Abstract
The dopamine transporter (DAT1) gene has been associated with impulsivity and executive functioning. Further, DAT1 has been associated with brain structural characteristics and resting state connectivity. This study tested an indirect effect model in which DAT1 genotype (9-repeat carriers vs. 10-repeat homozygotes) is linked to phenotypes representing impulsivity and executive function (planning behavior) through effects on white matter (WM) volumes in prefrontal cortex (PFC), particularly orbitofrontal cortex (OFC). Adolescents (ages 14–18, n=38), were recruited from substance use treatment (n=22) and the community (n=16) to increase phenotype variation. Results indicated that DAT1 10/10 genotype was associated with lower WM volume in the PFC, specifically the left OFC. Further, lower WM volume in the left OFC predicted more difficulties in self-reported planning behavior, but not impulsivity. Indirect effect analysis indicated that lower WM volume in the left OFC mediated the association between DAT1 10/10 genotype and difficulties in planning behavior. Results suggest a brain structural mechanism, involving lower WM volume in the left OFC, as a link in the association between DAT1 genotype and a specific aspect of executive function. Genetic effects on regional WM volume that are linked to behavioral outcomes could ultimately inform the development of tailored interventions that address an individual’s unique risk factors.
Keywords: DAT1, Impulsivity, Executive function, White matter volume, Adolescent, Substance use
1. Introduction
Neuroimaging measures have been used as an intermediate phenotype to explore neurobiological mechanisms in relationships between genes and behaviors (Hariri and Weinberger, 2003). In this model, genetic influence on a behavioral phenotype may be indirect, or mediated by the neuroimaging intermediate phenotype. Testing an indirect effect model can help to identify links, such as brain structural characteristics (white matter volume), in a pathway that connects the DAT1 genotype with behavioral phenotypes of impulsivity and executive function. Since single genes are likely to have small effects, which are biased toward one extreme of a continuous phenotype, analytic samples that increase phenotypic variation by including a mix of affected and healthy cases can facilitate detection of genetic effects (Durston et al., 2005). This study tested an indirect effects model in which DAT1 genotype is linked to behavioral phenotypes (e.g., impulsivity, goal setting and planning) through effects on white matter (WM) volumes in the prefrontal cortex (PFC) using a mixed sample of youth recruited from substance use treatment and the community to increase variation in the behavioral phenotypes of interest.
The dopamine transporter (DAT1) gene has a variable number of tandem repeats (VNTR) in its 3′ untranslated region that may influence DAT expression. The 9-repeat (9R) and 10-repeat (10R) alleles are most common. In vitro studies generally suggest lower DAT expression for 9R, relative to 10R, resulting in increased DA signaling for 9R carriers (Mill et al., 2002; Madras et al., 2005). In vivo results, however, have been inconsistent (Heinz et al., 2000; Jacobsen et al., 2000; Krause et al., 2006). Research on the DAT1 genotype indicates that 10R homozygotes (10/10 genotype), relative to 9R carriers, demonstrated greater impulsive responding (Gizer and Waldman, 2012). Other studies have reported worse executive functioning among 10R homozygotes relative to 9R carriers (Loo et al., 2003; Stollstorff et al., 2010). The 10R allele also has a modest association with attention-deficit/hyperactivity disorder (Yang et al., 2007; Gizer et al., 2009; Hawi et al., 2010), a disorder involving impulsivity and impairment in executive function.
The prefrontal cortex (PFC) plays a key role in the behavioral phenotypes of impulsivity and executive function that have been associated with DAT1 genotype (Miller and Cohen, 2001). In particular, the orbitofrontal cortex (OFC), as part of the PFC, has been associated in human lesion studies with impulsivity, that is, a preference for smaller but rapid rewards relative to larger but delayed rewards (Bechara et al., 1994). The OFC, together with other PFC regions, is involved in planning and monitoring goal-directed behavior, specifically by encoding the reward value of temporally distant goals (Wallis, 2007). In one model (Wallis, 2007), the OFC serves as a major hub that integrates sensory (e.g., temporal cortex), affective (e.g., amygdala), and motivational (e.g., hypothalamus) inputs to compute the value of possible outcomes and their reward values. Information on outcomes and associated reward values is then transferred from the OFC to other areas of the PFC to prioritize goals and determine action plans (Kringelbach, 2005; Wallis, 2007).
The OFC’s role as a key information-processing hub that is highly connected, functionally and anatomically (Ongur and Price, 2000; Kringelbach, 2005; Kahnt et al., 2012), with other brain regions suggests the importance of efficient communication between relevant brain regions, as well as within the OFC. In this regard, integrity of white matter (WM) tissue facilitates efficient communication within and across brain regions (Fields, 2008; Filley, 2010). As a classic index of WM tissue integrity (Salat et al., 2005), WM volume reflects, for example, number of axons and degree of myelination (Paus, 2010). Regional WM volume has been associated with, for example, processing speed (Ferrer et al., 2013) and cognitive control as indicated by reduced interference on the Stroop task (Takeuchi et al., 2012). Total brain WM volume has been found to be positively associated with working memory performance (Posthuma et al., 2003). Of particular interest for the current study, WM volume in the area of the OFC adjacent to Brodmann’s area 11 was positively correlated with executive functioning involved in everyday events in healthy adults (Takeuchi et al., 2013). Results from these studies suggest a role for the OFC in executive functioning and impulsive behavior, and that WM volume in OFC may be associated with aspects of executive functioning.
Emerging research suggests associations of the DAT1 genotype with regional brain structure. Specifically, youth with the 10/10 genotype had smaller caudate gray matter volumes than 9R carriers, a result which was tentatively interpreted as related to reduced DAT1 expression (Durston et al., 2005). Little is known regarding DAT1 genotype and regional WM integrity, particularly WM integrity within specific regions of interest such as the PFC and the OFC. Although DAT1 may be expressed more in striatum than in prefrontal areas (Madras et al., 2005), due to the functional and anatomical connectivity of these areas (e.g., fronto-striatal circuit), dopamine signaling that originates in the striatum may have effects in the PFC as part of a network of brain regions, in which striatum gates and updates information in the PFC (Hazy et al., 2007; Braskie et al., 2011). For example, high midbrain dopamine D3 receptor availability was associated with lower resting state functional connectivity between the OFC and networks involved in cognitive control and reward in healthy males (Cole et al., 2012). As another example, a recent study found that resting state connectivity of a striato-frontal circuit mediated the association of DAT1 with working memory and impulsivity in healthy adults (Gordon et al., 2015). These studies suggest effects of midbrain dopamine signaling on the OFC and other cortical areas through network connectivity, although connectivity within a given region (e.g., the OFC) is also important to understanding circuit dynamics (Sporns et al., 2005; Filley, 2010).
The finding reported by Gordon and colleagues (2015) that adults with 10/10 DAT1 genotype, relative to 9R carriers, had weaker striato-frontal connectivity, which, in turn, was associated with worse executive functioning (working memory) and marginally associated with higher trait impulsivity, suggests that a DAT1 effect on functional connectivity might occur through genetic association with WM integrity, indexed by regional WM volume. Although inter-region anatomical connectivity may be of interest, we focus here on connectivity within a priori selected regions (PFC and OFC) that have been associated with executive functioning and impulsivity (Kringelbach, 2005; Wallis, 2007). In support of a possible association of brain dopamine and WM characteristics, in vitro work indicates an association between levels of brain dopamine and brain myelination (e.g., Karadottir and Attwell, 2007). Further, psychotropic medication effects on dopamine neurotransmission involve complex signaling pathways that can affect myelination (Bartzokis, 2012), which suggests an association between dopaminergic activity and WM characteristics, such as WM volume (e.g., Bartzokis et al., 2011).
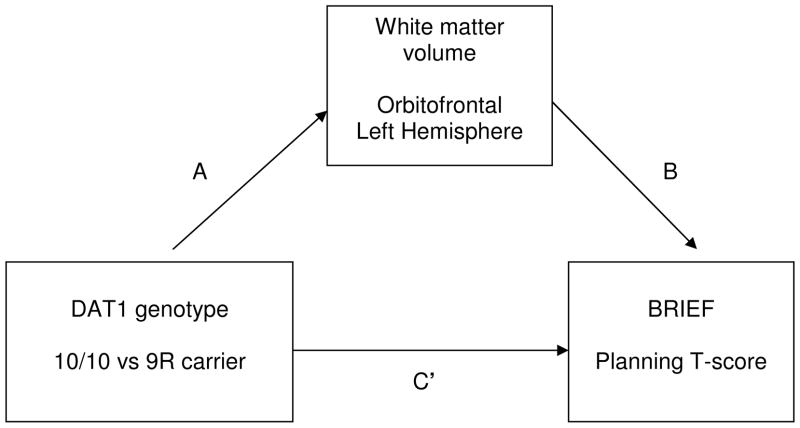
Based on research suggesting effects of the DAT1 genotype on regional brain structure and striatal-frontal connectivity (Durston et al., 2005; Gordon et al., 2015), we hypothesized that the 10/10 genotype would be associated with lower prefrontal WM volume. We focus on regional WM volumes illuminate the role of selected regions of interest (PFC and OFC) in relation to specific behavioral phenotypes, and WM volume as an indicator of WM integrity within these specific regions of interest. We predicted that lower prefrontal WM volume, particularly in the OFC (due to its role in planning goal-directed behavior and its implication in impulsivity), would be associated with phenotypes representing trait impulsivity and difficulties in executive function related to planning goal-directed behavior. This study of youth from substance use treatment and the community tested a model of gene-to-brain-to-behavior relations (see Fig. 1) in which DAT1 genotype (10/10) has an indirect effect on phenotypes of higher trait impulsivity and difficulties in planning goal-directed behavior through lower prefrontal WM volume.
Figure 1.
Hypothesized mediation (indirect effects) model
Notes: n=38
10/10= DAT1 10R/10R homozygotes; 9R carrier= DAT1 10R/9R or 9R/9R
BRIEF= Behavior Rating Inventory of Executive Function-Self-Report Version
2. Methods
2.1. Participants
Adolescents ages 14–18 were recruited from community-based intensive outpatient treatment for substance use, and from the community using random digit dialing, to participate in a naturalistic longitudinal study. Inclusion criteria for these analyses were self-reported Caucasian race (only Caucasians were included to minimize differences in minor allele frequency by race), valid neuroimaging data, valid DAT1 genotype data, and complete data on the dependent variables. Imaging data from six participants were excluded due to excess motion (n=3), faulty cortical segmentation (n=2) or a problem with resampling of diffusion tensor imaging data (n=1). Two cases with valid neuroimaging data were excluded because the DNA sample was not successfully genotyped. One case with valid neuroimaging and genotype data was excluded due to missing data for a dependent variable.
The analysis sample included 38 adolescents (58% male), 22 recruited from substance use treatment (57.9%) and 16 recruited from the community (42.1%). Table 1 reports sample descriptive statistics. Participants were, on average, 16.6 (SD=1.2) years old. The sample represented a full range of socio-economic status (SES; range=1–5) and was, on average, middle-class (Hollingshead, 1975). In the total sample, the mean full scale IQ score was in the average range (Wechsler Abbreviated Scale of Intelligence; Wechsler, 1999). The Edinburgh Handedness Inventory (EHI) (Oldfield, 1971) indicated that 34 participants were right handed (score >39), one participant was ambidextrous (score=10), and three participants were left handed (score < −39).
Table 1.
Descriptive statistics for treatment and community subsamples
| Demographics | TOTAL N=38 |
Treatment N=22 |
Community N=16 |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Female | 16 | 42.1 | 9 | 40.9 | 7 | 43.8 |
| Male | 22 | 57.9 | 13 | 59.1 | 9 | 56.2 |
| DAT1 genotype | ||||||
| 10/10 | 17 | 44.7 | 10 | 45.5 | 7 | 43.8 |
| 9 carrier | 21 | 55.3 | 12 | 54.5 | 9 | 56.2 |
| Baseline | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age | 16.6 (1.2) | 16.8 (1.2) | 16.3 (1.0) | |||
| Socio-economic status (Hollingshead, 1975) | 2.4 (1.0) | 2.6 (1.1) | 2.1 (0.8) | |||
| WAIS Full Scale IQ | 102.7 (13.5) | 97.0 (12.3) | 110.6 (11.2)** | |||
| Planning T score | 53.9 (13.1) | 60.5 (11.3) | 44.8 (9.4)** | |||
| Barratt Impulsivity Scale score | 69.2 (13.0) | 76.0 (11.3) | 59.9 (8.6)** | |||
| Frequency of substance use (past 6-months)† | ||||||
| Alcohol use | 2.7 (2.0) | 3.4 (1.9) | 1.7 (1.8)** | |||
| Marijuana use | 3.5 (3.3) | 5.5 (2.6) | 0.7 (1.6)** | |||
| Tobacco use | 4.1 (3.6) | 5.9 (3.1) | 1.5 (2.6)** | |||
| n | % | n | % | n | % | |
| Lifetime DSM-IV alcohol use disorder | 15 | 39.5 | 14 | 63.6 | 1 | 6.2** |
| Alcohol Abuse | 12 | 31.6 | 1 | 50.0 | 1 | 6.2 |
| Alcohol Dependence | 3 | 7.9 | 3 | 13.6 | 0 | 0.0 |
| Lifetime DSM-IV cannabis use disorder | 24 | 63.2 | 22 | 100.0 | 2 | 12.5** |
| Cannabis Abuse | 18 | 47.4 | 16 | 72.7 | 2 | 12.5 |
| Cannabis Dependence | 6 | 15.8 | 6 | 27.3 | 0 | 0.0 |
| Lifetime DSM-IV nicotine use disorder | 7 | 18.4 | 0 | 0.0 | 7 | 31.8* |
| Lifetime DSM-IV psychopathology | ||||||
| Conduct disorder | 11 | 28.9 | 11 | 50.0 | 0 | 0.0** |
| Attention deficit hyperactivity disorder | 10 | 26.3 | 9 | 40.9 | 1 | 6.3* |
| Major depression | 9 | 23.7 | 8 | 36.4 | 1 | 6.3* |
| White matter volume | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Prefrontal volume | 7.12 (0.44) | 7.01 (0.43) | 7.29 (0.41)* | |||
| Prefrontal volume (excluding orbitofrontal) | 5.72 (0.38) | 5.62 (0.36) | 5.87 (0.38)* | |||
| Orbitofrontal volume | 1.40 (0.10) | 1.39 (0.10) | 1.42 (0.10) | |||
| Left hemisphere | 0.68 (0.05) | 0.68 (0.05) | 0.69 (0.06) | |||
| Right hemisphere | 0.72 (0.05) | 0.71 (0.06) | 0.73 (0.05) | |||
Notes: Baseline N=38; SD= standard deviation.
Frequency coded: 0=never used, 1=no use in the last 6-months, 2=used < once per month, 3= once per month, 4=2–3 times per month, 5=once per week, 6= 2–3 times per week, 7= 4–6 times per week, 8=daily;
comparison of treatment vs community p<0.05;
p<0.01
2.2. Procedure
Youth from substance use treatment and the community, enrolled in the longitudinal study (King et al., 2009; Maisto et al., 2011), were invited to participate in an add-on neuroimaging protocol (Thatcher et al., 2010; Chung et al., 2011; Clark et al., 2012; Chung et al., 2013). The University’s Institutional Review Board approved the study protocol. Treated youth attended three 3-h group sessions per week for 6–8 weeks, with content (e.g., relapse prevention, 12-step facilitation) that supported a goal of abstinence from alcohol and illicit drugs. Community youth served as a locally representative comparison to treated youth. Informed consent (from 18 year olds) or assent (from minors, with informed consent for the minor’s participation provided by the minor’s parent) was obtained before initiating study procedures. For treated youth, baseline assessment was typically completed within 2 weeks of starting treatment. Highly trained research associates, with a bachelor’s or master’s degree, collected substance use and psychiatric data, and saliva DNA according to protocol.
The neuroimaging protocol was completed shortly after baseline assessment, typically within 2 weeks. Youth were instructed to abstain from alcohol and illicit substance use for at least 24 h before the imaging session. No adolescent included in the analyses reported alcohol or illicit drug use <24 h before the scan. In the total sample, average number of days before the scan was 22.2 (SD=11.3) since last alcohol use, 21.9 days (SD=12.8) for marijuana use, 15.5 (SD=14.3) for tobacco use, and 24.8 (SD=9.3) for other drug use. Before magnetic resonance imaging (MRI), participants were screened for any MRI contraindications. Adolescents received compensation for study participation.
2.3. Measures of substance involvement, psychopathology, and cognitive functioning
The Drug Consumption Questionnaire assessed frequency of alcohol and marijuana use in the past 6 months using a 9-point scale (0=never used, 1=no use in the last 6-months, 2=used less than once per month, 3=used once per month, 4=used 2–3 times per month, 5=used once per week, 6=used 2–3 times per week, 7=used 4–6 times per week, 8=daily use) with satisfactory reliability and validity (Chung et al., 2004). An adapted Structured Clinical Interview for DSM-IV SUDs (SCID: First et al., 2002) assessed lifetime diagnoses of substance use disorder with acceptable reliability and validity (Chung et al., 2004). The Kiddie-Schedule for Affective Disorders and Schizophrenia (Kaufman et al., 1997) assessed lifetime DSM-IV psychopathology.
The Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) has been shown to reliably assess full-scale IQ. Behavior Rating Inventory of Executive Function-Self-Report Version (BRIEF-SR) (Guy et al., 2004), which has good psychometric properties, was used to assess Planning/Organization (“Planning”) with 13 items rated “never,” “sometimes,” or “often.” Planning subscale items assess the ability to develop and apply appropriate steps in carrying out a task or accomplishing a goal (e.g., “I don’t plan ahead for future activities”, “I have trouble prioritizing my activities”). A normalized T-score was used, with higher scores representing greater difficulties in Planning. The Barratt Impulsivity Scale (Patton et al., 1995) includes 30 items (e.g., “I do things without thinking”) rated on a 4-point scale (1=rarely/never to 4=almost always/always); higher scores reflect greater impulsivity.
2.4. DNA collection and genotyping
A mouthwash protocol was used to collect DNA from saliva (King et al., 2002). Samples were subjected to whole genome amplification using multiple displacement amplification (Dean et al., 2002), quantified by the pico green protocol, and diluted to 40 ng/μl for storage. Length polymorphisms were genotyped by polymerase chain reaction using unique sequence flanking primers and resolution of the amplimers on agarose polyacrylamide gel. Polymerase chain reaction amplification and gel separation were accomplished using published methods (Vandenbergh et al., 1992). For DAT1, alleles were categorized as 10R, 9R and other. Participants were categorized as 10R homozygotes or 10/10 (n=17) or 9R carriers (n=21, which included n=19 9R carriers [9R heterozygous] and n=2 9R homozygotes). Allele frequencies did not deviate significantly from Hardy-Weinberg equilibrium.
2.5. Neuroimaging protocol
MR images were acquired on a Siemens 3T Allegra Scanner. T1 weighted magnetization-prepared rapid gradient echo (MPRAGE) images were acquired for morphometric analyses (scan parameters: repetition time (TR)=1400ms; echo time (TE)=2.48 ms; field of view (FOV)=256×256; 176 1-mm slices × 2; matrix 256 × 256). In addition, diffusion images were acquired using standard fast echo-planar imaging (TR=6500 ms; TE=88 ms; FOV=205×205; b=1000 s/mm2; 46 3-mm slices × 12 directions in addition to b=0), with images collected twice to optimize the signal-to-noise ratio.
Image processing has been detailed elsewhere (Clark et al., 2012; Chung et al., 2013). In brief, processing involved the creation of WM regions of interest (ROIs) using Freesurfer (Dale et al., 1999; Fischl et al., 2002, Fischl et al., 2004). To ensure data quality, we visually assessed motion artifacts (i.e., striping), and examined motion parameters graphically, as generated by FSL’s eddy correct program (fmrib.ox.ac.uk/fsl) to exclude cases with excess motion. Cortical reconstruction processing and volumetric segmentation were run according to standard procedures in Freesurfer. Registration to standard space was performed using individual cortical folding patterns to match cortical geometry across subjects. Parcellation of the cerebral cortex into units based on gyral and sulcal structure was done. Next, WM volumes were calculated for each cortical parcellation. Freesurfer measurements and white/gray matter parcellation have been validated against histological and manual measurements (Rosas et al., 2002; Kuperberg et al., 2003; Han et al., 2006). ROIs created by Freesurfer WM parcellation were visually inspected to ensure anatomical accuracy. The ROI approach used here, compared with whole-brain voxel-based approaches (e.g., Tract-Based Spatial Statistics), permits tests of hypotheses involving specific brain regions with greater sensitivity (Niogi et al., 2007).
Prefrontal and orbitofrontal ROIs were created by combining non-overlapping Freesurfer-defined bilateral WM regions, and were adjusted for total intracranial volume. The prefrontal ROI (excluding the OFC) included the frontal pole, frontal superior, frontal caudal middle, frontal rostral middle, pars opercularis, and pars triangularis. Orbitofrontal ROIs included frontal lateral orbital, frontal medial orbital, and pars orbitalis areas. The “prefrontal ROI” variable represented the sum of “prefrontal ROIs (excluding OFC)” and “orbitofrontal ROI”.
2.6. Data analysis
Comparisons between substance use treatment and community youth, and DAT1 10/10 versus 9R carrier groups, were conducted using chi-square test or t-tests. Correlations, controlling for recruitment source, age, and sex, were examined to determine the utility of testing hypothesized mediation (indirect effect) models (MacKinnon, 2008). Indirect effect analyses used an SPSS macro that ran a bootstrapping procedure (Preacher and Hayes, 2004). The mediation model tested an “A path,” which is the path from the independent variable (DAT1 genotype) to the intervening variable (e.g., regional WM volume); a “B path,” which represents the direct effect of the intervening variable (e.g., regional WM volume) on the dependent variable (Planning score); a “C path,” which represents the total effect of the independent variable on the dependent variable; and a “C′ path,” which represents the direct effect of the independent variable on the dependent variable, after controlling for the intervening variable. A significant indirect effect is indicated when the 95% bias-corrected and accelerated (BCa) confidence interval around the unstandardized coefficient does not include zero (Preacher and Hayes, 2004).
In this sample, fractional anisotropy (FA) in the PFC (excluding OFC) and OFC were not associated with DAT1 genotype (r= −0.14 to 0.08, p>0.3). Further, WM volume and FA in the PFC and OFC ROIs were not significantly correlated (r= −0.02 to 0.19; p>0.2). In this regard, WM volume and FA have been shown to be “moderately to weakly related,” and reflect different (complementary) aspects of WM integrity (Salat et al., 2005; Fjell et al., 2008; Taki et al., 2013). In further support of a focus on regional WM volume, a study of healthy college students found a significant association between executive functioning and WM volume (but not FA) in the left OFC (Takeuchi et al., 2013). Thus, analyses focus on WM volume in the PFC and OFC as an indicator of regional WM tissue integrity.
Regression analyses controlled for recruitment source (treatment=1, community=0), sex (1=female, 2=male), age, lifetime alcohol diagnosis (0=no, 1=yes), lifetime marijuana diagnosis (0=no, 1=yes), and IQ score. Recruitment source was covaried given differences between treatment and community youth in mental health and substance use. Due to differences in brain development by age and sex (Gogtay et al., 2007), these demographic characteristics were covaried. Alcohol and marijuana diagnosis was covaried due to possible effects of substance use on WM volume (Squeglia et al., 2009); results were similar using frequency of alcohol and marijuana use as covariates. Inclusion of other drug and nicotine use did not change the overall pattern of findings. Other drug and nicotine use were not significant covariates (p>0.2), and so were not included in the model reported. IQ score was covaried due to its potential relationship to planning and organization of behavior (Guy et al., 2004). Handedness was examined in preliminary analyses, but since it was not significantly associated with DAT1 genotype (p=0.5) or WM volumes (p>0.6), handedness was not included as a covariate in the analyses.
3. Results
3.1. Comparison of treatment and community youth
Treatment and community youth did not differ (p>0.05) in sex, age, SES, or DAT1 genotype (Table 1). However, treated youth, compared with community youth, had lower full scale IQ (t36= 3.51, p<0.01), more difficulties in Planning (t36= −4.53, p<0.001), and reported greater impulsivity (t36= −4.79, p<0.001). Youth in treatment, compared with community youth, also reported more frequent use (6 months before the assessment) of alcohol (t34= −2.67, p<0.05), marijuana (t35= −6.35, p<0.001), and tobacco (t36= −4.63, p<0.001); and were more likely to meet criteria for a lifetime DSM-IV diagnosis of alcohol (χ2[df=1]= 12.77, p<0.001), marijuana (χ2[df=1]= 30.48, p<0.001), and nicotine (χ2[df=1]= 6.24, p<0.05) use disorder. Treated youth also were more likely than their community counterparts to have lifetime DSM-IV diagnoses of conduct disorder (χ2[df=1]= 11.26, p<0.01), attention deficit hyperactivity disorder (χ2[df=1]= 5.74, p<0.05), and major depression (χ2[df=1]= 4.65, p<0.05). In addition, youth in treatment had lower WM volume in prefrontal (t36= 2.04, p<0.05) and prefrontal excluding orbitofrontal (t36= 2.05, p<0.05) ROIs compared with community youth (Table 1).
3.2. Comparison of DAT1 10/10 and 9c genotypes
DAT1 10/10 and 9R carrier genotypes did not differ (p>0.05) on demographic characteristics (sex, age, SES); IQ score; Planning T-score; impulsivity; alcohol, marijuana, or nicotine use disorder diagnosis; or psychiatric conditions that were commonly observed in the treatment sample (Table 2). However, carriers of the 9R carrier genotype, compared with 10/10, had greater WM volume in prefrontal (t36= −2.41, p<0.05), prefrontal excluding orbitofrontal (t36= −2.19, p<0.05), and orbitofrontal left hemisphere (t36= −2.72, p<0.01) ROIs.
Table 2.
Descriptive statistics for DAT1 10/10 homozygotes and 9R carriers
| Demographics | 10/10 n=17 |
9R carriers n=21 |
||
|---|---|---|---|---|
| n | % | n | % | |
| Female | 8 | 47.1 | 8 | 38.1 |
| Male | 9 | 52.9 | 13 | 61.9 |
| Substance use treatment | 10 | 58.8 | 12 | 57.1 |
| Community | 7 | 41.2 | 9 | 42.9 |
| Baseline | Mean (SD) | Mean (SD) | ||
| Age | 16.8 (1.2) | 16.4 (1.2) | ||
| Socio-economic status (Hollingshead, 1975) | 2.6 (1.1) | 2.2 (1.0) | ||
| WAIS Full Scale IQ | 105.4 (14.2) | 100.6 (12.9) | ||
| Planning T score | 54.9 (12.6) | 53.0 (13.6) | ||
| Barratt Impulsivity Scale score | 67.4 (13.4) | 70.7 (12.8) | ||
| Frequency of substance use (past 6-months)† | ||||
| Alcohol use | 2.8 (2.3) | 2.6 (1.9) | ||
| Marijuana use | 4.2 (3.5) | 3.0 (3.1) | ||
| Tobacco use | 3.9 (3.4) | 4.2 (3.9) | ||
| n | % | n | % | |
| Lifetime DSM-IV alcohol use disorder | 7 | 41.1 | 8 | 38.1 |
| Alcohol Abuse | 4 | 23.5 | 8 | 38.1 |
| Alcohol Dependence | 3 | 17.6 | 0 | 0.0 |
| Lifetime DSM-IV cannabis use disorder | 12 | 70.6 | 12 | 57.1 |
| Cannabis Abuse | 7 | 41.2 | 11 | 52.4 |
| Cannabis Dependence | 5 | 29.4 | 1 | 4.7 |
| Lifetime DSM-IV nicotine use disorder | 4 | 23.5 | 3 | 14.3 |
| Lifetime DSM-IV psychopathology | ||||
| Conduct disorder | 6 | 35.3 | 5 | 23.8 |
| Attention deficit hyperactivity disorder | 3 | 17.6 | 7 | 33.3 |
| Major depression | 5 | 29.4 | 4 | 19.0 |
| White matter volume | Mean (SD) | Mean (SD) | ||
| Prefrontal volume | 6.94 (0.36) | 7.27 (0.45)* | ||
| Prefrontal volume (excluding orbitofrontal) | 5.58 (0.31) | 5.84 (0.40)* | ||
| Orbitofrontal volume | 1.37 (0.10) | 1.43 (0.10) | ||
| Left hemisphere | 0.66 (0.05) | 0.70 (0.05)** | ||
| Right hemisphere | 0.71 (0.05) | 0.73 (0.06) | ||
Notes: Baseline N=38; SD= standard deviation.
Frequency coded: 0=never used, 1=no use in the last 6-months, 2=used < once per month, 3=used once per month, 4=used 2–3 times per month, 5=used once per week, 6=used 2–3 times per week, 7=used 4–6 times per week, 8=daily
comparison of 10/10 vs 9 carrier p<0.05;
comparison of 10/10 vs 9 carrier p=0.01
3.3. Correlations of DAT1 genotype, white matter volume, and self-report measures
Partial correlations (controlling for recruitment source, sex, and age) indicated significant associations (“moderate” effect size; Cohen, 1988) between the DAT1 genotype and WM volume in prefrontal and left orbitofrontal ROIs (p<0.05; see Table 3). The left orbitofrontal ROI was negatively associated with the Planning T-score (p<0.05), indicating that lower WM volume in this region was associated with greater self-report of Planning difficulties. Although the partial correlation of Planning and impulsivity was 0.69 (p<0.01; bivariate r=0.83, p<0.001), the left orbitofrontal ROI was only significantly associated with Planning. The DAT1 genotype was not significantly associated with either Planning or impulsivity. The pattern of correlations supported the utility of testing an indirect effects model in which the DAT1 genotype is indirectly associated, through WM volume in the left orbitofrontal ROI, with the Planning T-score (see Fig. 1).
Table 3.
Partial correlations of DAT1 genotype, white matter volumes in regions of interest, and self-report measures
| DAT1 genotype | Prefrontal | Prefrontal (no orbitofrontal) | Orbitofrontal | Orbitofrontal Left Hemisphere | Orbitofrontal Right Hemisphere | |
|---|---|---|---|---|---|---|
| DAT1 genotype | 0.38* | 0.35* | 0.30 | 0.41* | 0.15 | |
| Brief Planning T-score | −0.03 | −0.08 | −0.02 | −0.26 | −0.36* | −0.12 |
| Barratt Impulsivity Scale score | 0.23 | 0.03 | 0.06 | −0.07 | −0.07 | −0.06 |
Notes: N=38,
p<0.05
DAT1 genotype: 10/10 homozygote coded 0, 9R carrier coded 1
Partial correlations controlling for recruitment source (treatment vs community), sex, and age
3.4. Indirect effect of DAT1 genotype on Planning T-score through WM volume
Table 4 reports the parameter estimates for the indirect effects regression model. Results indicate that the DAT1 9R genotype is associated with greater WM volume in the left orbitofrontal ROI (A path), and that greater WM volume in this ROI is associated with less self-reported difficulties in Planning (B path). The direct association between the DAT1 genotype and Planning was not significant. A significant indirect effect linking the DAT1 genotype with the Planning T-score, through WM volume in the left hemisphere orbitofrontal ROI, was detected, point estimate=−3.92 (BCa 95% CI: −10.59, −0.88). Among the covariates, only IQ score was significant, such that higher IQ was associated with fewer difficulties in Planning (p<0.05).
Table 4.
Parameter estimates for regression model testing indirect effects of regional white matter volume on the association between DAT1 genotype and BRIEF Planning T-score
| B | SE | t | p | ||
|---|---|---|---|---|---|
|
|
|||||
| A path | “mediator”=LH OF | 0.05 | 0.02 | 2.81 | 0.01 |
| B path | −76.01 | 33.46 | −2.27 | 0.03 | |
| C path | −2.91 | 3.58 | −0.81 | 0.42 | |
| C′ path | 1.01 | 3.78 | 0.27 | 0.79 | |
| Controls | Recruitment source | 8.91 | 8.08 | 1.10 | 0.28 |
| Sex | −4.98 | 3.26 | −1.52 | 0.14 | |
| Age | 0.83 | 1.56 | 0.53 | 0.60 | |
| IQ score | −0.29 | 0.14 | −2.06 | 0.048 | |
| Alcohol diagnosis | 3.23 | 4.34 | 0.74 | 0.46 | |
| Marijuana diagnosis | −0.30 | 8.39 | −0.04 | 0.97 | |
Model summary: R2=0.59, F(8, 29)=5.13, p=0.0005
Notes: n=38
DAT1 genotype: 10R/10R coded 0, 9R carrier coded 1
B = unstandardized coefficient, SE=standard error. “Mediator”=intervening variable being tested.
LH OF=white matter volume in left hemisphere of orbitofrontal region of interest
Recruitment source: treatment coded 1, community coded 0
Sex: female coded 1, male coded 2
Alcohol diagnosis= lifetime DSM-IV alcohol use disorder, absent coded 0, present coded 1
Marijuana diagnosis=lifetime DSM-IV marijuana use disorder, absent coded 0, present coded 1
Replacing lifetime alcohol and marijuana diagnosis with frequency of alcohol and marijuana use in the past 6 months did not change the pattern of results; and alcohol and marijuana use frequency were not significant covariates.
A path (see Figure): independent variable (DAT1 genotype) to intervening variable (LH OF). B path: direct effect of intervening variable (LH OF) on dependent variable (Planning T-score). C path: Total effect of independent variable (DAT1 genotype) on dependent variable (Planning T-score). C′ path: Direct effect of independent variable (DAT1 genotype) on dependent variable (Planning T-score), after controlling for the intervening variable (LH OF). Controls: partial effect of control variables on dependent variable.
4. Discussion
The results provide some support for the hypothesized indirect effects model. As predicted, the DAT1 10/10 genotype was associated with lower PFC WM volume, and lower PFC WM volume, specifically in the left OFC, was associated with difficulties in Planning. These associations represent “moderate” effect sizes. Although the DAT1 genotype was not directly associated with either impulsivity or Planning, an indirect effect of the 10/10 genotype on difficulties in Planning was detected, with lower WM volume in the left OFC serving as a linking mechanism. Although impulsivity and Planning measures were correlated, significant associations were specific to Planning, and to the left OFC, instead of to the PFC more generally.
Regarding the specificity of findings to difficulties in Planning, the BRIEF Planning scale assesses an adolescent’s ability to manage future-oriented demands, such as the ability to anticipate future events, set a goal, and develop a sequence of steps to be accomplished in achieving the goal (Guy et al., 2004). Although there is some overlap in the domains covered by the Planning scale and the Barratt Impulsivity Scale (BIS), such as planning ahead, the BIS provides less coverage of the cognitive aspects of goal setting and planning. The BRIEF Planning scale’s greater focus on cognitive aspects of planning that have been related specifically to the OFC, and more generally to PFC function, may partially explain the specificity of the association between OFC WM volume and the Planning T-score. Given that other studies have observed direct associations of the DAT1 genotype and risk taking behavior (e.g., Mata et al., 2012) and impulsive responding (Gizer and Waldman, 2012), other aspects of OFC and PFC structure (such as gray matter volume), or functioning, which were not examined here, might be associated with specific facets of impulsivity and executive functioning.
This study’s finding of an association with OFC WM volume and the Planning score is in line with results from a study of healthy young adults, which found that greater left OFC WM volume was associated with better executive functioning in everyday activities (Takeuchi et al., 2013). The localization of findings to the left OFC in adolescent and young adult samples warrants further investigation, particularly since this asymmetry did not appear to be associated with handedness in this adolescent sample, and the DAT1 effect on resting state striato-frontal connectivity in a study of healthy adults was symmetrical (Gordon et al., 2015). Of note, however, a recent functional MRI study of youth (ages 12–15) found that greater activation in the left lateral OFC in order to achieve correct performance on an antisaccade task (a result which was interpreted as lower efficiency of processing) was associated with self-reported difficulties in flexibly in using problem-solving strategies to adapt to changing circumstances (Zhai et al., in press). The importance of structural and functional deviations specifically in left, versus right, OFC in relation to planning, problem solving, and other executive functions warrants further study.
Study results did not support a direct association of the 10/10 DAT1 genotype with a deficit in executive function, as found in some studies (Loo et al., 2003; Stollstorff et al., 2010). In this adolescent sample, the DAT1 genotype was only indirectly associated with managing future-oriented goals, through left OFC WM volume. The finding of lower WM volume in the left OFC among youth with the 10/10 genotype is novel, and suggests possible downstream effects on, for example, weaker striato-frontal connectivity, which was observed among adults with the 10/10 genotype (Gordon et al., 2015). For example, lower regional WM volume may reduce efficiency of communication within, as well as among, brain regions, serving as a mechanism by which the DAT1 genotype might be associated with more complex behavioral phenotypes involving executive function. Results from earlier work indicating DAT1 effects on caudate gray matter volume (Durston et al., 2005) suggest multiple potential, and possibly converging, pathways that could contribute to DAT1 effects on measures of executive function.
The study sample included youth from both substance use treatment and the wider community, which provided a range in severity of the behavioral phenotypes. This phenotypic variation likely increased the ability to detect associations between the DAT1 genotype and phenotype (Durston et al., 2005). Notably, treatment and community samples did not differ in DAT1 genotype distribution, despite between-group differences in impulsivity and Planning, level of substance involvement, and co-occurring psychopathology. In addition, treatment and community youth differed in prefrontal WM volume, but not left OFC WM volume. Importantly, the DAT1 genotype was associated with left OFC WM volume in analyses that controlled for age, sex, and recruitment source. Further, the effect of the DAT1 10/10 genotype and lower left OFC WM volume was observed when controlling for recent substance use, supporting a more general DAT1 genetic effect on left OFC WM volume that was independent of recruitment source. The DAT1 effect on left OFC WM volume is notable, since greater WM integrity as indicated by regional fractional anisotropy has been associated with better substance use treatment outcome in an overlapping sample of youth (Chung et al., 2013). Since WM volume and FA are only moderately to weakly related, and appear to reflect different aspects of WM integrity (Fjell et al., 2008), further research examining various indicators of WM integrity is needed.
Study limitations warrant comment. Only Caucasian youth, sampled from substance use treatment and the community, were included, which limits generalizability. Although the sample provided a broad range in the behavioral phenotypes of interest, sample size was relatively small. Only one candidate gene was examined, although multiple genes likely contribute to WM volume and complex behavioral phenotypes. Substance use may reduce WM volume in youth (Squeglia et al., 2009), although recent frequency of substance use was not correlated with regional WM volume in this mixed sample of treated and community youth. Only WM volume within selected brain regions (PFC and OFC) was examined, such that directions for future research include the use of other methods to examine WM integrity such as tract-based analyses to examine inter-region connectivity, other measures of regional brain structure (e.g., WM fractional anisotropy, gray matter volume), and more narrowly defined structural regions (e.g., separate analyses of the three component OFC segments that were combined in this study). Measures of functional activation (e.g., resting state or task-related connectivity) also remain to be examined. Self-reports of impulsivity and Planning were used, which could be supplemented by laboratory-based measures in future research. Although we tested a specific gene-to-brain-to-behavior model, alternative models that could account for the pattern of observed correlations remain to be tested. A relatively large number of statistical tests were conducted. Procedures to minimize Type I error included examining a limited number of ROIs and behavioral measures based on earlier research findings, including relevant covariates, and describing effect sizes for statistically significant associations. Replication of results is needed.
The current study’s findings suggest a possible brain structural mechanism, involving lower WM volume in the left OFC, which links the 10/10 DAT1 genotype to impaired executive functioning, specifically in relation to planning goal-directed behavior. The novel finding that lower WM volume mediated the association between the DAT1 genotype and an indicator of executive functioning supports the use of neuroimaging measures as an intermediate phenotype, which could ultimately inform the development of interventions that aim to improve executive functioning in youth.
Supplementary Material
Highlights.
DAT1 10/10 (vs 9 carrier) was associated with lower orbitofrontal white matter (WM) volume
Lower left orbitofrontal WM volume predicted difficulties in planning (executive function)
DAT1 10/10 was linked with difficulties in planning through lower left orbitofrontal WM volume
Acknowledgments
Support for the conduct of the research and preparation of the manuscript was provided by funding from the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse (R01 AA014357, R21 AA016272, R21 AA017128, K02 AA018195, P50DA005605, U01AA021690).
Footnotes
Contributors
T.C. collaborated on study design, data collection, and data analysis, conducted the statistical analyses, and wrote the draft manuscript. R.F. conducted genotyping and provided feedback on the manuscript. D.C. collaborated on study design and data collection, and provided feedback on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartzokis G. Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology. 2012;62:2137–2153. doi: 10.1016/j.neuropharm.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Amar CP, Raven EP, Detore NR, Altshuler LL, Mintz J, Ventura J, Casaus LR, Luo JS, Subotnik KL, Nuechterlein KH. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophrenia Research. 2011;132:35–41. doi: 10.1016/j.schres.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O’Neil JP, Baker SL, Madison CM, Jagust WJ. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Human Brain Mapping. 2011;32:947–961. doi: 10.1002/hbm.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, Clark DB. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug and Alcohol Dependence. 2011;115:43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, San Pedro R, Shriberg RF, Cornelius JR. Retest reliability and discrepancy interview for DSM-IV alcohol, cannabis, and nicotine diagnoses in treated adolescents. Alcoholism: Clinical and Experimental Research. 2004;28:Abstract 111A. [Google Scholar]
- Chung T, Pajtek S, Clark DB. White matter integrity as a link the association between motivation to abstain and treatment outcome in adolescent substance users. Psychology of Addictive Behaviors. 2013;27:533–542. doi: 10.1037/a0026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher D, Pajtek S, Long E. Psychological dysregulation, white matter organization and substance use disorders in adolescence. Addiction. 2012;107:206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum; Mahwah, NJ: 1988. [Google Scholar]
- Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, Gunn RN, Matthews PM, Rabiner EA, Beaver JD. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cerebral Cortex. 2012;22:2784–2793. doi: 10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L, Wu X, Faruqi AF, Bray-Ward P, Sun Z, Zong Q, Du Y, Du J, Driscoll M, Song W, Kingsmore SF, Egholm M, Lasken RS. Comprehensive human genome amplification using multiple displacement amplification. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Molecular Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Whitaker KJ, Steele JS, Green CT, Wendelken C, Bunge SA. White matter maturation supports the development of reasoning ability through its influence on processing speed. Developmental Science. 2013;16:941–951. doi: 10.1111/desc.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM. White matter: organization and functional relevance. Neuropsychology Review. 2010;20:158–173. doi: 10.1007/s11065-010-9127-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bjornerud A, Due-Tonnessen P, Walhovd KB. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Waldman ID. Double dissociation between lab measures of inattention and impulsivity and the dopamine transporter gene (DAT1) and dopamine D4 receptor gene (DRD4) Journal of Abnormal Psychology. 2012;121:1011–1023. doi: 10.1037/a0028225. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, 3rd, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Devaney JM, Bean S, Vaidya CJ. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cerebral Cortex. 2015;25 (2):336–345. doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy SC, Isquith PK, Gioia GA. BRIEF-SR: Behavior Rating Inventory of Executive Function--Self-Report Version Professional Manual. Psychological Assessment Resources, Inc; Lutz, FL: 2004. [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. British Medical Bulletin. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Hawi Z, Kent L, Hill M, Anney RJ, Brookes KJ, Barry E, Franke B, Banaschewski T, Buitelaar J, Ebstein R, Miranda A, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Faraone SV, Asherson P, Gill M. ADHD and DAT1: further evidence of paternal over-transmission of risk alleles and haplotype. American Journal of Medical Genetics: Part B. 2010;153B:97–102. doi: 10.1002/ajmg.b.30960. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-Factor Index of Social Status. Author; New Haven, CT: 1975. [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. The American Journal of Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. The Journal of Neuroscience. 2012;32:6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King IB, Satia-Abouta J, Thornquist MD, Bigler J, Patterson RE, Kristal AR, Shattuck AL, Potter JD, White E. Buccal cell DNA yield, quality, and collection costs: comparison of methods for large-scale studies. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:1130–1133. [PubMed] [Google Scholar]
- King KM, Chung T, Maisto SA. Adolescents’ thoughts about abstinence curb the return of marijuana use during and after treatment. Journal of Consulting and Clinical Psychology. 2009;77:554–565. doi: 10.1037/a0015391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Dresel SH, Krause KH, La Fougere C, Zill P, Ackenheil M. Striatal dopamine transporter availability and DAT-1 gene in adults with ADHD: no higher DAT availability in patients with homozygosity for the 10-repeat allele. The World Journal of Biological Psychiatry. 2006;7:152–157. doi: 10.1080/15622970500518444. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature reviews: Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. Lawrence Erlbaum; New York: 2008. [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Krenek M, Chung T, Martin CS, Cornelius JR, Clark DB. Comparison of the concurrent and predictive validity of three measures of readiness to change alcohol use in a clinical sample. Psychological Assessment. 2011;23:983–994. doi: 10.1037/a0024136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R, Hau R, Papassotiropoulos A, Hertwig R. DAT1 polymorphism is associated with risk taking in the Balloon Analogue Risk Task (BART) PloS One. 2012;7:e39135. doi: 10.1371/journal.pone.0039135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. American Journal of Medical Genetics. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, McCandliss BD. Diffusion tensor imaging segmentation of white matter structures using a Reproducible Objective Quantification Scheme (ROQS) Neuroimage. 2007;35:166–174. doi: 10.1016/j.neuroimage.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain and Cognition. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Research. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavioral Research Methods and Instrumentation. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLoS Computational Biology. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert S. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollstorff M, Foss-Feig J, Cook EH, Jr, Stein MA, Gaillard WD, Vaidya CJ. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53:970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Brain structures associated with executive functions during everyday events in a non-clinical sample. Brain Structure & Function. 2013;218:1017–1032. doi: 10.1007/s00429-012-0444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R. Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry. Neuroimage. 2012;59:2899–2907. doi: 10.1016/j.neuroimage.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Hashizume H, Sassa Y, Takeuchi H, Wu K, Kotozaki Y, Nouchi R, Asano M, Asano K, Fukuda H, Kawashima R. Linear and curvilinear correlations of brain white matter volume, fractional anisotropy, and mean diffusivity with age using voxel-based and region-of-interest analyses in 246 healthy children. Human Brain Mapping. 2013;34:1842–1856. doi: 10.1002/hbm.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark DB. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug and Alcohol Dependence. 2010;110:55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wexler D. Wechsler Abbreviated Scale of Intelligence (WASI) manual. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. American Journal of Medical Genetics: Part B. 2007;144B:541–550. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- Zhai ZW, Pajtek S, Luna B, Geier C, Ridenour TA, Clark DB. Reward modulated response inhibition, cognitive shifting and the orbitofrontal cortex in early adolescence. Journal of Research on Adolescence. doi: 10.1111/jora.12168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.