Abstract
Objective
To identify clinical and serologic correlates of cutaneous ulcers in dermatomyositis (DM).
Methods
We retrospectively examined a cohort of 152 DM patients. We compared the features of patients with ulcers to those without ulcers using chi-square or Fisher’s exact tests and used univariate and multivariate logistic regression models to assess the association between ulcers and clinical features such as malignancy, interstitial lung disease (ILD), and amyopathic disease.
Results
Forty-three patients (28%) had cutaneous ulcers. Nearly half the patients had ulcers present in more than 1 location: 24 (56%) had ulcers over the extensor surfaces of joints, 18 (42%) at the digital pulp or periungual areas, and 25 (58%) had ulcers located elsewhere. In univariate analysis ulcers were associated with Asian race, but not with other clinical and demographic features, including malignancy or ILD. In multivariate analysis ulcers were significantly associated with anti–melanoma differentiation gene 5 (anti-MDA5) antibodies (odds ratio 10.14, 95% confidence interval 1.95–52.78, P = 0.0059) and this was greatest for ulcers located at the digital pulp. In patients with cutaneous ulcers, ILD risk was specifically increased only in patients with anti-MDA5+ antibodies.
Conclusion
We confirmed the strong association between anti-MDA5 antibodies and cutaneous ulcers, with the novel finding that the association of cutaneous ulcers with ILD depends upon the presence of anti-MDA5 antibodies. DM patients who display this cutaneous phenotype should undergo appropriate evaluation for ILD.
INTRODUCTION
Dermatomyositis (DM) is a systemic autoimmune disease that affects the muscles and skin. Internal malignancy affects approximately 25% of DM patients (1), while interstitial lung disease (ILD) can occur in up to 50% of patients (2). The skin manifestations of DM are heterogeneous, and include macular erythema, papules and plaques, nodules, and skin ulceration (3). Skin disease can lead to substantial morbidity (4). Given the wide variety of patterns of cutaneous involvement and the fact that the skin is readily examined, careful observation of particular cutaneous manifestations may provide the opportunity to classify DM patients with regards to their systemic risk factors at the time of the physical examination. Despite this, the correlation between various cutaneous features and systemic manifestations has not been well studied.
Cutaneous ulcers have been reported in 3–19% of DM patients (1,5–7). They are associated with significant pain and disability and are at risk for secondary infection. Ulcers may also portend a poor prognosis for disease control, as they have been associated with increased resistance of both skin and muscle disease to immunosuppressive therapies (8,9). Cutaneous ulcerations in DM patients vary with regards to location and severity. Common locations for ulcers in DM patients include extensor surfaces overlying joints (particularly over the fingers, elbows, and knees), lateral nailfolds or digital pulp, and sun-exposed areas such as the anterior chest and ear helix. There are multiple potential factors involved in ulcer development in DM, including vasculopathy, vasculitis, excessive inflammation at the interface between the dermis and epidermis, or excoriation in response to pruritus.
Few large-scale studies have examined the systemic significance of cutaneous ulcerations in DM patients. Interestingly, several small studies have demonstrated a correlation between cutaneous ulcerations and internal malignancy (1,10,11). Studies in Asian populations have found an association between cutaneous ulceration and lung disease; specifically, the association was found between pneumomediastinum (6,11) as well as poorer long-term survival (7), the latter largely due to rapidly progressive lung disease.
Autoantibodies in patients with connective tissue diseases tend to be mutually exclusive and are associated with certain clinical features. Several DM-specific autoantibodies have been identified in recent years, including the antibody to melanoma differentiation–associated gene 5 (MDA5) (13). Anti-MDA5 antibodies have been associated with mild (or absent) muscle inflammation as well as a high frequency of ILD (14,15). We have previously described that patients with anti-MDA5 antibodies have a characteristic cutaneous phenotype that includes mucocutaneous ulcers, alopecia, and palmar papules (16). However, it is unclear if ulceration is associated with any of the other DM-specific autoantibodies.
In this study we examined the association between the presence and location of cutaneous ulceration in DM with internal organ complications such as malignancy and ILD, as well as all of the major DM-specific autoantibodies that have recently been described.
PATIENTS AND METHODS
We retrospectively examined a cohort of 152 DM patients seen in the Stanford University interdisciplinary rheumatology-dermatology clinic from July 2004 through April 2013. Patients were only included if they had a diagnosis of definite DM based on the criteria of Bohan and Peter (17), or in the case of clinically amyopathic patients, if they had the characteristic rash of DM as defined by Sontheimer (3). Clinically amyopathic patients were defined as those patients with the characteristic rash of DM for at least 6 months without clinical weakness attributable to inflammatory myopathy or elevation of muscle enzymes >20% over the upper limit of normal at any time (3,18). All patients had skin biopsy findings consistent with DM. Clinical data were collected during routine medical care. A patient was considered positive for a given clinical feature if it was present at any time during their disease course, and these features may have preceded or followed the time of blood draw for antibody analysis (see below). ILD was defined as evidence of fibrosis or ground-glass opacities on computed tomography of the chest in the absence of infection. Age-appropriate cancer screening and/or computed tomography of the chest, abdomen, and pelvis were performed in all patients at least once either at presentation to our clinic or during followup. All malignancies were confirmed by tissue diagnosis. Patients were considered to have cancer-associated DM if they had a diagnosis (or specific signs) of malignancy (excluding nonmelanoma skin cancer) within 3 years of the onset of the first DM symptom. Anatomic location of cutaneous ulcerations was defined as ulcerations over joints (Gottron’s papules, knees, or elbows), ulcerations of the digital pulp or periungual region, and ulcers elsewhere on the body, including those in sun-exposed areas.
Determination of autoantibodies
Patients generally had plasma collected for antibody analysis at their initial visit to Stanford, which was performed at a mean ± SD time of 3.7 ± 4.2 years following their diagnosis. Assays to determine the presence of circulating autoantibodies were performed as previously described (19).
Statistical analysis
We compared the features of patients with ulcers to those without ulcers using Student’s t-test for age and chi-square or Fisher’s exact tests where appropriate for others. We used univariate and multivariate logistic regression models to assess the association between cutaneous ulcers and clinical features. Corresponding interactions terms were also examined. All P values were 2-sided, with P less than 0.05 considered statistically significant. Analyses were performed using SAS (version 9.4).
RESULTS
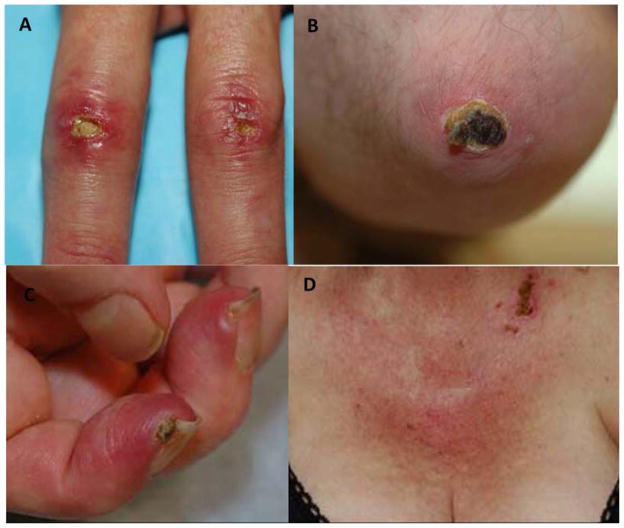
The clinical features of our cohort are shown in Table 1. Our adult DM cohort is typical in being mostly female (71%) and middle-aged (mean ± SD 49.5 ± 14.98 years). Mean ± SD disease duration at the time of the most recent followup visit was 5.35 ± 4.41 years. Approximately 14.5% of patients had malignancy-associated DM and 19.1% had ILD. Forty-three patients (28%) had cutaneous ulcers (Table 1). Of the patients with ulcers, 24 (56%) had ulcers over the Gottron’s papules and extensor surfaces, 18 (42%) at the digital pulp or periungual areas, and 25 (58%) had ulcers located elsewhere (Figure 2). Nearly half of the patients had ulcers present in more than 1 location: 30% of patients had ulcers over both Gottron’s papules/extensor surfaces and the digital pulp/periungual area, 35% had ulcers over the Gottron’s papules/extensor surfaces and elsewhere, 15% had ulcers over both the digital pulp/periungual area and elsewhere, and 20% had ulcers in all 3 distributions.
Table 1.
Demographics and clinical features of dermatomyositis patients
| No. (%) | |
|---|---|
| Demographics | |
| Age of disease onset, mean ± SD years | 49.5 ± 15.0 |
| Female | 108 (71.0) |
| Smoker | 30 (20.8) |
| Race | |
| White | 89 (65.0) |
| Asian | 25 (18.3) |
| African American | 6 (4.4) |
| Hispanic | 17 (12.4) |
| Time to followup, mean ± SD years | 2.42 ± 3.0 |
| Clinical features | |
| Cutaneous ulcers | 43 (28) |
| Raynaud’s phenomenon | 30 (19.9) |
| Dysphagia | 61 (40.1) |
| Calcinosis | 15 (9.9) |
| Cancer | 22 (14.5) |
| Interstitial lung disease | 29 (19.1) |
| Clinically amyopathic | 31 (20.4) |
| Arthritis/arthralgia | 68 (45.3) |
Figure 2.
Location of ulcers in dermatomyositis patients: A) joint ulceration, Gottron’s papules, B) joint ulceration olecranon, C) periungual ulcer, and D) sun-exposed ulceration, anterior chest.
We next looked for an association between ulceration and various clinical features. The distribution of ethnicities was different between the patients with ulcers compared to those without ulcers (P = 0.21); in particular, patients with cutaneous ulcers were more likely to be Asian and less likely to be Hispanic (Table 2). There was also a trend for an association between ulcers and calcinosis. There was no significant association with ulcers and the presence of other clinical features of DM such as arthritis, Raynaud’s phenomenon, or dysphagia. In addition, there was no significant difference in the percentage of patients with cutaneous ulcers in the classic DM group (30.6%) versus the clinically amyopathic group (19.3%) (P = 0.27). In univariate analysis, we did not find a significant association between ulcers and malignancy, ILD, or mortality.
Table 2.
Comparison of ulcer-positive and ulcer-negative dermatomyositis patients*
| Ulcer positive (n = 43) | Ulcer negative (n = 109) | P | |
|---|---|---|---|
| Demographics | |||
| Age at disease onset | 49.3 ± 16.8 | 49.5 ± 14.2 | 0.95 |
| Male | 10 (23.3) | 34 (31.2) | 0.33 |
| Race | |||
| White | 25 (58.1) | 64/94 (68.1)† | 0.021 |
| Asian | 13 (30.2) | 12/94 (12.8)† | |
| African American | 3 (7.0) | 3/94 (3.2)† | |
| Hispanic | 2 (4.7) | 15/94 (16.0)† | |
| Smoker | 8/40 (20)† | 22/104 (21.2)† | 0.88 |
| Clinical features | |||
| Raynaud’s phenomenon | 9 (20.9) | 21/108 (19.4)† | 0.84 |
| Dysphagia | 18 (41.9) | 43 (39.5) | 0.78 |
| Calcinosis | 7 (16.3) | 8 (7.3) | 0.10 |
| Cancer | 8 (18.6) | 14 (12.8) | 0.36 |
| Interstitial lung disease | 9 (20.9) | 20 (18.4) | 0.72 |
| Amyopathic | 6 (14.0) | 25 (22.9) | 0.22 |
| Arthritis | 19 (44.2) | 49/107 (45.8)† | 0.86 |
Values are the mean ± SD or the number (percentage) unless indicated otherwise.
If data points were missing, the modified no. available is noted as a denominator.
We next looked for any association between ulcers and DM-associated autoantibodies. Patients with ulcers were more likely to be anti-MDA5+ (36.1% in ulcer positive versus 4.4% in ulcer negative; P < 0.0001) and less likely to be anti-Mi2 positive (5.6% in ulcer positive versus 21.1% in ulcer negative; P = 0.04) (Figure 1). A positive trend was also found between cutaneous ulcers and anti–Ro52 antibodies (P = 0.07).
Figure 1.
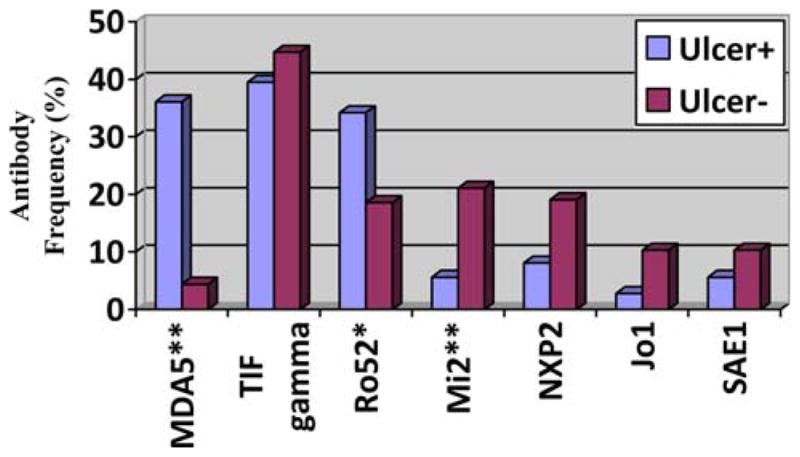
Dermatomyositis (DM)–specific autoantibody distributions in ulcer-positive and ulcer-negative DM patients. MDA5 = melanoma differentiation gene 5; **= P < 0.5; TIF = transcription intermediary factor; * = P < 0.1; NXP2 =; SAE1 =.
In multivariate analysis, anti-MDA5 antibodies were associated with ulcers (odds ratio [OR] 10.14, 95% confidence interval [95% CI] 1.95–52.78, P = 0.0059) (Table 3). Examining only the ulcer-positive patients, ulcers located at the digital pulp or periungual areas were the most highly associated with anti-MDA5 antibodies (OR 19.8, 95% CI 3.26–120.3, P = 0.0012) (data not shown). No other associations were found between location of ulcers and other DM-specific autoantibodies. In addition, although Asians were overrepresented in the MDA5+ patients, there was not a significant association between Asian ethnicity and ulcers in multivariate analysis after correcting for MDA5 status (Table 3).
Table 3.
Multivariate model for predictors of cutaneous ulcers in dermatomyositis patients*
| OR | 95% CI | P | |
|---|---|---|---|
| Asian | 2.58 | 0.72–9.17 | 0.14 |
| Hispanic | 0.29 | 0.03–2.55 | 0.26 |
| NXP2 positive | 0.38 | 0.07–1.98 | 0.25 |
| MDA5 positive | 10.14 | 1.95–52.78 | 0.0059 |
| Ro52 positive | 2.52 | 0.83–7.69 | 0.10 |
| Mi-2 | 0.65 | 0.12–3.43 | 0.61 |
OR = odds ratio; 95% CI = 95% confidence interval; NXP2 = nuclear matrix protein 2; MDA5 = melanoma differentiation gene 5.
Consistent with previous reports, we also found that anti-MDA5 antibodies were associated with an increased risk for ILD (OR 6.26, 95% CI 2.02–19.43, P = 0.0015) (data not shown). We next examined why ulcers themselves are not associated with ILD (Table 2), despite the fact that they are associated with anti-MDA5 antibodies (Table 3) and anti-MDA5 antibodies are associated with ILD. In order to examine this, we examined ILD status as a function of ulcers, stratified by anti-MDA5 status (Table 4). We found that, in the anti-MDA5− patients, only 1 of 23 patients (4.3%) had ILD, while in the anti-MDA5+ patients, 8 of 13 patients (61.5%) had ILD. Therefore, ulcer association with ILD depends on anti- MDA5 status, i.e., ulcers and anti-MDA5 status interact, although a formal test for interaction between fell just short of statistical significance (P = 0.085; data not shown). Multivariate regression analysis demonstrated that, in ulcer negative patients, anti-MDA5 antibodies were not significantly associated with ILD (OR 1.82, 95% CI 0.15–21.58, P = 0.63), while in the ulcer positive patients, anti-MDA5 status was highly associated with ILD (OR 35.19, 95% CI 3.55–3.49, P = 0.0024) (not shown).
Table 4.
Risk for development of ILD stratified by ulcer and anti-MDA5 antibody status*
| ILD negative | ILD positive | |
|---|---|---|
| Anti-MDA5 negative | ||
| Ulcer negative | 51 | 14 |
| Ulcer positive | 22 | 1 |
| Anti-MDA5 positive | ||
| Ulcer negative | 2 | 1 |
| Ulcer positive | 5 | 8 |
ILD = interstitial lung disease; anti-MDA5 = anti–melanoma differentiation gene 5.
DISCUSSION
Cutaneous ulcerations in DM patients can be extremely painful and disabling, leading to gangrene or osteomyelitis and are associated with poor treatment response. Despite this, cutaneous ulcerations in DM have been rarely studied and little is known about their etiology or prognostic significance. We previously described a phenotype associated with antibodies to MDA5 that includes cutaneous ulceration, although it was unclear if this association was confounded by other factors. This present study confirms this association and provides evidence that the association is direct and not confounded by other clinical or serologic factors that are typical of anti-MDA5+ patients (16). In addition, we demonstrate a strong and novel association between the location of cutaneous ulcers at the digital pulp/periungual region and positive anti-MDA5 antibodies. Also, we found that anti-MDA5 antibodies were associated with ILD largely only in patients with cutaneous ulcers (Table 4).
In contrast to other studies (1,10,11), we did not find an association between cutaneous ulceration and internal malignancy. We surmised that this might be due to the fact that a significant proportion of patients with ulceration in our cohort had antibodies to MDA5, which has not been associated with internal malignancy in our cohort (16). However, even after exclusion of the anti-MDA5+ patients, we still did not find a significant association between cutaneous ulcerations and cancer (OR 1.83, 95% CI 0.60–5.58, P = 0.29). Another possibility is that prior studies define cutaneous necrosis (rather than ulceration) as the clinical feature that portends malignancy; this definition might not correspond exactly with ulceration. Our definition of ulcers is one that includes any breakdown of epidermal integrity.
Most previous studies examining cutaneous ulceration in the DM population have been limited to isolated case reports. Two of these case reports have demonstrated evidence of vasculitis on histopathologic examination of ulcerated lesions located over the digits and extensor surfaces in DM patients (20,21). However, cutaneous ulceration in DM may also be a result of underlying vasculopathy; for example, skin biopsies from anti-MDA5+ patients show an underlying vasculopathy with infiltration of mononuclear cells, endothelial cell swelling and ballooning, and fibrin deposition in the vessel walls (16). In fact, a more general vasculopathy may be a characteristic finding of skin disease in DM (22).
Beyond the association between vasculopathy and DM skin disease, several studies have also postulated a role for endothelial injury in the development of ILD (23–25). There have been several case reports describing patients with extensive cutaneous ulceration who also had prominent pulmonary disease, including ILD and recurrent pneumomediastinum, findings suggestive of MDA5 positivity (23–26). An underlying systemic vasculopathy in anti-MDA5+ DM patients may explain the link we have demonstrated between cutaneous ulcers and increased risk of ILD in this autoantibody subgroup. DM and polymyositis patients with interstitial pneumonitis have been found to have higher serum levels of endothelin, thrombomodulin, and plasminogen activator inhibitor, which are all known to reflect the extent of endothelial damage (23). In addition to serologic markers of endothelial damage, there have been multiple reports of lung biopsy results supporting a role for vascular inflammation or damage in ILD. A case report of a DM patient with ILD demonstrated endothelial cell injury with necrotizing pulmonary capillaritis and interstitial spaces expanded by infiltrating neutrophils (24). In another case series, lung biopsies of 16 connective tissue disease patients with ILD (including 7 DM patients) demonstrated microvascular injury with intraparenchymal fibrosis (25).
ILD is often subclinical in the early stages of injury, and fibrosis can best be detected by high resolution computed tomography of the chest; by contrast, cutaneous ulcers are highly symptomatic, painful, and readily visible to the clinician. There are currently no validated guidelines regarding clinical screening for ILD in DM patients, in particular regarding whether all patients should have baseline pulmonary function testing and/or lung imaging performed and at what frequency they should be repeated. Our data suggest that careful attention to the presence of ulcers may serve as a sign for ILD. We should note that we found that the association between cutaneous ulcers and ILD is different when considering the MDA5+ patients versus the MDA5− patients. In MDA5− patients, ulcers were not associated with increased risk of ILD (and in fact they were protective of ILD); we surmise that because the MDA5 negative patients were the majority of our study population, ulcers were not found to be associated with ILD when we examine the study population as a whole. As the anti-MDA5 antibody assay becomes clinically available, guidelines for the assessment of ILD in DM patients based on autoantibody status will be highly relevant for the clinical care of these patients.
A unique strength of our study is that it includes a large cohort of clinically phenotyped DM patients who have undergone comprehensive autoantibody testing. It should be noted, however, that our results are from a single tertiary academic medical center in the US, and our findings may not be generalizable to other patient populations, particularly from different countries of origin or ethnic backgrounds. For example, we have a larger cohort of Asian patients in our geographic area, which may affect our particular clinical findings. Because our study was retrospective, some missing data were unavoidable and followup time was variable among the patients. For instance, we did not have information on the timing of onset of particular clinical features or autoantibodies. For example, it is unclear what percentage of patients with anti-MDA5 antibodies develops ulcers before versus after the diagnosis of ILD. Further study is required to determine the temporal relationship between these features; without this information, we are unable to postulate whether ulcers could be used as a predictor for developing ILD or whether they are simply a marker of already ongoing ILD. Additional details such as depth and size of cutaneous ulcers or validated scoring of ILD severity would have been interesting to evaluate and may play a role in risk stratification for both development and severity of ILD. Nevertheless, our study supports aggressive screening for ILD in anti-MDA5+ patients with cutaneous ulcerations.
Therefore, our study has identified that skin ulceration is not uncommonly seen in adult DM patients and has potential associations with ethnicity, autoantibody status, and the risk of ILD. Our data suggest that these relationships are complex and interrelated, and further studies will confirm what role cutaneous ulceration plays in systemic prognosis for adult patients with DM.
Significance & Innovations.
We confirmed the strong association between anti-melanoma differentiation gene 5 (anti-MDA5) antibodies and cutaneous ulcers.
Ulcers are associated with the presence of interstitial lung disease primarily in anti-MDA5+ patients.
Acknowledgments
Dr. Casciola-Rosen’s work was supported by the NIH (grant RO1-AR-4684) and the Donald and Dorothy Stabler Foundation. Drs. Chung and Fiorentino’s work was supported by the Scleroderma Research Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Fiorentino had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Narang, Chung, Fiorentino.
Acquisition of data. Narang, Casciola-Rosen, Chung, Fiorentino.
Analysis and interpretation of data. Narang, Casciola-Rosen, Li, Chung, Fiorentino.
References
- 1.Ponyi A, Constantin T, Garami M, Andras C, Tallai B, Vancsa A, et al. Cancer-associated myositis: clinical features and prognostic signs. Ann N Y Acad Sci. 2005;1051:64–71. doi: 10.1196/annals.1361.047. [DOI] [PubMed] [Google Scholar]
- 2.Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28:451–8. doi: 10.1055/s-2007-985666. [DOI] [PubMed] [Google Scholar]
- 3.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20:387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 4.Goreshi R, Chock M, Foering K, Feng R, Okawa J, Rose M, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman D, Hochberg MC, Zizic TM, Stevens MB. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85–9. [PubMed] [Google Scholar]
- 6.Kono H, Inokuma S, Nakayama H, Suzuki M. Pneumomediastinum in dermatomyositis: association with cutaneous vasculopathy. Ann Rheum Dis. 2000;59:372–6. doi: 10.1136/ard.59.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamasaki Y, Yamada H, Ohkubo M, Yamasaki M, Azuma K, Ogawa H, et al. Longterm survival and associated risk factors in patients with adult-onset idiopathic inflammatory myopathies and amyopathic dermatomyositis: experience in a single institute in Japan. J Rheumatol. 2011;38:1636–43. doi: 10.3899/jrheum.101002. [DOI] [PubMed] [Google Scholar]
- 8.Yosipovitch G, Feinmesser M, David M. Adult dermatomyositis with livedo reticularis and multiple skin ulcers. J Eur Acad Dermatol Venereol. 1998;11:48–50. [PubMed] [Google Scholar]
- 9.Tsujimura S, Saito K, Tanaka Y. Complete resolution of dermatomyositis with refractory cutaneous vasculitis by intravenous cyclophosphamide pulse therapy. Intern Med. 2008;47:1935–40. doi: 10.2169/internalmedicine.47.1289. [DOI] [PubMed] [Google Scholar]
- 10.Mautner GH, Grossman ME, Silvers DN, Rabinowitz A, Mowad CM, Johnson BL., Jr Epidermal necrosis as a predictive sign of malignancy in adult dermatomyositis. Cutis. 1998;61:190–4. [PubMed] [Google Scholar]
- 11.Mahe E, Descamps V, Burnouf M, Crickx B. A helpful clinical sign predictive of cancer in adult dermatomyositis: cutaneous necrosis [commentary] Arch Dermatol. 2003;139:539. doi: 10.1001/archderm.139.4.539-a. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Hong YK, Yoo WH. Dermatomyositis, complicated with pneumomediastinum, successfully treated with cyclosporine A: a case report and review of literature. Rheumatol Int. 2009;29:1101–4. doi: 10.1007/s00296-008-0822-2. [DOI] [PubMed] [Google Scholar]
- 13.Gunawardena H, Betteridge ZE, McHugh NJ. Myositisspecific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 2009;48:607–12. doi: 10.1093/rheumatology/kep078. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res (Hoboken) 2012;64:1602–10. doi: 10.1002/acr.21728. [DOI] [PubMed] [Google Scholar]
- 15.Chaisson NF, Paik J, Orbai AM, Casciola-Rosen L, Fiorentino D, Danoff S, et al. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 2012;91:220–8. doi: 10.1097/MD.0b013e3182606f0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohan A, Peter J. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 18.Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1γ. Arthritis Rheum. 2013;65:2954–62. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicuttini FM, Fraser KJ. Recurrent pneumomediastinum in adult dermatomyositis. J Rheumatol. 1989;16:384–6. [PubMed] [Google Scholar]
- 21.Yamamoto T, Ohkubo H, Katayama I, Nishioka K. Dermatomyositis with multiple skin ulcers showing vasculitis and membrano-cystic lesion. J Dermatol. 1994;21:687–9. doi: 10.1111/j.1346-8138.1994.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 22.Crowson AN, Magro CM. The role of microvascular injury in the pathogenesis of cutaneous lesions of dermatomyositis. Hum Pathol. 1996;27:15–9. doi: 10.1016/s0046-8177(96)90132-x. [DOI] [PubMed] [Google Scholar]
- 23.Funauchi M, Shimadsu H, Tamaki C, Yamagata T, Nozaki Y, Sugiyama M, et al. Role of endothelial damage in the pathogenesis of interstitial pneumonitis in patients with polymyositis and dermatomyositis. J Rheumatol. 2006;33:903–6. [PubMed] [Google Scholar]
- 24.Kawakami T, Mizoguchi M, Saito R, Soma Y. Histopathological evidence of small-vessel vasculitis within the skin and lungs associated with interstitial pneumonia in an adult patient with dermatomyositis. Clin Exp Dermatol. 2008;33:415–7. doi: 10.1111/j.1365-2230.2008.02729.x. [DOI] [PubMed] [Google Scholar]
- 25.Magro CM, Ross P, Marsh CB, Allen JN, Liff D, Knight DA, et al. The role of anti-endothelial cell antibody-mediated microvascular injury in the evolution of pulmonary fibrosis in the setting of collagen vascular disease. Am J Clin Pathol. 2007;127:237–47. doi: 10.1309/CNQDMHLH2WGKL32T. [DOI] [PubMed] [Google Scholar]
- 26.Bradley JD. Spontaneous pneumomediastinum in adult dermatomyositis. Ann Rheum Dis. 1986;45:780–2. doi: 10.1136/ard.45.9.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmody E, McNicholl J, Chadwick G, Bresnihan B, Fitzgerald MX. Prolonged spontaneous pneumomediastinum in adult dermatomyositis. Ann Rheum Dis. 1987;46:566. [PMC free article] [PubMed] [Google Scholar]