated with each other (p-values > .47).
Table 3.
Striatal levels of glutamate minimally contaminated by glutamine and γ-aminobutyric acid
| OCD (n=15)a | Healthy control (n=16)a | 95% CI for difference in means | |
|---|---|---|---|
|
| |||
| Striatal subregions | Mean (SD), i.u. | Mean (SD), i.u. | |
|
| |||
| Dorsal caudate | 5.30 (1.60) | 4.75 (2.20) | −1.98, 0.86 |
| Dorsal putamen | 6.22 (2.05) | 7.10 (2.63) | −1.08, 2.85 |
| Ventral striatum | 4.70 (2.50) | 5.35 (2.38) | −1.28, 2.59 |
Abbreviations: CI, confidence interval; i.u., Institutional units; SD, standard deviation
Samples sizes were smaller for the dorsal putamen (12 OCD, 13 healthy control) and the ventral striatum (13 OCD, 14 healthy control).
The rms of the background noise used for glutamate normalization was found to have outlier values (more than three standard deviations from the mean) for several subjects. To assess the sensitivity of the results to these outliers, subjects meeting this criterion were removed and the analyses rerun on the remaining 10 healthy controls and 13 OCD subjects. This re-analysis did not alter the results: glutamate levels did not significantly differ by group (dorsal caudate: t=− .49, df=21, p=.62; dorsal putamen: t=1.94, df=20, p=.07; ventral striatum: t=1.39, df=21, p=.18).
3.3. Clinical correlations
In the OCD sample, glutamate levels in left striatal subregions were not significantly correlated with OCD severity (p-values: dorsal caudate > .54; dorsal putamen > .29; ventral striatum > .48), age of OCD onset (p-values: dorsal caudate > .38; dorsal putamen > .60; ventral striatum > .09), or any of the five symptom dimensions (all p-values: dorsal caudate > .13; dorsal putamen >.16; ventral striatum >.24; except for symmetry and ordering [r=−.51, p=.07]).
4. Discussion
We compared glutamate levels in striatal subregions in unmedicated adults with OCD with those in matched healthy controls using MRS methods designed to measure glutamate with minimal glutamine and GABA contamination. Contrary to our hypothesis, we did not find glutamate abnormalities in the left dorsal caudate in unmedicated adults with OCD; there were no significant associations between glutamate levels and OCD severity, age of OCD onset, or OCD symptom dimensions. There also were no significant group differences in glutamate levels in the left dorsal putamen or ventral striatum.
The lack of evidence for striatal glutamate abnormalities in OCD agrees with two prior studies in unmedicated patients, including one in 11 treatment-naïve pediatric patients using a 1.5T instrument (Lazaro et al., 2012) and another in 13 unmedicated adults using a 4.0T instrument (Bartha et al., 1998b). However, the voxel in both studies straddled the caudate, putamen, and internal capsule. In contrast, our study measured minimally contaminated glutamate simultaneously in three striatal subregions (i.e., dorsal caudate, dorsal putamen, ventral striatum) in unmedicated adults with OCD; this is important since these striatal subregions receive glutamatergic projections from different cortical regions (Haber and McFarland, 1999), and since medications can change MRS glutamatergic findings (Rosenberg et al., 2000). On the other hand, our results disagree with a single-voxel MRS study in 11 psychotropic drug-naïve pediatric OCD patients (Rosenberg et al., 2000) that also achieved a relatively high spatial resolution and found increased glutamatergic compound levels in the left caudate compared to matched controls at baseline; in their study, these abnormalities normalized with successful treatment, supporting the glutamatergic hypothesis of OCD.
Several factors could explain the discrepancy with this study and ours, even though both sampled striatal subregions. First, we examined subjects 19 years and older, whereas the other study examined subjects ages 8 to 17. Thus, the discrepancy could reflect age-dependence in glutamate abnormalities in OCD. Second, the prior 1H MRS study was conducted on a 1.5 T instrument, which did not enable the discrimination of glutamate from glutamine and GABA; this could yield confounding glutamatergic compound levels if GABA levels were altered, as we previously found in another brain region (Simpson et al., 2012). Finally, the samples could have differed in important clinical characteristics, such as symptom dimensions, comorbidity, or genetic variation that might influence brain glutamatergic systems (Arnold et al., 2009).
This is the first study targeting unmedicated adults with OCD to use a MRSI method designed specifically to measure minimally contaminated glutamate and to examine three different striatal subregions (dorsal caudate, dorsal putamen, ventral striatum). However, the relatively small sample size and the inherent inability of MRS to directly measure glutamatergic neurotransmission are significant limitations. Moreover, while the MRSI method implemented in this study minimized the contamination of glutamate by glutamine and GABA, it did not completely eliminate it (Mullins et al., 2008).
In summary, this study found no evidence of significant striatal glutamatergic abnormalities in unmedicated adults with OCD. If replicated, this would require revisions to the glutamatergic hypothesis of OCD. Future studies in larger samples are warranted to confirm the present findings in adult OCD patients and to determine whether glutamatergic abnormalities in OCD occur only in pediatric OCD or in subjects with gene variants that might influence brain glutamatergic systems.
Supplementary Material
Acknowledgments
We thank members of the Anxiety Disorders Clinic for help with this study, including expert administrative support (Ms. Donna Vermes), clinical assessments (Drs. James Bender, Raphael Campeas, Carolyn Rodriguez), subject coordination (Mr. Jose Hernandez), and manuscript preparation (Ms. Ashley Greene, Ms. Olivia Pascucci). We thank the subjects for participating.
This study was supported by a NARSAD Independent Investigator Award to HBS (with HBS named the NARSAD Dylan Tauber Investigator). Additional support came from NIMH (K24 MH091555 [HBS]), and the New York State Office of Mental Health.
In the last three years, Dr. Simpson has received research funds from Janssen Pharmaceuticals and Transcept Pharmaceuticals, consulting fees from Quintiles Inc, and royalties from UpToDate, Inc and Cambridge University Press; Dr. Slifstein has received research funds from Forest Laboratories, Otsuka, and Pierre-Fabre Inc., and consulting fees from Amgen; Dr. Kegeles has received research funds from Amgen and Pfizer Inc. None of the other authors have financial disclosures to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold PD, MacMaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, Mirza Y, Easter PC, Rose M, Kennedy JL. Glutamate receptor gene (< i> GRIN2B</i>) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive–compulsive disorder. Psychiatry Research: Neuroimaging. 2009;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PB, Lin DD. In vivo proton MR spectroscopy of the human brain. Progress in Nuclear Magnetic Resonance Spectroscopy. 2006;49:99–128. [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, Canaran G, Densmore M, Anderson G, Siddiqui AR. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. American Journal of Psychiatry. 1998a;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, Canaran G, Densmore M, Anderson G, Siddiqui AR. A short echo -sup-1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. American Journal of Psychiatry. 1998b;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Rauch SL, Jensen JE, Pope HG., Jr A Critical Review of Magnetic Resonance Spectroscopy Studies of Obsessive-Compulsive Disorder. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby K, Hawryszko C, Tropp J. Baseline deconvolution, phase correction, and signal quantification in Fourier localized spectroscopic imaging. Magnetic resonance in medicine. 1989;12:235–240. doi: 10.1002/mrm.1910120209. [DOI] [PubMed] [Google Scholar]
- Ebert D, Speck O, Konig A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Research. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Archives of General Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Annals of the New York Academy of Sciences. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Next-generation treatments for mental disorders. Science Translational Medicine. 2012;4:155ps119–155ps119. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Shungu D, Sano M, Jhung S, Engelstad K, Mitsis E, Mao X, Shanske S, Hirano M, DiMauro S. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;62:1297–1302. doi: 10.1212/01.wnl.0000120557.83907.a8. [DOI] [PubMed] [Google Scholar]
- Lazaro L, Bargallo N, Andres S, Falcon C, Morer A, Junque C, Castro-Fornieles J. Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: longitudinal study before and after treatment. Psychiatry Research. 2012;201:17–24. doi: 10.1016/j.pscychresns.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Markwardt C. Non-linear least squares fitting in IDL with MPFIT. Proceedings of Astronomical Data Analysis Software and Systems XVIII 2008. Vol 411. Astronomical Society of the Pacific; San Francisco, CA. 2009. pp. 251–254. The IDL fitting routine, ‘MPFIT’, is available at http://purl.com/net/mpfit; Last modified on 2011-2012-2021 by Craig Markwardt. [Google Scholar]
- Mathew SJ, Mao X, Keegan KA, Levine SM, Smith EL, Heier LA, Otcheretko V, Coplan JD, Shungu DC. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: An in vivo 3.0 T 1H MRS imaging study. NMR in Biomedicine. 2009;22:251–258. doi: 10.1002/nbm.1315. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in cognitive sciences. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magnetic Resonance in Medicine. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Piacentini JC, Chang S, Levitt JG, Rozenman M, Bergman L, Salamon N, Alger JR, McCracken JT. MRSI correlates of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36:161–168. doi: 10.1016/j.pnpbp.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Murphy DL, Nestadt G, Rasmussen SA. Using individual items to clarify OCD symptom structure: the case for five factors. American Journal of Psychiatry. 2009;166:728–729. doi: 10.1176/appi.ajp.2009.09020287. author reply 729–731. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacology and Therapeutics. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Potter W, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster F, McKinzie D, Felder C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. American Journal of Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, Kegeles LS. Investigation of Cortical Glutamate-Glutamine and gamma-Aminobutyric Acid in Obsessive-Compulsive Disorder by Proton Magnetic Resonance Spectroscopy. Neuropsychopharmacology. 2012;37:2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jonsson L, Lundberg S, Ivarsson T, Ribbelin S, Ekholm S, Carlsson A, Forssell-Aronsson E, Carlsson ML. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. Journal of Neural Transmission. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Kaufmann P, Mao X, Engelstad KM, Hinton V, DiMauro S, De Vivo D, Shungu D. Cerebral metabolic abnormalities in A3243G mitochondrial DNA mutation carriers. Neurology. 2014;82:798–805. doi: 10.1212/WNL.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiduschat N, Mao X, Beal MF, Nirenberg MJ, Shungu DC, Henchcliffe C. Usefulness of Proton and Phosphorus MR Spectroscopic Imaging for Early Diagnosis of Parkinson’s Disease. Journal of Neuroimaging. 2013 doi: 10.1111/jon.12074. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Archives of General Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Abramowitz JS, Port JD. The effect of behavior therapy on caudate N-acetyl-l-aspartic acid in adults with obsessive-compulsive disorder. Psychiatry Research. 2012;201:10–16. doi: 10.1016/j.pscychresns.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Research. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.