Abstract
In addition to its high affinity for antibody Fc domains, staphylococcal Protein A has been shown to bind certain Fab domains. We investigated this in order to develop a small, recombinant Protein A-binding alternative to IgG from nanobodies, single-domain antibodies derived from a camelid variant IgG’s variable region. We engineered a nanobody with affinity solely for Protein A, as well as a dimerized version of higher affinity for typical multi-domain Protein A constructs. As this recombinant nanobody can be immobilized using a cleavable crosslinker, it has proven suitable for the isolation and mild elution of protein complexes in native conditions.
Keywords: Protein A, ProA, affinity isolation, immunoprecipitation, nanobody, native elution
Staphylococcal Protein A (ProA or PrA) and its derivatives continue to be widely used biological tools, due to their high affinity for the Fc domain of many antibodies. It is a commonly used protein tag, with multiple copies of its five homologous antibody-binding domains often used to increase overall avidity to IgG binding partners. Curiously, in addition to its well-known affinity for the Fc region, PrA has been found to bind certain immunoglobulins through their Fab domains using a distinct binding mechanism [1; 2]. It has similarly been observed that recombinant nanobodies, which are derived from camelid heavy chain-only VHH antibody variants, can sometimes bind PrA through their Fab-like single variable domain [3]. In their typical role as antigen binding proteins, nanobodies have proven to be valuable reagents in various proteomic and cell biological applications [4; 5; 6]. These small recombinant proteins can bind antigens with high affinity, and their versatility and ease of production have made them a valuable and accessible tool for many investigators [7].
To date, standard purified IgG protein has been the reagent of choice for isolating PrA-tagged complexes [8; 9], recently supplemented by an engineered high affinity affibody [10]. However, the heterogeneous nature of IgG preparations, high molecular weight, lot-to-lot variation, and cost make these less than ideal for many applications, particularly in proteomics. Additionally, we have found that any particular recombinant protein used for affinity binding of a single target can display unpredictable off-target binding in certain systems, making it valuable to have more than one available option [11]. Given the well-established ease of use and versatility of nanobodies, we sought to generate such a reagent with high affinity for PrA.
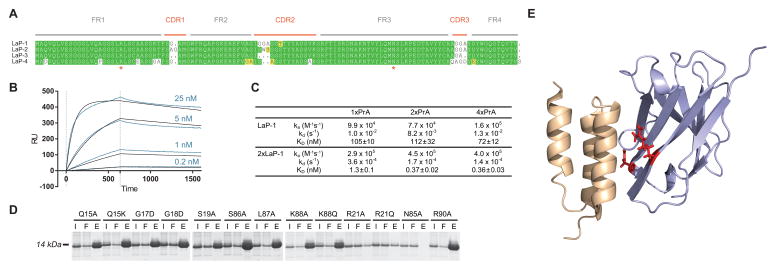
In the course of generating nanobody repertoires against various antigens, we observed that approximately half of identified nanobody clones bound robustly to PrA, consistent with previous studies [3; 11]. Sequence alignments of PrA binders revealed highly conserved framework regions. These sequences all contained CDR loops specific for their original antigen, however, and to generate a nanobody with no extraneous binding activity, we synthesized four sequence variants derived from these consensus sequences with minimal or absent CDR loops (Fig. 1A). Once synthesized and expressed in E. coli with a periplasmic leader sequence, all four proteins (termed llama antibody against Protein A, or LaP 1–4) showed high levels of overexpression, and high solubility after periplasmic purification. These crude periplasm preparations were incubated with PrA-Sepharose, and all constructs displayed high affinity, particular LaP-1, which was selected for follow-up studies (Supplementary Fig. 1A). In all cases, no detectable binding was observed for the original antigen (data not shown).
Figure 1.
Design and characterization of PrA nanobodies. (A) Highly conserved regions from multiple identified nanobody sequences were used as a framework for four engineered nanobodies against PrA (LaP-1–4), with varying minimal linkers used in place of existing CDR regions. Asterisks: residues whose mutation eliminates PrA binding. (B) SPR sensorgrams are shown for injections of multiple concentrations of 2xLaP-1 nanobody over immobilized 4xPrA. Curves fit from a Langmuir model are shown in black. (C) Binding constants determined by SPR for either LaP-1 nanobody or 2xLaP-1 dimerized nanobody against immobilized 1xPrA, 2xPrA, or 4xPrA. Corresponding association rates (ka), dissociation rates (kd), and dissociation constants (Kd) are shown. (D) LaP-1 nanobodies with the designated point mutant were expressed in bacteria, and periplasmic extracts were incubated with PrA-Sepharose. For each mutant, input (I), flow-through (F), and elution (E) samples from the Sepharose binding were run by SDS-PAGE and Coomassie-stained. (E) The PrA:LaP-1 interaction was modeled using I-TASSER, via LaP-1’s homology to Fab in a PrA:Fab crystal structure (PDB ID: 1DEE). The structure was visualized in PyMOL. Grey: LaP-1; Beige: PrA; Red: R21 and N85 residues, which are required for PrA binding.
Surface plasmon resonance (SPR) analysis was performed to determine the binding kinetics of LaP-1’s interactions with multiple recombinant PrA constructs, containing 1, 2, or 4 repeats of the IgG-binding domain (Fig. 1C and Supplementary Fig. 2). Regardless of the number of PrA repeats, LaP-1 bound these proteins with a KD of 70–120 nM. While suitable for many applications, it was reasoned that this affinity could be increased by generating a homodimeric form of the nanobody. Two copies of the LaP-1 sequence were therefore fused using a glycine-rich peptide linker (3 repeats of GGGGS). As the LaP-1 monomer is only 13 kDa, even this dimerized form remains a relatively small 27 kDa. After purification, the PrA affinities of this 2xLaP-1 fusion protein were similarly assessed by SPR (Fig. 1B, 1C and Supplementary Fig. 2). When binding to 2xPrA or 4xPrA constructs, the affinity of the dimer was more than 300-fold stronger than the LaP-1 monomer, with a KD of 360–370 pM.
Given the structural similarity of the nanobody variable region to other mammalian Fab fragments, it was hypothesized that the LaP-1 nanobodies interacted with PrA through an analogous binding surface, rather than an Fc-like binding mechanism [1; 12]. To test this, mutagenesis was done across the homologous sequences corresponding to this binding region (Fig. 1D) [13]. Mutations in two residues, R21 and N85, eliminated PrA binding. These are both present in the homologous binding region, and expected to be necessary for PrA binding via an Fab-like interaction. A model of the proposed PrA:LaP-1 interaction was also generated via homology to a PrA:Fab crystal structure (PDB ID: 1DEE) [13] using the program I-TASSER [14; 15; 16], and is consistent with the mutagenesis results (Fig. 1E).
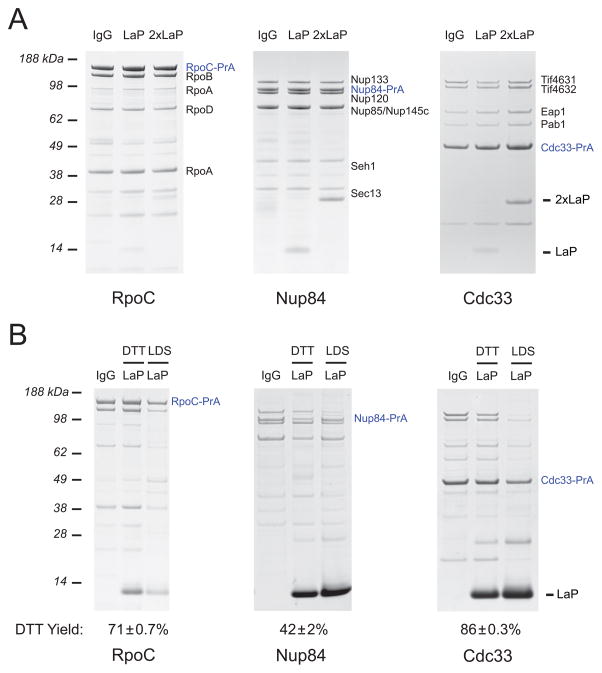
To investigate the specificity and versatility of these anti-PrA nanobodies, we assessed their effectiveness in ex vivo affinity isolations of PrA-tagged protein complexes from yeast and bacteria. LaP-1 and 2xLaP-1 proteins were conjugated to magnetic beads and used to isolate tagged RNA polymerase from E. coli [17], the S. cerevisiae Nup84 subcomplex of the nuclear pore complex (NPC) [18; 19] and mRNA cytoplasmic cap binding complex [20] (Fig. 2A). In all cases, both the monomeric and dimeric LaP-1 proteins were able to efficiently isolate the targeted complex with yield and purity comparable to control affinity isolations with polyclonal IgG, and negligible non-specific binding or contamination. The tandem affinity purification (TAP) tag is a frequently used PrA alternative, containing two artificial PrA Z domains [21]. However, consistent with other studies showing weak binding between this Z domain and Fab fragments [1; 22], our LaP-1 proteins are only able to recover very limited amounts of TAP-tagged protein in affinity isolations (Supplementary Fig. 1B). The PrA Z domains in the TAP tag have only a single glycine to alanine point mutation however, so binding should be restored by reverting this mutation.
Figure 2.
Affinity isolations performed with LaP-1 nanobodies. (A) LaP-1, 2xLaP-1, or rabbit IgG were conjugated to epoxy-activated magnetic beads and used to isolate E. coli RpoC-PrA (an RNA polymerase subunit), as well as S. cerevisiae Nup84-PrA (an NPC subcomplex component) and Cdc33-PrA (a protein in the mRNA cytoplasmic cap binding complex). Bands previously determined by MS are labeled. (B) LaP-1 was reversibly immobilized to magnetic beads using SPDP crosslinker, and used for affinity isolations as in (A). Bound protein was first eluted in 25 mM DTT (DTT), before release of remaining bound protein in LDS (LDS). The yield of protein recovered in the DTT elution step, as compared to total eluted protein, is listed with the s.e.m. All experiments were performed in duplicate.
In addition to these original LaP-1 proteins, we generated constructs containing an additional free cysteine at the C-terminus. Thiol chemistry can thus be targeted to the C-terminus, which allowed us to reversibly immobilize LaP-1 to magnetic amine-coated Dynabeads using an N-Succinimidyl 3-(2-pyridyldithio)-propionate (SPDP) crosslinker with a 12 unit PEG spacer. This produces a disulfide-containing crosslink to the bead, which is easily cleavable in mild reducing conditions [23; 24]. Beads with LaP-1 immobilized in this manner were tested in isolations of RNA polymerase, Nup84, and Cdc33 as before. Again, yield and purity comparable to IgG was observed, and by a short incubation with 25 mM DTT, 42–86% of the isolated protein could be eluted (Fig. 2B). This level of elution efficiency is comparable to that seen in other native methods of PrA release [21; 25]. The complex-to-complex difference in yield is also a consistent phenomenon, likely due to differences in interactions with the bead surface. This approach does necessarily lead to release of both PrA-associated and unassociated LaP-1 nanobody in the elution. Due to the small size (13 kDa) of LaP-1 however, an additional purification step, such as gradient centrifugation, which is often necessary for sensitive downstream applications like electron microscopy, can easily remove unbound nanobody.
In either monomeric or dimeric form, the LaP-1 nanobody is suitable for highly efficient isolation of PrA-tagged protein targets. As a small recombinant protein, it is an especially flexible reagent, as shown in its use in native elutions of protein complexes, making it a convenient option for any application making use of a PrA-based tag.
Supplementary Material
Acknowledgments
We acknowledge support from NIH grants U54 GM103511 and P41 GM109824. We thank members of the Rout and B. Chait labs for helpful discussions and assistance, particularly Y. Li, H. Jiang, J. LaCava, and B. Chait.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jansson B, Uhlen M, Nygren PA. All individual domains of staphylococcal protein A show Fab binding. FEMS immunology and medical microbiology. 1998;20:69–78. doi: 10.1111/j.1574-695X.1998.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 2.Sasso EH, Silverman GJ, Mannik M. Human IgA and IgG F(ab′)2 that bind to staphylococcal protein A belong to the VHIII subgroup. Journal of immunology. 1991;147:1877–83. [PubMed] [Google Scholar]
- 3.Frenken LG, van der Linden RH, Hermans PW, Bos JW, Ruuls RC, de Geus B, Verrips CT. Isolation of antigen specific llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. Journal of biotechnology. 2000;78:11–21. doi: 10.1016/s0168-1656(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 4.Ries J, Kaplan C, Platonova E, Eghlidi H, Ewers H. A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nature Methods. 2012;9:582–4. doi: 10.1038/nmeth.1991. [DOI] [PubMed] [Google Scholar]
- 5.Rothbauer U, Zolghadr K, Tillib S, Nowak D, Schermelleh L, Gahl A, Backmann N, Conrath K, Muyldermans S, Cardoso MC, Leonhardt H. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods. 2006;3:887–9. doi: 10.1038/nmeth953. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Muyldermans S, Saerens D. Nanobodies(R): proficient tools in diagnostics. Expert review of molecular diagnostics. 2010;10:777–85. doi: 10.1586/erm.10.62. [DOI] [PubMed] [Google Scholar]
- 7.Muyldermans S. Nanobodies: Natural Single-Domain Antibodies. Annual Review of Biochemistry. 2013;82:775–97. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 8.Moks T, Abrahmsen L, Nilsson B, Hellman U, Sjoquist J, Uhlen M. Staphylococcal protein A consists of five IgG-binding domains. European journal of biochemistry / FEBS. 1986;156:637–43. doi: 10.1111/j.1432-1033.1986.tb09625.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindmark R, Thoren-Tolling K, Sjoquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. Journal of immunological methods. 1983;62:1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 10.Lindborg M, Dubnovitsky A, Olesen K, Bjorkman T, Abrahmsen L, Feldwisch J, Hard T. High-affinity binding to staphylococcal protein A by an engineered dimeric Affibody molecule. Protein engineering, design & selection : PEDS. 2013;26:635–44. doi: 10.1093/protein/gzt038. [DOI] [PubMed] [Google Scholar]
- 11.Fridy PC, Li Y, Keegan S, Thompson MK, Nudelman I, Scheid JF, Oeffinger M, Nussenzweig MC, Fenyo D, Chait BT, Rout MP. A robust pipeline for rapid production of versatile nanobody repertoires. Nat Methods. 2014;11:1253–60. doi: 10.1038/nmeth.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nature structural biology. 1996;3:803–11. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 13.Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, Silverman GJ. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5399–404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–38. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy A, Yang J, Zhang Y. COFACTOR: an accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res. 2012;40:W471–7. doi: 10.1093/nar/gks372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westblade LF, Minakhin L, Kuznedelov K, Tackett AJ, Chang EJ, Mooney RA, Vvedenskaya I, Wang QJ, Fenyo D, Rout MP, Landick R, Chait BT, Severinov K, Darst SA. Rapid isolation and identification of bacteriophage T4-encoded modifications of Escherichia coli RNA polymerase: a generic method to study bacteriophage/host interactions. Journal of proteome research. 2008;7:1244–50. doi: 10.1021/pr070451j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brohawn SG, Partridge JR, Whittle JR, Schwartz TU. The nuclear pore complex has entered the atomic age. Structure. 2009;17:1156–68. doi: 10.1016/j.str.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Martinez J, Phillips J, Sekedat MD, Diaz-Avalos R, Velazquez-Muriel J, Franke JD, Williams R, Stokes DL, Chait BT, Sali A, Rout MP. Structure-function mapping of a heptameric module in the nuclear pore complex. The Journal of Cell Biology. 2012;196:419–34. doi: 10.1083/jcb.201109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyer C, Altmann M, Trachsel H, Sonenberg N. Identification and characterization of cap-binding proteins from yeast. The Journal of biological chemistry. 1989;264:7603–10. [PubMed] [Google Scholar]
- 21.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nature biotechnology. 1999;17:1030–2. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 22.Ljungberg UK, Jansson B, Niss U, Nilsson R, Sandberg BE, Nilsson B. The interaction between different domains of staphylococcal protein A and human polyclonal IgG, IgA, IgM and F(ab′)2: separation of affinity from specificity. Molecular immunology. 1993;30:1279–85. doi: 10.1016/0161-5890(93)90044-c. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson J, Axen R, Unge T. Reversible, covalent immobilization of enzymes by thiol-disulphide interchange. European journal of biochemistry / FEBS. 1975;59:567–72. doi: 10.1111/j.1432-1033.1975.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 24.Kiefer H. Separation of antigen-specific lymphocytes. A new general method of releasing cells bound to nylon mesh. European journal of immunology. 1975;5:624–8. doi: 10.1002/eji.1830050909. [DOI] [PubMed] [Google Scholar]
- 25.LaCava J, Chandramouli N, Jiang H, Rout MP. Improved native isolation of endogenous Protein A-tagged protein complexes. BioTechniques. 2013;54:213–6. doi: 10.2144/000114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.