Abstract
Purpose
Intermittent androgen deprivation therapy (IADT) for patients with PSA progression after treatment for localized prostate cancer is an alternative to the standard continuous ADT. IADT allows for the recovery of testosterone during off-cycles to stimulate regrowth and differentiation of the regressed prostate tumor in order to lessen the side effects of continuous ADT and potentially prolong survival. Previously, IADT coupled with finasteride was shown to prolong survival of animals bearing androgen-sensitive prostate tumors when off-cycle duration was not prolonged and fixed at 10–14 days. Regressed prostate tumor xenografts with testosterone replacement were initially responsive to 5α-reductase inhibition, but resumed growth after several days in the animal models. 5α-reductase inhibition in shorter off-cycles of testosterone recovery could maximize tumor growth inhibition during IADT and perhaps increase survival.
Materials and Methods
The LNCaP xenograft tumor model was utilized to evaluate the effectiveness of short off-cycles of 4 days coupled with 5α-reductase inhibition on IADT on survival and tumor regrowth.
Results
Dutasteride inhibited initial testosterone-induced tumor regrowth during both the first and second off-cycle and significantly increased survival.
Conclusions
These results further support the potential for IADT combined with 5α-reductase inhibition to improve survival in prostate cancer patients when off cycle durations are short or very short.
Keywords: Prostate Cancer, Intermittent Androgen Deprivation Therapy, 5α-reductase inhibition, LNCaP
INTRODUCTION
Androgen deprivation therapy (ADT) is the standard treatment for patients with advanced prostate caner 1–4. ADT is initially quite effective, however most patients develop resistance 5, 6. Additionally ADT is associated with known side effects, including cognitive and sexual dysfunction, anemia, hot flashes, endocrine abnormalities and metabolic syndrome, cardiovascular disease, and loss of bone mineral density and muscle mass 7–10. Intermittent androgen deprivation therapy (IADT), which allows for periods of intermittent testosterone recovery, was originally developed with the intention to prolong tumor androgen dependence. Recovery of testosterone levels can restore the apoptotic potential of prostate tumor cells resulting in delay of tumor progression to castration resistance 11–14. IADT consists of multiple cycles of androgen suppression, termed “on-cycle” where prostate tumors undergo regression, followed by a period of testosterone recovery and tumor regrowth, or “off-cycle” 9, 15. In advanced prostate cancer, several studies have reported that IADT results in significant improvement in quality of life while achieving survival comparable to that observed in patients on continuous ADT 14, 16–20. Two large non-inferiority phase III trials compared continuous ADT to IADT in men with rising PSA, without evidence of metastases after primary or salvage radiation following prostatectomy (NCIC PR-7) and in men with newly diagnosed hormone naïve, prostate adenocarcinoma (SWOG 9346). In the PR-7 study, which evaluated 1386 men with biochemical recurrence, established that overall survival for men on the IADT arm was not-inferior to men on the continuous ADT arm 16. There was a non-statistically significant trend for improved overall survival in the continuous ADT arm for patients with high Gleason score (8–10) in a post-hoc subset analysis. Urinary and sexual side-effects and hot-flashes were significantly better in the IADT arm on PR-7. The multi-institutional SWOG 9346 study was inconclusive on whether IADT is non-inferior to continuous ADT in 1535 patients with metastatic hormone-naive prostate adenocarcinoma 21. A non-planned subset analysis by extent of disease (minimal vs. extensive disease) showed reduced overall survival (5.4 versus 6.9 years) for IADT compared to continuous ADT in the minimal disease group. In the sub-group with extensive disease, there was no statistically-significant difference in overall survival for the IADT versus continuous ADT arms (4.9 years versus 4.4 years respectively). The PR-7 study demonstrated non-inferiority of IADT in non-metastatic disease, whereas the SWOG 9346 study failed to demonstrate non-inferiority of IADT compared to ADT in patients with hormone naïve metastatic prostate adenocarcinoma. Cumulative assessment of results from these phase III studies suggest that particularly for patients with non-metastatic advanced prostate cancer with a biochemical recurrence, IADT has fewer side effects and better quality of life and offers non-inferior survival to ADT.
In a recent review, we discussed the potential survival benefit of 5α-reductase inhibition in IADT in animal models and the potential translation of this finding into clinic 22. 5α-reductase inhibitors (5ARIs) such as dutasteride or finasteride could enhance prostatic cell differentiation, reduce cell proliferation and delay prostate tumor progression. We have previously investigated the impact of finasteride or dutasteride on the efficacy of IADT in animal models. Our initial studies showed that addition of finasteride to the off-cycle increased survival over IADT alone in LNCaP xenografts when the off-cycle interval was fixed 8. Subsequent studies showed that finasteride doubled the off-cycle interval in IADT, when the off-cycle was terminated based on tumor volume 23. However, prolongation of the off-cycle in the presence of finasteride did not translate into a survival benefit 23. Similarly, in a retrospective clinical study, Scholz et al., reported that use of finasteride during the off-cycle doubled its duration from a median of 15 months to a median of 31 months with no effect on prostate cancer progression to castration resistance, when off-cycle termination was based on serum PSA 20. One important and clinically relevant question is whether time to castrate resistant prostate cancer and eventually disease-specific overall survival can be prolonged by 5ARI treatment in human patients on IADT when off-cycle duration is fixed in a manner similar to that in the animal model.
Recently, we demonstrated that 5ARI treatment profoundly inhibited the initial phase of prostate tumor regrowth after testosterone replacement suggesting that regressed tumors behave differently from intact tumors in response to testosterone in the presence of 5ARIs. Finasteride and dutasteride treatment during the off-cycle of IADT inhibited initial regrowth of regressed xenograft tumors and increased expression of androgen-responsive genes compared to IADT alone 24. This study showed that early testosterone-induced regrowth of regressed prostate tumors was susceptible to 5ARI treatment, but not after extended exposure to androgens. Short off-cycle intervals combined with 5ARI treatment could stimulate the differentiation of prostate tumor cells while minimizing proliferation. We used dutasteride for our experiments since it inhibits both type I and II 5ARI and has a longer half-life compared to finasteride, which inhibits type II 5ARI. In the current study, we extended our findings to examine the potential for IADT in the presence of 5ARI dutasteride to prolong survival in the LNCaP xenograft tumor model specifically when off cycle durations are very short.
MATERIALS AND METHODS
Animals
BALB/c strain of athymic SCID mice were obtained from Charles River Laboratory, Wilmington, MA, USA and were kept in accordance with the National Institute of Health guidelines under standard animal housing conditions for the Care and Use of Experimental Animals. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and were conducted in strict accordance with the standards for humane animal care and use as set by the Animal Welfare Act and the National Institutes of Health guidelines for the use of laboratory animals.
Xenograft tumor implantation
LNCaP cells were obtained from American Type Culture Collection. LNCaP cells were maintained in RPMI, supplemented with 10% fetal bovine serum (FBS), glutamine, penicillin and streptomycin. LNCaP cells underwent 4–8 passages in culture prior to mouse inoculation. Approximately 106 LNCaP cells suspended in 250 μL media were gently mixed with 250 μL Matrigel (Becton Dickinson Labware, Bedford, MA) and then inoculated subcutaneously in the flank region of 6~8 week old male athymic SCID mice using a 25-gauge needle.
Treatment protocol and measurement of tumor growth
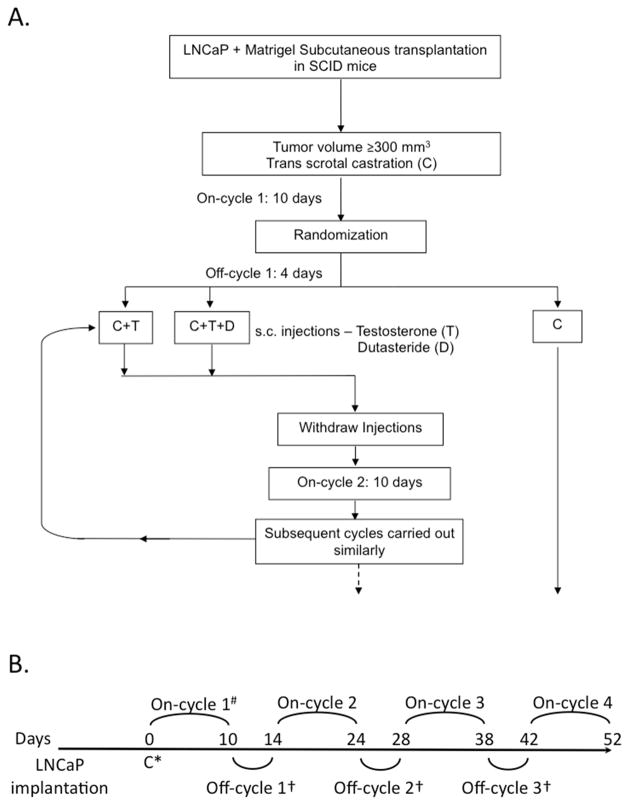
The experimental design and treatment regimen is outlined in Figure 1. Tumor volume was evaluated 3 times per week and calculated by the modified ellipsoid formula: length x width2 x 0.52 25. When tumors reached ≥ 200 mm3 in size, trans-scrotal castration was performed under isoflurane anesthesia with proper aseptic and antiseptic technique as previously 23. Castrated mice were randomized into 3 groups: castration (C), castration + testosterone replacement (C+T), and castration + testosterone + dutasteride (C+T+D). The average time to castration was 33.7 days for group C, 35.7 days for group C+T and 35.75 days for group C+T+D, median time to castration was 35 days for all groups. Actual average tumor size at castration was 355 mm3 for C, 358 mm3 for C+T, and 351 mm3 for C+T+D. There was no statistically significant difference in time to tumor development or tumor size at castration among the groups. The castration only group (C) was followed without any additional intervention. At 10 days post-castration, testosterone alone (C+T) or testosterone combined with dutasteride (C+T+D) were injected s.c. daily for 4 days (off-cycle) followed by 10 days with no injections (on-cycle). Testosterone (Sigma Chemical, St. Louis, MO) at 30 μg/day and dutasteride (gift from GlaxoSmithKline) at 1 mg/kg of body weight were suspended in egg phospholipids (EPL, NSC 704057, National Cancer Institute). At least 8 animals per group were generated and tumor volume was measured 3 times per week. Euthanasia was performed when tumor diameter exceeded 20 mm, ulcerated or caused severe tumor-related morbidity. Study endpoints were tumor volume and animal survival.
Fig. 1.
Experimental protocol. A. Schematic diagram of protocol. LNCaP xenograft tumors were established in SCID mice and animals were castrated when tumor volume reached ≥ 200 mm3. Castrated animals (C) were randomized into groups on day of castration (Day 0) and subsequently treated with multiple cycles of intermittent androgen deprivation therapy 10 days of no treatment other than castration (on-cycle) followed by 4 days of 30 μg/day testosterone replacement (C+T), or 30 μg/day testosterone plus 1 mg/kg body weight dutasteride (C+T+D) (off-cycle). Tumor volume was evaluated 3 times per week and calculated by the modified ellipsoid formula: (length x width2 x 0.52)25. Control animals were treated by castration only. Study endpoints were tumor volume and animal survival. B. Protocol timeline. Animals bearing LNCaP xenograft tumors from each group were subjected to treatment protocol from castration (Day 0) until tumor diameter exceeded 20 mm or tumor volume exceeded 2000 mm3.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc) was used for statistical analysis and graphical composition. The Kaplan-Meier test was used to calculate survival, and the log-rank test was used for evaluation of significance. Survival data were expressed as the median and 95% confidence interval ± SEM. Tumor volume data were expressed as the mean ± SEM, and statistical significance was determined by Mantel-Cox log-rank or Student’s t-test as appropriate. A p value of < 0.05 was considered statistically significant.
RESULTS
Survival analysis of IADT plus dutasteride
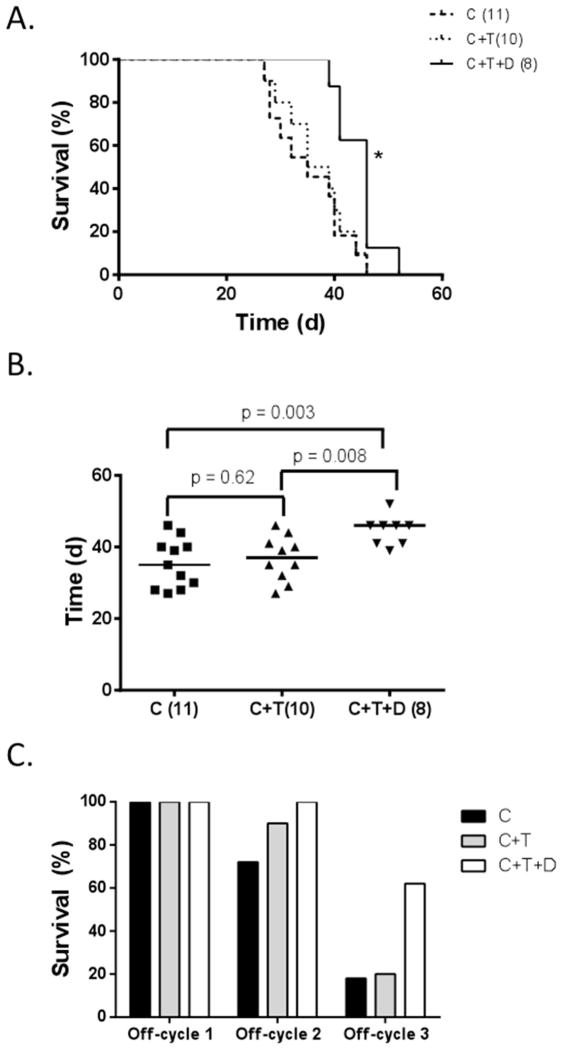
The survival of mice with LNCaP xenografts was significantly improved by the addition of dutasteride during the off-cycle (Figure 2A). Overall survival for castrated mice treated with intermittent testosterone replacement plus dutasteride (C+T+D) was statistically greater than for castrated (C) or castrated plus testosterone (C+T) (p < 0.05). Median survival for castrated mice treated with intermittent testosterone replacement (C+T) was 37 days, which was not significantly different than the median survival of 35 days (p=0.62) in castrated (C) mice. The median survival for C+T+D mice was 46 days after castration, which was significantly greater than that of both C+T and C mice (p < 0.05) (Figure 2B). The percentage of mice surviving at the end of each off-cycle was determined for each group (Figure 2C). All mice in all groups were alive at the end of off-cycle 1 (Day 14). At the end of off-cycle 2, 72% of mice in group C were still alive, 90% of group C+T and 100% of group (C+T+D). At the end of off-cycle 3, 18% of group C were still alive, 20% of group C+T and 60% of group C+T+D. No animals survived through the end of off-cycle 4 as the last animal in group C+T+D died at 52 days post-castration.
Fig. 2.
Survival of castrated mice bearing LNCaP xenograft tumors. A. Kaplan–Meier Survival Curve of castrated mice (C), castrated mice treated with multiple cycles of short testosterone-induced off-cycles (C+T) and with dutasteride (C+T+D). Number of mice in each treatment group given in parentheses, *, p < 0.05 when comparing C+T+D to C or C+T by Mantel-Cox log-rank test, p> 0.05 when comparing C to C+T. B. Median survival of mice in group C was 35 days (95% confidence interval [CI], 28–44), 37 days in group C+T (95% CI, 29–44), and 46 days in group C+T+D (95% CI, 39–52), p value for indicated comparisons by Student’s t-test. C. Survival of mice bearing tumors at the end of each off-cycle. Groups include castrated mice (C), castrated mice treated with multiple cycles of short testosterone-induced off-cycles (C+T) with dutasteride (C+T+D).
Tumor response to dutasteride during the short off-cycle
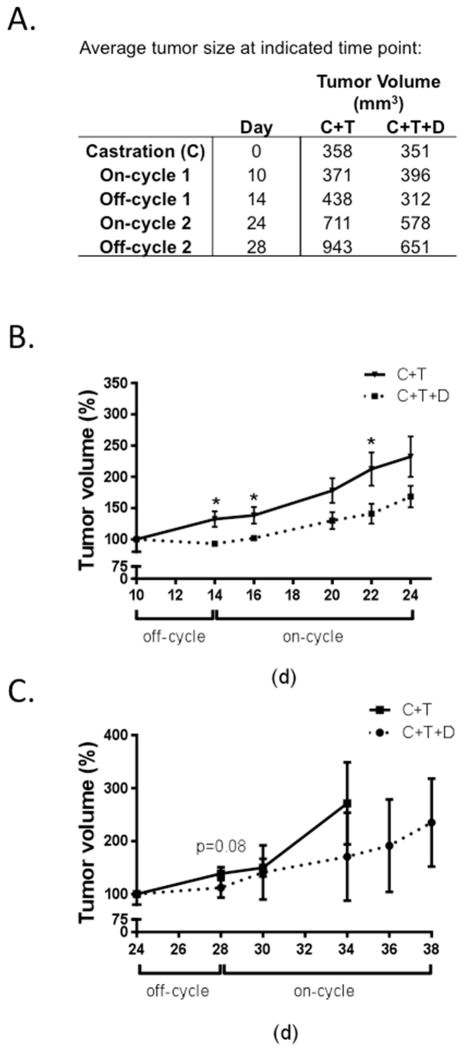
Previously in LNCaP xenograft tumors, finasteride was shown to inhibit testosterone-induced tumor regrowth during the initial 4 days of treatment of the first off-cycle. As expected, dutasteride inhibited testosterone-induced initial tumor regrowth (Figure 3). Average tumor volumes during the first two off-cycles were decreased in group C+T+D compared to group C+T (Figure 3A). Dutasteride significantly inhibited tumor regrowth during the initial 4 days of the first off-cycle (Figure 3B). Furthermore, although the inhibition was not statistically significant (p = 0.08), dutasteride also decreased tumor regrowth during the initial 4 days of the second off-cycle (Figure 3C).
Fig. 3.
Response of LNCaP xenograft tumors to multiple short off-cycles of testosterone recovery. A. Average xenograft tumor volume at indicated time point for animals treated with multiple cycles of short testosterone-induced off-cycles (C+T) and with dutasteride (C+T+D). B. Response of LNCaP xenograft tumors to dutasteride during off-cycle 1. C. Response of LNCaP xenograft tumors to dutasteride during off-cycle 2. Error bars represent SEM, *, p < 0.05 when comparing C+T+D to C+T by Student’s t-test at each time point.
DISCUSSION
IADT is an alternative treatment to ADT that, if optimized, has the potential to improve prostate cancer outcomes while reducing the treatment cost. Multiple clinical trials have demonstrated that IADT may reduce the adverse effects associated with continuous ADT without any clear difference in survival 26. Long term efficacy remains to be determined and methods for optimization of IADT will be a critical step in establishing it as a viable treatment option, especially in patients with advanced metastatic disease. Rising serum PSA levels are frequently used to determine off-cycle duration 27. In a recent review, we discussed the potential survival benefit of 5α-reductase inhibition in IADT, only when off-cycles are short, in animal models and the potential translation of this finding into clinic 22. 5α-reductase inhibitors (5ARIs) such as dutasteride or finasteride could enhance prostatic cell differentiation, reduce cell proliferation and delay prostate tumor progression. In a retrospective clinical study, the addition of finasteride during the off-cycle of IADT was shown to significantly inhibit rising PSA serum levels, thereby extending the duration of the off-cycle from a median of 15 months to 31 months 20. However, the extension of off-cycle duration by finasteride in prostate cancer patients did not translate into an improvement in time to development of castrate-resistant prostate adenocarcinoma 20. Similarly, in xenograft tumor models, prolongation of off cycle duration did not improve overall survival 23. However, fixed off-cycles of about 10 days coupled with finasteride treatment prolonged overall survival in xenograft tumor models 9. Finasteride or dutasteride treatment during the off-cycle of IADT significantly inhibited testosterone-stimulated regrowth of regressed prostate tumors during the first 4 days of testosterone replacement, but tumor growth resumed after 4 days 24. Regressed prostate tumors treated with finasteride or dutasteride had enhanced expression of growth suppressive androgen-responsive genes at day 4 and reduced expression at day 14, suggesting that initial testosterone replacement induced differentiation rather than proliferation 24. Masoodi et al., showed that early testosterone-induced growth of regressed prostate tumors was susceptible to 5ARI treatment, but not after extended exposure to androgens.
In addition to the treatment of advanced prostate cancers, 5ARIs might also decrease disease progression in low-risk, localized prostate cancer. The reduction by dutasteride of clinical progression events in expectant management (REDEEM) trial consisted of a randomized study to determine whether the use of a 5ARI could delay the time to treatment or pathological progression in men undergoing active surveillance for low-risk prostate cancer 28. This study found that dutasteride significantly delayed time to prostate cancer progression thus delaying the initiation of primary therapy. In the dutasteride group, 38% of men had prostate cancer progression compared to 48% of men in the control group during the 3 year study 28. Although tumor biology may differ among low-risk and high-risk prostate cancers, these studies and ours suggest that dutasteride might be a promising alternative treatment for inhibiting prostate cancer progression in certain patient populations.
CONCLUSIONS
In the current study, we show that short off-cycles of 4 days in IADT combined with dutasteride enhanced survival in the LNCaP xenograft tumor model, suggesting that the addition of dutasteride could significantly enhance survival in tumor-bearing mice. Tumor growth was inhibited during the first 4 days of testosterone-stimulated regrowth during both off-cycle 1 and off-cycle 2. In a phase II clinical trial, proliferation marker Ki-67 in prostate cancer biopsies was significantly inhibited by dutasteride during the early phase of off-cycles of IADT when testicular function was recovering 29, which is consistent with our findings using the animal models 24.
In summary, our animal studies suggest that 5ARI treatment inhibits the initial regrowth of regressed prostate tumors and has the potential to enhance the efficacy of IADT as long as the off-cycles are not permitted to prolong or can be terminated very short soon after testicular function recovery. This finding can be used to help design future clinical trials testing whether 5ARI coupled with short off-cycles can prolong the survival of prostate cancer patients on IADT.
Acknowledgments
We are grateful to Aiyuan Zhang, Katie Leschak, Dailing Zhao, and Megan Lambert for technical support. This investigation was supported in part by National Institutes of Health Grants 1 P50 CA90386 and R01CA186780. This project used the UPCI CCSG-supported Animal Facility and was supported in part by award P30CA047904. LEP is a Tippins scholar. KMZ is a recipient of the Mellam Family Foundation Scholarship.
Key of Definitions
- IADT
intermittent androgen deprivation therapy
- PSA
prostate specific antigen
- ADT
androgen deprivation therapy
- 5 ARI
5α-reductase inhibitor
- C
castration
- C+T
castration + testosterone replacement
- C+T+D
castration + testosterone + dutasteride
Footnotes
Disclosure statement: Authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sato N, Gleave ME, Bruchovsky N, et al. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996;58:139. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 3.2012 Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:1081. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 3.Schulman C, Irani J, Aapro M. Improving the management of patients with prostate cancer receiving long-term androgen deprivation therapy. BJU Int. 2012;109(Suppl 6):13. doi: 10.1111/j.1464-410X.2012.11216.x. [DOI] [PubMed] [Google Scholar]
- 4.Ismail M, Ferroni M, Gomella LG. Androgen suppression strategies for prostate cancer: is there an ideal approach? Curr Urol Rep. 2011;12:188. doi: 10.1007/s11934-011-0178-0. [DOI] [PubMed] [Google Scholar]
- 5.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 7.Dadras SS, Cai X, Abasolo I, et al. Inhibition of 5alpha-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr. 2001;9:183. doi: 10.3727/000000001783992551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggener SE, Stern JA, Jain PM, et al. Enhancement of intermittent androgen ablation by “off-cycle” maintenance with finasteride in LNCaP prostate cancer xenograft model. Prostate. 2006;66:495. doi: 10.1002/pros.20297. [DOI] [PubMed] [Google Scholar]
- 9.Buchan NC, Goldenberg SL. Intermittent androgen suppression for prostate cancer. Nat Rev Urol. 2010;7:552. doi: 10.1038/nrurol.2010.141. [DOI] [PubMed] [Google Scholar]
- 10.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer. 2006;42:1083. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Bruchovsky N, Rennie PS, Coldman AJ, et al. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50:2275. [PubMed] [Google Scholar]
- 12.Akakura K, Bruchovsky N, Goldenberg SL, et al. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Bruchovsky N, Snoek R, Rennie PS, et al. Control of tumor progression by maintenance of apoptosis. Prostate - Supplement. 1996;6:13. [PubMed] [Google Scholar]
- 14.Bruchovsky N, Klotz LH, Sadar M, et al. Intermittent androgen suppression for prostate cancer: Canadian Prospective Trial and related observations. Mol Urol. 2000;4:191. [PubMed] [Google Scholar]
- 15.De La Taille A, Zerbib M, Conquy S, et al. Intermittent androgen suppression in patients with prostate cancer. BJU Int. 2003;91:18. doi: 10.1046/j.1464-410x.2003.04015.x. [DOI] [PubMed] [Google Scholar]
- 16.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crook JM, Szumacher E, Malone S, et al. Intermittent androgen suppression in the management of prostate cancer. Urology. 1999;53:530. doi: 10.1016/s0090-4295(98)00547-0. [DOI] [PubMed] [Google Scholar]
- 18.Gleave M, Bruchovsky N, Goldenberg SL, et al. Intermittent androgen suppression for prostate cancer: rationale and clinical experience. European Urology. 1998;34:37. doi: 10.1159/000052297. [DOI] [PubMed] [Google Scholar]
- 19.Klotz LH, Herr HW, Morse MJ, et al. Intermittent endocrine therapy for advanced prostate cancer. Cancer. 1986;58:2546. doi: 10.1002/1097-0142(19861201)58:11<2546::aid-cncr2820581131>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Scholz MC, Jennrich RI, Strum SB, et al. Intermittent use of testosterone inactivating pharmaceuticals using finasteride prolongs the time off period. J Urol. 2006;175:1673. doi: 10.1016/S0022-5347(05)00975-4. [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh RA, Pascal LE, Davies BJ, et al. Improving intermittent androgen deprivation therapy: lessons learned from basic and translational research. Asian J Androl. 2014 doi: 10.4103/1008-682X.125410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Gupta S, Hua V, et al. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate. 2010;70:147. doi: 10.1002/pros.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masoodi KZ, Ramos Garcia R, Pascal LE, et al. 5alpha-reductase inhibition suppresses testosterone-induced initial regrowth of regressed xenograft prostate tumors in animal models. Endocrinology. 2013;154:2296. doi: 10.1210/en.2012-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Euhus DM, Hudd C, LaRegina MC, et al. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 26.Kratiras Z, Konstantinidis C, Skriapas K. A review of continuous vs intermittent androgen deprivation therapy: Redefining the gold standard in the treatment of advanced prostate cancer. Myths, facts and new data on a “perpetual dispute”. Int Braz J Urol. 2014;40:3. doi: 10.1590/S1677-5538.IBJU.2014.01.02. [DOI] [PubMed] [Google Scholar]
- 27.Wright JL, Higano CS, Lin DW. Intermittent androgen deprivation: clinical experience and practical applications. Urol Clin North Am. 2006;33:167. doi: 10.1016/j.ucl.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Fleshner NE, Lucia MS, Egerdie B, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1103. doi: 10.1016/S0140-6736(11)61619-X. [DOI] [PubMed] [Google Scholar]
- 29.Shevrin D, MacVicar G, Kuzel T, et al. Effect of dutasteride on tumor proliferation during the regrowth phase of intermittent androgen ablation therapy in men with advanced prostate cancer. ASCO 2013 Genitourinary Cancers Symposium; 2013. [Google Scholar]