Abstract
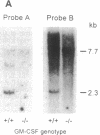
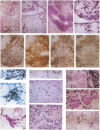
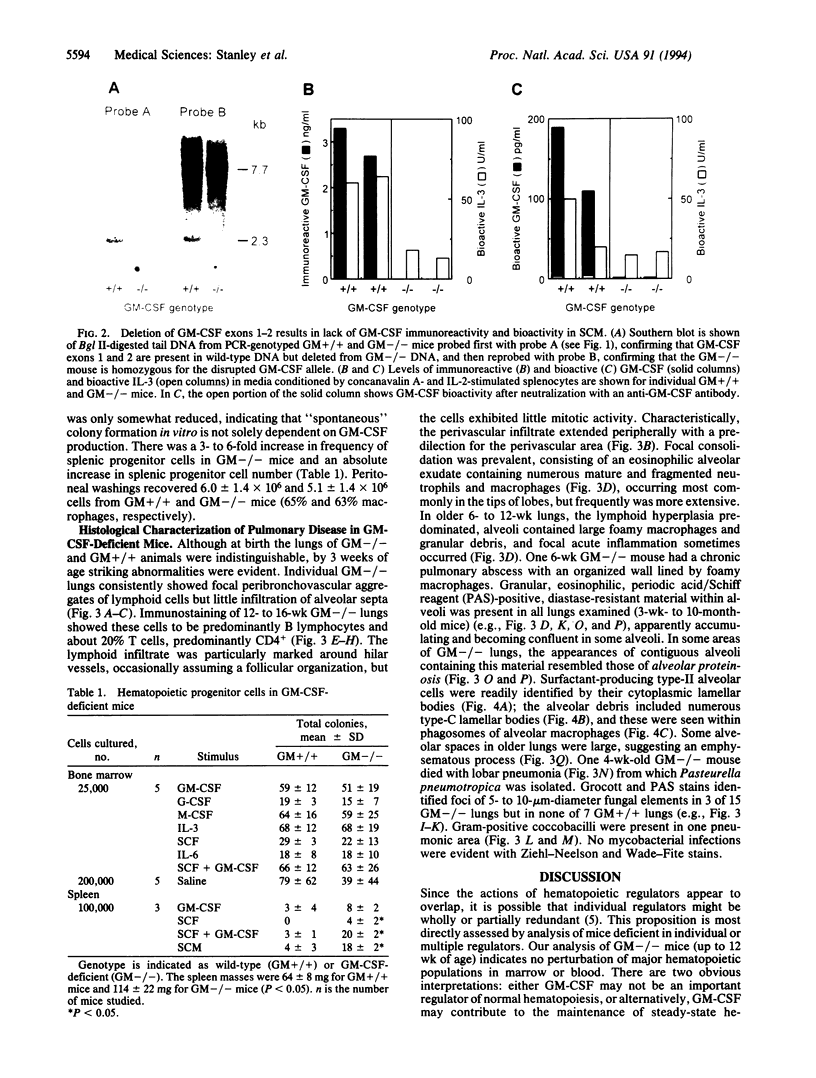
Mice homozygous for a disrupted granulocyte/macrophage colony-stimulating factor (GM-CSF) gene develop normally and show no major perturbation of hematopoiesis up to 12 weeks of age. While most GM-CSF-deficient mice are superficially healthy and fertile, all develop abnormal lungs. There is extensive peribronchovascular infiltration with lymphocytes, predominantly B cells. Alveoli contain granular eosinophilic material and lamellar bodies, indicative of surfactant accumulation. There are numerous large intraalveolar phagocytic macrophages. Some mice have subclinical lung infections involving bacterial or fungal organisms, occasionally with focal areas of acute purulent inflammation or lobar pneumonia. Some features of this pathology resemble the human disorder alveolar proteinosis. These observations indicate that GM-CSF is not essential for the maintenance of normal levels of the major types of mature hematopoietic cells and their precursors in blood, marrow, and spleen. However, they implicate GM-CSF as essential for normal pulmonary physiology and resistance to local infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. M., Warnock M. L., Curtis J. L., Sniezek M. J., Arraj-Peffer S. M., Kaltreider H. B., Shellito J. E. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol. 1991 Aug;5(2):186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- Bilyk N., Holt P. G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993 Jun 1;177(6):1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gasson J. C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991 Mar 15;77(6):1131–1145. [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Gough N. M. Differential inhibition by cyclosporin A reveals two pathways for activation of lymphokine synthesis in T cells. Growth Factors. 1989;1(2):165–177. doi: 10.3109/08977198909029126. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Williamson D. J., Nice E. C., De Lamarter J., Mermod J. J., Thatcher D., Schmidt A. Hemopoietic responses in mice injected with purified recombinant murine GM-CSF. Exp Hematol. 1987 Jan;15(1):1–9. [PubMed] [Google Scholar]
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993 Dec 15;82(12):3515–3523. [PubMed] [Google Scholar]
- Metcalf D. Lineage commitment of hemopoietic progenitor cells in developing blast cell colonies: influence of colony-stimulating factors. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11310–11314. doi: 10.1073/pnas.88.24.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Coffman R. L. A cell-surface antigen shared by B cells and Ly2+ peripheral T cells. Cell Immunol. 1982 Jul 1;70(2):311–320. doi: 10.1016/0008-8749(82)90332-x. [DOI] [PubMed] [Google Scholar]
- Nogee L. M., de Mello D. E., Dehner L. P., Colten H. R. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993 Feb 11;328(6):406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- ROSEN S. H., CASTLEMAN B., LIEBOW A. A. Pulmonary alveolar proteinosis. N Engl J Med. 1958 Jun 5;258(23):1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Lee D. K., Lacy J., Coleman D. L. Rat tracheal epithelial cells produce granulocyte/macrophage colony-stimulating factor. Am J Respir Cell Mol Biol. 1990 Jan;2(1):59–68. doi: 10.1165/ajrcmb/2.1.59. [DOI] [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]