Abstract
Background & Aims
Low-grade chronic inflammation is a cardinal feature of the metabolic syndrome, yet its pathogenesis is not well defined. The purpose of this study was to examine the role of TRAIL receptor (TR) signaling in the pathogenesis of obesity-associated inflammation utilizing mice with the genetic deletion of TR.
Methods
TR knockout (TR−/−) mice and their littermate wild-type (WT) mice were fed a diet high in saturated fat, cholesterol and fructose (FFC) or chow. Metabolic phenotyping, liver injury, and liver and adipose tissue inflammation were assessed. Chemotaxis and activation of mouse bone marrow-derived macrophages (BMDMϕ) was measured.
Results
Genetic deletion of TR completely repressed weight gain, adiposity and insulin resistance in FFC-fed mice. Moreover, TR−/− mice suppressed steatohepatitis, with essentially normal serum ALT, hepatocyte apoptosis and liver triglyceride accumulation. Gene array data implicated inhibition of macrophage-associated hepatic inflammation in the absence of the TR. In keeping with this, there was diminished accumulation and activation of inflammatory macrophages in liver and adipose tissue. TR−/− BMDMϕ manifest reduced chemotaxis and diminished activation of nuclear factor-κ B signaling upon activation by palmitate and lipopolysaccharide.
Conclusions
These data advance the concept that macrophage-associated hepatic and adipose tissue inflammation of nutrient excess requires TR signaling.
Keywords: nonalcoholic fatty liver disease, metainflammation, lipoapoptosis, macrophage activation
Chronic low-grade tissue inflammation occurs in obesity-related diseases including type 2 diabetes mellitus and nonalcoholic fatty liver disease (NAFLD). Obesity-associated inflammation appears to be triggered by the recruitment and activation of macrophages within adipose and liver tissues [27]. Inflammation promotes insulin resistance by enhancing lipolysis in adipose tissue and liberating free fatty acids (FFAs) and other lipid mediators into the circulation. Many of these lipids are proinflammatory and can cause cellular demise termed lipotoxicity [30]. For example, elevated concentrations of saturated FFAs cause death of hepatocytes, pancreatic β cells, and adipocytes by apoptosis, a process termed lipoapoptosis [30]. Lipoapoptosis is a histologic hallmark of nonalcoholic steatohepatitis (NASH) and correlates with disease severity [11]. More importantly, the liver contains abundant resident macrophages, Kupffer cells, and their activation or an influx of recruited macrophages has been implicated in the progression of NASH-associated liver injury.[25] Of note, cell death by apoptosis has recently been associated with release of cytokines including monocyte chemotactic protein 1 (MCP-1) which could provide a signal for monocyte recruitment into the liver [8]. Activated macrophages within the tissue may in turn express death ligands such as Fas ligand (FasL), tumor necrosis factor alpha (TNF-α), and TNF-related apoptosis-inducing ligand (TRAIL), further aggravating lipoapoptosis.
Activated macrophages induce inflammation and apoptosis of adjacent cells, in part, by secreting TNF superfamily ligands [32]. Of these ligands, the most potent apoptosis inducers are FasL, TNF-α, and TRAIL. Genetic deletion of Fas protects against adipose tissue inflammation, insulin resistance and hepatic steatosis in mice fed a high fat diet [37]. TNF-α has also been implicated in obesity-associated metabolic syndrome [28]. However, the role of TRAIL and its cognate death receptors in lipotoxicity has not been explored. Yet, several observations implicate a critical role for TRAIL and/or its cognate receptors in lipotoxicity. TRAIL has been implicated in the genesis of hepatic steatosis [24], and its receptors are upregulated in human NASH specimens [1], dietary models of NASH [10, 14], in genetically obese ob/ob mice [18], and in FFA-treated hepatocytes [21]. Serum TRAIL concentrations are associated with anthropometric variables and serum lipids in humans [6], and subcutaneous adipose tissue expression of TRAIL and it’s cognate receptors are increased in human obesity [18]. Finally, the saturated FFA palmitate also promotes ligand-independent, TR-initiated hepatocyte cytotoxicity [4]. Thus, the role of TRAIL signaling in obesity-associated inflammation merits further investigation. Mice possess only a single ortholog of the two closely related human TRs (TNFRSF10A and TNFRSF10B).[12], [36] Although the mouse receptor has been referred to as death receptor 5, we have referred to it as TR.
MATERIALS AND METHODS
Animal studies
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Heterozygotes in a C57BL/6J background were bred to obtain TR−/− and wild-type littermate mice as described [12, 36]. Mice were housed 4–5/cage with a 12 h light-dark cycle, and ad libitum access to food and water. For the dietary studies, the animals were assigned to one of two groups: 1) standard chow diet (Purina LabDiet, St. Louis, MO); or 2) a fructose-fat-cholesterol (FFC) diet (AIN-76A Western Diet 1/2, TestDiet, Richmond, IN) as previously described [5]. This diet provides 40% kcal from fat, 45% kcal from carbohydrate, 15% kcal from protein and has 0.2% cholesterol. Total body weight was measured using a standard balance. Quantitative magnetic resonance was used to quantify lean and fat mass described previously [20]. At the completion of the study, mice were euthanized, blood, liver and adipose tissues were collected [14, 22]. The adipose depots were individually excised and weighed as previously described [29].
Bone marrow derived macrophage isolation and cell migration assays
Bone marrow-derived macrophages (BMDMϕ) were isolated as described [22]. Cell migration assays were performed with BMDMϕ using Corning Transwell plates with 5 μm pores (Corning Inc, Corning, NY). BMDMϕ were serum starved for 2 hours, detached with 5 mM EDTA in PBS, and resuspended in RPMI-complete media (RPMI-1640 media with 10% FBS), containing 400 μM PA [4] and/or 10 ng/ml LPS, and applied to the upper chamber. After allowing 1 hour for attachment, TRAIL 100 ng/ml, or fMLP 100 nM were added to the lower chamber. Migration was assessed after an additional 5.5 hours by counting DAPI stained nuclei of migrated and total cells.
Immunofluorescence of p65 NF-κB
BMDMϕ cells were treated with palmitate and/or LPS as above, for 1 hour. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS, blocked with 1% BSA in PBS, and the primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was applied overnight at 4°C. Secondary antibody used was Alexa fluor chicken anti-rabbit antisera (Molecular Probes, Eugene, OR) for 1 h at 37°C. Cells were mounted with ProLong antifade with DAPI (Molecular Probes) and images acquired by confocal microscopy (Carl Zeiss, Jena, Germany) with excitation and emission wavelengths of 488 and 507 nm, respectively. Cells with nuclear translocation of p65 NF-κB were quantified and expressed as a percentage of the total cell number.
Immunoblot analysis
Total protein from liver, adipose tissue, and BMDMϕs was isolated, resolved and detected as previously described [22]. Primary antibodies used were: caspase-8 (Enzo Life Sciences, Farmingdale, NY); Ser32/36 IκB-α (Cell Signaling Technology, Danvers, MA); C/EBP-α or C/EBP-β (Santa Cruz Biotechnology). GAPDH (Millipore, Billerica, MA) or β-actin (Santa Cruz Biotechnology) was used as loading control.
ELISA for Monocyte chemotactic protein 1 (MCP-1)
MCP-1 was measured in cell culture supernatants of isolated primary hepatocytes with mouse CCL2/JE/MCP-1 DuoSet ELISA Development kit (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. Primary hepatocytes from WT and TR−/− were treated with 400 μM PA for 8 h for this assay. The measured MCP-1 concentration was as expressed as pg/ml.
Statistical analysis
Data are presented as mean ± SEM except where indicated. Statistical significance between multiple groups was determined by two-tailed ANOVA while statistical difference between two groups was defined by unpaired t-test using GraphPad Prism software. Statistical analysis of the microarray data including gene ontology and pathway analysis (MetaCore software, Thomson Reuters/Genego, St Joseph, MI and IPA software, Ingenuity Systems, Redwood City, CA) was performed in collaboration with the Division of Biomedical Statistics & Informatics, Mayo Clinic, Rochester, MN.
Supplementary materials and methods
Additional methods including glucose tolerance tests, insulin tolerance tests, indirect calorimetry, adipose tissue characterization, isolation of liver cell subpopulations, RNA isolation, quantitative real-time PCR (qPCR), histologic analyses and biochemical analyses are provided in the supplementary materials and methods section.
RESULTS
TR−/− mice are resistant to diet induced obesity
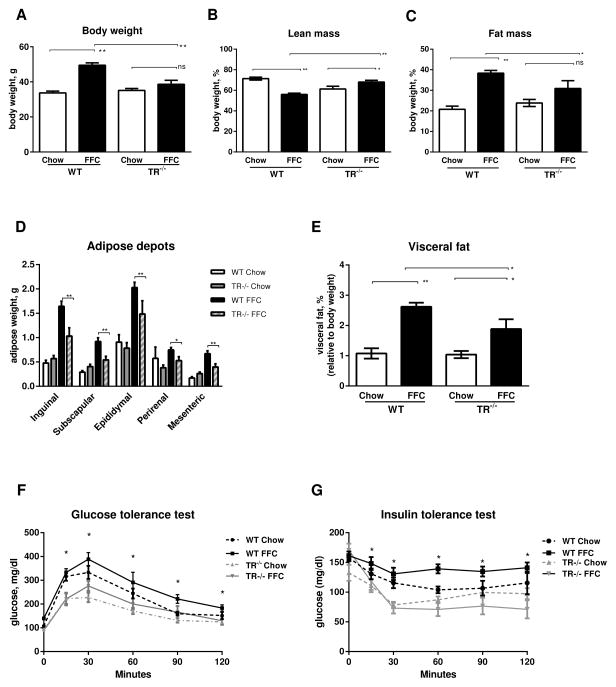
In accordance with our prior observations [5], mice fed the FFC diet for 6 months weighed significantly more than mice fed standard chow; however, the body weight of wild-type mice was 29% greater than that of TR−/− mice (Fig. 1A). TR−/− mice had greater relative lean mass and less relative fat mass on the FFC diet compared to wild-type mice (Fig. 1B and C). Chow-fed mice showed no differences between genotypes with regard to total body mass, lean mass or fat mass. Given the reduced fat mass of FFC-fed TR−/− mice, we characterized the specific adipose depots. Compared to chow-fed wild-type mice, FFC-fed wild-type animals had larger inguinal, subscapular, epididymal, and mesenteric adipose depots (Fig. 1D); these adipose depots were decreased in size in FFC-fed TR−/− mice. Overall, significant visceral fat accumulation occurred in both wild-type and TR−/− mice fed the FFC diet compared to respective chow-fed mice; however, this was significantly reduced in FFC-fed TR−/− compared to FFC-fed wild-type (Fig. 1E).
Fig. 1. TR−/− mice are resistant to diet induced obesity.
(A) Body weight in grams (g), (B) Lean mass (%), (C) Fat mass (%), (D) White adipose tissue (g), (E) Visceral fat (%), (F) Serum glucose determinations following an intraperitoneal injection of glucose, and (G) Serum glucose determinations following an intraperitoneal injection of insulin in wild-type (WT) and TR knockout (TR−/−) mice fed chow or FFC diet for 6 months (n=7 per group). For B and C the percent lean and fat mass totals to approximately 90%, as QMR excludes skin and bones which accounts for the remaining 10% body mass. For A–E *p<0.05, **p<0.01. For F and G the differences between FFC-fed WT versus TR−/− mice are statistically significant by two-tailed ANOVA (*p<0.05).
To determine whether the protective effects of TR−/− on diet-induced obesity were associated with metabolic benefits, we examined glucose tolerance and insulin sensitivity. The 12 hour fasted blood glucose of chow-fed TR−/− was lower than wild-type mice (Fig. 1F). The FFC diet caused a significant elevation in fasting blood glucose levels in wild-type mice, but not in TR−/− mice, 139.6 ± 5.5 mg/dl and 89.5 ± 7.2 mg/dl, respectively (p<0.01). Moreover, following an intraperitoneal bolus of glucose, chow- and FFC-fed TR−/− mice had significantly lower glucose concentrations compared to diet-matched wild-type mice. The FFC diet diminished the response of wild-type mice to an exogenous intraperitoneal bolus of insulin (Fig. 1G). In contrast, TR−/− mice were protected from diet-induced insulin resistance and displayed preserved insulin sensitivity, comparable to chow-fed TR−/− mice. Collectively, these results suggest deletion of TR confers salutary effects in mice against diet-induced obesity and associated insulin-resistant state. To explain resistance to body weight gain and preserved insulin sensitivity in FFC-fed TR−/− mice we looked for differences in caloric intake, physical activity, respiratory exchange ratio and energy expenditure (Supplementary Fig. 1). There were no significant differences in these metabolic parameters between FFC-fed wild-type and FFC-fed TR−/− mice. To investigate the tissue injury profiles in these mice, given our interest in the pathogenesis of NASH, and the liver being a key target organ in the metabolic syndrome we first interrogated parameters of liver injury.
Hepatic steatosis and liver injury are reduced in FFC-fed TR−/− mice
FFC-fed wild-type mice displayed hepatic steatosis as assessed by histology and CARS microscopy (Fig. 2A and B), increased relative (normalized to body weight) liver weight, (Fig. 2C) hepatic triglyceride content (Fig. 2D), and elevated serum ALT (Fig. 2E) compared to chow-fed mice. In contrast, FFC-fed TR−/− mice demonstrated minimal changes in liver histology and no increase in relative liver weight or hepatic triglycerides, and near normal serum ALT. Additional parameters of liver injury, including TUNEL positive apoptotic hepatocytes and hepatic fibrosis assessed by Sirius red staining (Fig. 2F–I) were also increased in FFC-fed wild-type mice. Liver collagen 1 alpha-1 mRNA expression was significantly increased in FFC-fed wild-type mice (Fig. 2J). In contrast, FFC-fed TR−/− mice were protected from liver injury and fibrosis much as they were protected from the obese, insulin resistant state. Indeed, a significant reduction in liver triglyceride accumulation, serum ALT elevation, TUNEL positive apoptotic hepatocytes and fibrosis were observed in the FFC-fed TR−/− mice. Thus, TR deficiency protected against the hepatic effects of the FFC diet.
Fig. 2. Hepatic steatosis and liver injury are reduced in FFC-fed TR−/− mice.
(A) H&E stained sections (scale bar 20 μm), (B) CARS and SHG microscopy for lipid droplets and extracellular matrix (scale bar 100 μm), (C) Liver weight, (D) Hepatic triglycerides (μg/mg of liver tissue), (E) Serum alanine aminotransferase (U/L), (F) TUNEL staining (scale bar 100 μm), (G) Sirius red staining (scale bar 100 μm), (H) Quantification of TUNEL positive cells, (I) Quantification of Sirius red chromogen, and (J) mRNA abundance of collagen-1α1 in livers from WT and TR−/− mice fed chow or FFC diet for 6 months (n=7/group). *p<0.05, **p<0.01.
Hepatic macrophage infiltration is attenuated in FFC-fed TR−/− mice
To define the mechanisms of the hepatoprotective effects of TR−/− in an unbiased manner we performed a gene array study in liver tissue from FFC-fed wild-type and TR−/− mice. The top ten upregulated and downregulated genes in wild-type mice relative to TR−/− mice are reported in Supplementary Table 2. One of the top twenty upregulated genes (26-fold increase) was Cd68, a membrane glycoprotein highly expressed by cells of the monocyte/macrophage lineage [23]. Pathway analysis networks also highlighted inflammation-associated genes and networks, especially those associated with phagocytosis (Supplementary Fig. 2). Informed by the gene array data implicating inflammation due to cells of the monocyte/macrophage lineage in this model of steatohepatitis, we next focused on the role of TR in macrophage biology during obesity-associated inflammation.
The substantial accumulation of hepatic macrophages in FFC-fed wild-type mice compared to TR−/− mice was confirmed by Mac-2 immunohistochemistry, a phenotypic marker for phagocytically active macrophages [17] (Fig. 3A). We saw exuberant macrophage accumulation around fatty hepatocytes in FFC-fed wild-type mice, consistent with the literature [31]. In addition, qPCR for macrophage markers, Cd68 (Fig. 3B) and F4/80 (Fig. 3C) was consistent with the histology, demonstrating a significant increase in these markers in FFC-fed wild-type mice. In contrast, these macrophage markers were significantly reduced in FFC-fed TR−/− mice compared to diet-matched wild-type mice. The accumulation of macrophages was accompanied by increased proinflammatory cytokine response in FFC-fed wild-type mice as assessed by measurement of Mcp1 (Fig. 3D) and Tnfα (Fig. 3E). In marked contrast, Tnfα and Mcp1 mRNA levels were substantially reduced in FFC-fed TR−/− mice. Correspondingly, Cd11c and Ly6c, markers of monocyte-derived macrophages, known to be proinflammatory, were significantly upregulated in FFC-fed wild-type mice compared to TR−/− mice (Fig. 3F and G).
Fig. 3. Liver and adipose tissue macrophage infiltration is attenuated in FFC-fed TR−/− mice.
(A) Liver section with Mac-2 immunohistochemistry for macrophages (scale bar 20 μm), mRNA abundance of (B) Cd68, (C) F4/80, (D) Mcp1, (E) Tnfα, (F) Cd11c and (G) Ly6c in liver tissue, (H) Cd68, (I) F4/80, (J) Tnfα and (K) Mcp1 in epididymal fat of WT and TR−/− mice fed the FFC diet for 6 months expressed relative to that observed in chow-fed WT mice (n=6 per group, *p<0.05, **p<0.01).
Hepatocyte and macrophage TR signaling is upregulated and contributes to the inflammation of nutrient excess
To further examine the role of TR signaling in our model, we quantified Trail and TR mRNA expression in liver from FFC-fed and chow-fed mice and found an increase in both in liver (Supplementary Fig. 3A and B). Next, we used a cell fractionation approach to define the cellular source of increased hepatic Trail and TR expression, and found that the FFC diet increased Trail mRNA abundance in hepatocytes and TR mRNA abundance in macrophages and hepatocytes (Supplementary Fig. 3C and E). These data suggest TR signaling in two cell types, hepatocytes and macrophages, contributes to tissue inflammation in this model. Given, the existing data on lipotoxic ligand-independent activation of TR signaling in fatty hepatocytes leading to lipoapoptosis [4], we further explored TR signaling in macrophages.
TR signaling in BMDMϕ promotes macrophage chemotaxis and activation
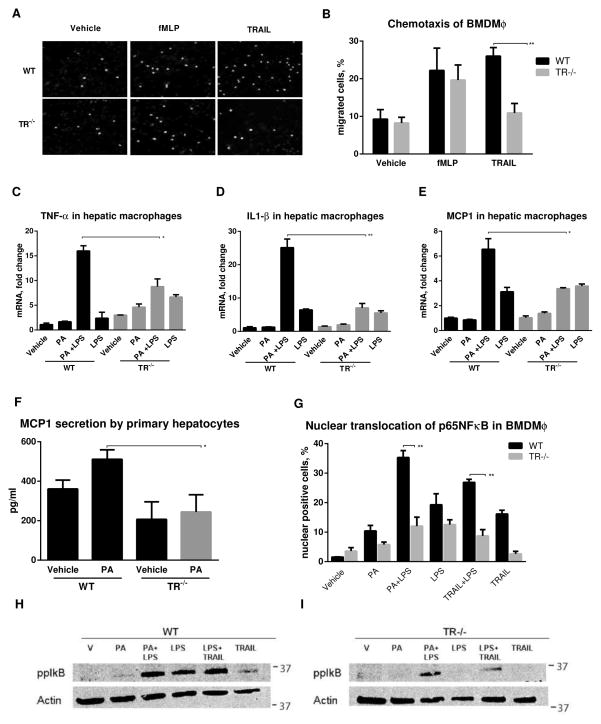
We first examined a role for TR signaling in macrophage chemotaxis and activation. TR−/− and wild-type BMDMϕ migrated equally to fMLP; however TR−/− BMDMϕ demonstrated reduced migration to TRAIL (Fig. 4A and B). Thus, TRAIL was a chemoattractant for macrophages consistent with our observations of enhanced macrophage accumulation in FFC-fed wild-type mice. Due to the enhanced liver injury and inflammation in FFC-fed wild-type mice, and its significant abrogation in TR−/− mice, we next explored if TRAIL signaling is proinflammatory in macrophages, in addition to its chemotaxis effects. We treated macrophages with palmitate, a known proinflammatory saturated fatty acid implicated in lipotoxicity, alone or in combination with low dose lipopolysaccharide (LPS)[34]. Palmitate and LPS induced Tnfα, Il1β and Mcp1, in wild-type, but not TR−/− hepatic macrophages (Fig. 4C–E). As hepatocyte apoptosis is associated with chemokine production, [8] we measured MCP-1 in cell culture supernatants of hepatocytes from wild-type and TR−/− mice treated with palmitate. Treatment with palmitate induced MCP-1 secretion in wild-type hepatocytes, but not in TR−/− cells (Fig. 4F). This correlates with the increased hepatocyte apoptosis and increased MCP-1 abundance seen in FFC-fed wild-type versus TR−/− mice, suggesting that chemokines from injured hepatocytes recruit inflammatory macrophages to the liver. Furthermore, TR−/− macrophages demonstrate reduced chemotaxis and activation under lipotoxic conditions.
Fig. 4. Macrophage TR signaling contributes to the inflammation of nutrient excess.
(A) Fluorescent photomicrographs (scale bar 20 μm) and (B) quantification of DAPI stained migrated WT and TR−/− BMDMϕ. (C–E) mRNA abundance of Tnfα, Il1β, and Mcp1 in cells treated with 400 μM palmitate (PA) and/or 10 ng/ml lipopolysaccharide (LPS), 8 h. (F) MCP1 levels in supernatants from cells treated with 400 μM PA, 8 h. (G) Quantification of nuclear translocation of NF-κB by immunofluorescence, (H) phosphorylation of IκB-α (Ser32/Ser36) in WT and TR−/− BMDMϕ treated with 400 μM PA ± 10 ng/ml lipopolysaccharide (LPS) or 10 ng/ml TRAIL for 1 h. *p<0.05, **p<0.01
To define the mechanisms of diminished recruitment of TR−/− BMDMϕ into the liver of FFC-fed mice we explored NF-κB signaling, a nonapoptotic signaling pathway activated by TRAIL and its cognate receptors [3]. TR−/− BMDMϕ manifest reduced p65 NF-κB subunit nuclear translocation when stimulated with a combination of palmitate plus LPS compared to wild-type cells (Fig. 4G and H). Because palmitate can induce TR signaling by a ligand-independent mechanism [4], we postulated that TRAIL could substitute for palmitate in this assay. Indeed, TRAIL plus LPS yielded similar results for p65 NF-κB subunit nuclear translocation to palmitate plus LPS. We confirmed NF-κB activation by examining IκB-α phosphorylation. Palmitate plus LPS induced a substantial increase in IκB-α (Ser32/36) phosphorylation (Fig. 4H). In contrast, IκB-α (Ser32/36) phosphorylation was significantly reduced in TR−/− cells. Similar to the nuclear translocation studies, TRAIL substitutes for palmitate in this assay. Thus, TR−/− BMDMϕ display reduced levels of NF-κB activation in response to palmitate plus LPS or TRAIL plus LPS stimulation. This likely contributes to the reduced activation of macrophages in vivo in FFC-fed mice. Having demonstrated a reduction in hepatic injury and inflammation in FFC-fed TR−/− mice, and reduced chemotaxis and activation in TR−/− macrophages, we next turned our attention to the expanded white adipose tissue to examine its role in the improved metabolic phenotype on TR−/− mice.
TR−/− mice exhibit decreased adiposity and adipose tissue inflammation
FFC feeding led to adipocyte expansion. H&E stained adipose tissue is shown in supplementary figure 4A. This was quantified by measuring adipocyte diameter in epididymal and inguinal fat pads; these fat pads demonstrated larger adipocytes with increased adipocyte diameter in FFC-fed wild-type mice as compared to chow-fed mice; however, adipocyte diameter in FFC-fed TR−/− mice was comparable to chow-fed mice (Supplementary Fig. 4B and C). As adipose tissue expansion is governed, in part, by master transcriptional regulators C/EBPα and β [19], we examined their expression by mRNA and immunoblot analysis. As anticipated, C/EBPα and β were both upregulated in FFC-fed wild-type mice (Supplementary Fig. 4D–G). In contrast, but likely contributing to the lack of adipose tissue expansion in FFC-fed TR−/−, we observed lower levels of C/EBPα and C/EBPβ.
To further characterize features of the expanded adipose tissue, we asked if development of beige or brown fat-like properties might account for the reduction in adipose tissue expansion in FFC-fed TR−/− mice. We observed no increase in markers of beige fat in adipose tissue from FFC-fed wild-type or TR−/− mice (Supplementary Fig. 4H and I). However, we detected a trend towards increased uncoupling protein-1 (UCP-1) mRNA expression in TR−/− mice (Supplementary Fig. 4K). Although there was not a statistically significant difference, this trend towards enhanced UCP-1 expression in the TR−/− mice, perhaps explains, in part, their improved metabolic phenotype. Correspondingly, we detected an increase in UCP-1 by immunohistochemistry in TR−/− mice (Supplementary Fig. 4L). We confirmed that the UCP1 antibody worked in our hands by testing it on brown adipose tissue (Supplementary Fig. 6). The immunohistochemistry for UCP-1 identifies a pattern of adipose tissue browning associated with fibroblast-like cells within the tissue, which may account for the improved metabolic phenotype in the TR−/− mice [2]. Lastly, we analyzed characteristics of infiltrating macrophages in adipose tissues. First, we found an increase in TR expression in FFC-fed wild-type mice without a corresponding increase in adipose tissue TRAIL (Supplementary Fig. 5A and B). There was a significant increase in Cd68 and F4/80 consistent with macrophage accumulation and Tnfα and Mcp1 mRNA abundance in FFC-fed wild-type white adipose tissues (Supplementary Fig. 5C–F), supporting the accumulation of pro-inflammatory macrophages in FFC-fed wild-type mice, and a significant reduction in the accumulation of pro-inflammatory macrophages in white adipose tissue in FFC-fed TR−/− mice. We did not detect an increase in alternatively activated, M2 macrophages in FFC-fed white adipose tissue (Supplementary Fig. 5G–I). Thus, consistent with the observations in liver tissue, we found increased pro-inflammatory macrophages in adipose tissue of FFC-fed obese mice.
DISCUSSION
The current observations provide key mechanistic insights into the signaling pathways that form the basis of the inflammation of dietary nutrient excess. We observed, in a murine model of obesity and insulin resistance, genetic deletion of TR provided several salutary effects including: i) protection against an increase in adiposity and insulin resistance; ii) reduction in steatohepatitis; iii) suppression of accumulation of pro-inflammatory macrophages in the liver; and iv) reduction in adipose tissue pro-inflammatory macrophage accumulation. In vitro, the genetic deletion of TR attenuated macrophage activation responses following co-stimulation with palmitate plus LPS. These data implicate TR signaling as a potent process in the inflammatory response occurring during nutrient excess.
We employed the FFC diet to mimic a fast food diet and obtain a model of obesity with steatohepatitis, which mimics human NASH [5]. Genetic deletion of the TR attenuates many of the adverse metabolic features observed in wild-type mice fed this diet. Interestingly, TR−/− mice have reduced body mass, reduced steatohepatitis, reduced macrophage accumulation in liver and adipose tissues in spite of equal energy intake and expenditure. These observations suggest TR signaling plays a pivotal role in initiating the inflammatory response to nutrient excess. Without an inflammatory response, insulin resistance, weight gain, and organ lipotoxicity are attenuated, highlighting the role of inflammation in the pathogenesis of the metabolic syndrome [26].
During nutrient excess the resulting surplus of circulating FFAs are extracted by the liver with adverse outcomes, namely lipoapoptosis [30]. This hepatocyte lipoapoptosis is, in part, mediated by TR signaling in vitro [4]. Our current data extends these concepts by demonstrating reduced steatohepatitis in FFC-fed animals with TR deletion. Consistent with our observations, it has been demonstrated that apoptosis resistant mice due to hepatocyte-specific deletion of caspase 8 also have reduced hepatocyte apoptosis and consequently reduced steatohepatitis when fed a methionine and choline deficient diet [13]. Proapoptotic TR signaling in hepatocytes likely elicits an inflammatory response activating macrophages and promoting the development of steatohepatitis [8]. Thus, inhibition of TR proapoptotic signaling may prevent inflammation and insulin resistance simply by attenuating cell death in the liver and other tissues, as has been demonstrated with pharmacologic caspase inhibition [35]. Alternatively, due to the improved metabolic profile of FFC-fed TR−/− mice, and the associated reduction in inflammation, we cannot exclude a reduction in hepatocyte lipoapoptosis occurring secondary to a better metabolic profile. Due to the whole body knockout mouse employed in these studies the tissue- and organ-specific roles cannot be teased out; however, we hope to address this in the future with tissue-specific TR−/− mice.
Although absence of cell death may be sufficient to prevent inflammation, death receptors also exert proinflammatory signaling cascades in multiple cell types [3]. Indeed, palmitate treatment of primary mouse hepatocytes resulted in MCP-1 generation, similar to Fas [8]. As palmitate plus LPS triggers secretion of proinflammatory cytokines in cells of the monocyte lineage [34], we employed these mediators in our study of macrophages. Interestingly, enhanced expression of Mcp-1, Tnfα, and Il1β by LPS plus palmitate was suppressed in TR−/− hepatic macrophages and BMDMϕ. This observation is likely explained by the decrease in canonical NF-κB activation in TR−/− cells. Both TR signaling, and LPS signaling via Toll-like receptor 4 (TLR4), can engage TRAF6 as a platform to activate NF-κB [15, 38]. Perhaps overlap in this signaling process results in synergy between palmitate induced TR signaling plus LPS-mediated TLR4 signaling. This latter testable concept will require further studies to identify how TR non-apoptotic signaling synergizes with LPS stimulation to activate pro-inflammatory NF-κB signaling in macrophages.
Our studies demonstrate an increase in TR expression in FFC-fed wild-type white adipose tissue, and an increase in both TRAIL and TR in FFC-fed wild-type livers, suggesting that TR signaling in both liver and adipose tissue may play a role in the pathogenesis of obesity associated tissue injury. Indeed, in FFC-fed TR−/− mice a reduction in adipose depots, fat mass, and adipocyte size was observed. In addition, we show that the induction of master regulators of adipogenic expansion is impaired in FFC-fed TR−/− mice suggesting that TR signaling is required for maximal adipose expansion under FFC feeding conditions. An increase in brown fat-like features of adipose tissue, characterized by fibroblast-like cells within the tissue, was detected in TR−/− mice, which may account for the improved metabolic phenotype in the TR−/− mice. Our data suggest that TR signaling is essential for the development of diet-induced obesity and its sequelae, and its deletion limits both lipoapoptosis and tissue inflammation.
One unifying explanation for these findings could be a primary macrophage defect, as the current study suggests that TR signaling contributes to the immune response of nutrient excess by promoting macrophage chemotaxis and the inflammatory response to FFAs. Thus, due to the lack of macrophage accumulation and activation in both adipose tissue and liver, TR−/− mice are protected from insulin resistance and the progressive deleterious effects of diet-induced obesity. The pathophysiology of TRAIL and its receptors has remained somewhat enigmatic. TRAIL signaling was originally identified as proapoptotic in malignant cell lines but was uneventful in healthy, nontransformed cells [16]. Recently, a role for non-apoptotic TR signaling in host immune responses has emerged [3, 9]. Our findings are in accord with this growing appreciation of the role of TR in inflammatory disorders [7].
We propose a model where TR signaling, by promoting both cell death and macrophage activation, results in tissue inflammation and insulin resistance during dietary nutrient excess. Both processes coordinately participate in the observed phenotype. Finally, we note that a small molecule TR agonist has been described, which suggests that identifying a small molecule TR antagonist is also possible [33]. An inhibitor of TR signaling could be salutary in human obesity related syndromes if the observations in mice can be extrapolated to humans.
Supplementary Material
Acknowledgments
Grant support: This work was supported by NIH grants DK41876 (to GJG), AG13925 and DK50456 (to JLK) DK97178 (to HM), the Deutsche Forschungsgemeinschaft SFB/TRR57 (to CT and CL) and the Mayo Foundation.
We thank Ms. Courtney Hoover for outstanding secretarial assistance.
Non-standard abbreviations
- ALT
alanine aminotransferase
- BMDMϕ
bone marrow derived macrophages
- CARS
coherent anti-Stokes Raman scattering
- C/EBPα
CCAAT/enhancer binding protein (C/EBP) alpha
- C/EBPβ
CCAAT/enhancer binding protein (C/EBP) beta
- FasL
Fas ligand
- FFA
free fatty acid
- FFC
fat fructose cholesterol
- fMLP
formyl-Methionyl-Leucyl-Phenylalanine
- IL1-β
interleukin 1 beta
- LPS
lipopolysaccharide
- MCP-1
monocyte chemotactic protein-1
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- PA
palmitate
- PCR
polymerase chain reaction
- SHG
second-harmonic generation
- TNF-α
tumor necrosis factor alpha
- TRAIL
TNF-related apoptosis-inducing ligand
- TR
TRAIL receptor
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild-type
Footnotes
Disclosures: The authors report no conflicts
Author Contributions: L.I., N.W.W., S.F.B., C.F., T.T., T.P., T.A.W., M.B.S., P.H., A.K. designed and performed experiments and acquired and analyzed data. N.K.L, C.L., C.T. analyzed and interpreted data, provided material support and contributed to manuscript preparation. N.F., W.S.E., J.L.K. provided material support, analyzed data and contributed to manuscript preparation. L.I., H.M., and G.J.G. conceptualized the study, designed experiments, analyzed and interpreted data and prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Affo S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2012 doi: 10.1136/gutjnl-2011-301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176(3):1364–1376. doi: 10.2353/ajpath.2010.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict CA, Ware CF. TRAIL: not just for tumors anymore? J Exp Med. 2012;209(11):1903–1906. doi: 10.1084/jem.20122235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazanave SC, Mott JL, Bronk SF, Werneburg NW, Fingas CD, Meng XW, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem. 2011;286(45):39336–39348. doi: 10.1074/jbc.M111.280420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. American journal of physiology Gastrointestinal and liver physiology. 2011;301(5):G825–834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JW, Song JS, Pai SH. Associations of serum TRAIL concentrations, anthropometric variables, and serum lipid parameters in healthy adults. Ann Clin Lab Sci. 2004;34(4):400–404. [PubMed] [Google Scholar]
- 7.Collison A, Foster PS, Mattes J. Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin Exp Pharmacol Physiol. 2009;36(11):1049–1053. doi: 10.1111/j.1440-1681.2009.05258.x. [DOI] [PubMed] [Google Scholar]
- 8.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, et al. Fas/CD95-Induced Chemokines Can Serve as “Find-Me” Signals for Apoptotic Cells. Mol Cell. 2013 doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21(6):877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Farrell GC, Larter CZ, Hou JY, Zhang RH, Yeh MM, Williams J, et al. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. Journal of gastroenterology and hepatology. 2009;24(3):443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 11.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 12.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Molecular and cellular biology. 2005;25(5):2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatting M, Zhao G, Schumacher F, Sellge G, Masaoudi MA, Gabetaler N, et al. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in mice. Hepatology. 2013 doi: 10.1002/hep.26271. [DOI] [PubMed] [Google Scholar]
- 14.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib Suppresses TRAIL-mediated Liver Injury in a Mouse Model of Nonalcoholic Steatohepatitis. PLoS One. 2013;8(7):e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue J, Gohda J, Akiyama T. Characteristics and biological functions of TRAF6. Adv Exp Med Biol. 2007;597:72–79. doi: 10.1007/978-0-387-70630-6_6. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 17.Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13(4):434–446. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 18.Keuper M, Wernstedt Asterholm I, Scherer PE, Westhoff MA, Moller P, Debatin KM, et al. TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell Death Dis. 2013;4:e474. doi: 10.1038/cddis.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20(3):107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8(4):310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56(8):1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, et al. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem. 2013;288(26):18624–18642. doi: 10.1074/jbc.M112.442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. American journal of physiology Gastrointestinal and liver physiology. 2012;302(11):G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, et al. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54(11):1590–1596. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, et al. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59(4):916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801(3):209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. 2013;59(1):144–152. doi: 10.1016/j.jhep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Vujanovic NL. Role of TNF superfamily ligands in innate immunity. Immunol Res. 2011;50(2–3):159–174. doi: 10.1007/s12026-011-8228-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Wang X, Yu H, Wei S, Williams N, Holmes DL, et al. Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat Chem Biol. 2013;9(2):84–89. doi: 10.1038/nchembio.1153. [DOI] [PubMed] [Google Scholar]
- 34.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 36.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59(12):2770–2775. [PubMed] [Google Scholar]
- 37.Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest. 2010;120(1):191–202. doi: 10.1172/JCI38388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen ML, Hsu PN, Liao HJ, Lee BH, Tsai HF. TRAF-6 dependent signaling pathway is essential for TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation. PLoS One. 2012;7(6):e38048. doi: 10.1371/journal.pone.0038048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.