Abstract
Introduction
Cardiac arrest commonly results in varying degrees of cognitive injury. Standard outcome measures used in the cardiac arrest cohort do not rigorously evaluate for these injury patterns. We examined the utility of the Computerized Assessment for Mild Cognitive Injury (CAMCI) in cardiac arrest (CA) survivors. We hypothesized that cognitive deficits would be more severe in patients who were comatose on hospital arrival.
Methods
Prospective cohort of CA survivors at a single tertiary care facility where participants received neurocognitive testing using CAMCI. CAMCI results were subdivided into memory, attention, and executive functions. Scores between subjects who were initially comatose and were not comatose following resuscitation were compared using the Mann-Whitney test.
Results
Of 72 subjects included, the majority (N=44) were initially comatose following resuscitation with mean age of 54 (+/-14) years. The majority experienced a good neurologic outcome based on Cerebral Performance Category (N=47; 66%) and Modified Rankin Scale (N=38; 53%). Time from resuscitation to CAMCI testing was not associated with total CAMCI score in this cohort (Pearson's r2 value -0.1941, p=0.20). Initially comatose and not comatose subjects did not differ in their CAMCI overall scores (p=0.33), or in any subtest areas. The not comatose cohort had 1 subtest for which there was a Moderate Risk for mild cognitive impairment (Nonverbal Accuracy), and 2 for which there was a Moderately Low Risk (Verbal Accuracy and Executive Accuracy). The Comatose cohort had 4 subtests, which were deemed Moderately Low Risk for cognitive impairment (Verbal Accuracy, Attention Accuracy, Executive Accuracy and Nonverbal Accuracy).
Conclusions
In-hospital CAMCI testing suggests memory, attention and executive impairment are commonly in patients following resuscitation from cardiac arrest. Outcome evaluations should test for deficits in memory, attention, and executive function.
Keywords: Heart Arrest, Patient Outcome Assessment, Hypothermia, Cognition, Resuscitation, Critical Care
Introduction
Cardiac arrest is common and results in approximately 300,000 deaths per year in the US.1 In patients successfully resuscitated from cardiac arrest, manifestation of neurological injury due to global brain ischemia and reperfusion ranges from brain death to normal cognition.2 Protocolized resuscitation strategies (including the use of targeted temperature management) have been shown to improve neurologic outcomes.3, 4 Although impairment has been demonstrated in each of the areas of memory, attention, and executive function, the frequency of these cognitive impairments varies depending on cohort and testing method.5-8 Most studies use a global outcome measure to determine outcome and cognitive testing is rarely employed as part of this assessment.
Traditional outcome measures used after resuscitation from cardiac arrest are the Cerebral Performance Category (CPC) and the Modified Rankin Scale (mRS). The former is a 5-category scale with 1 being the best score and 5 indicating death. The latter is a 7-point scale with 0 indicating no symptoms and 6 corresponding with death. Both of these tests, however, have come under criticism for lacking validation in this population, being subjective and too global to detect subtle but clinically important deficits, and not being well suited for the testing of the patient in the hospital instead of at home.9 For example, the criteria for an mRS score of 3 focuses on the patient's ability to carry out tasks such as cooking, managing finances, and shopping within the hospital setting where such tasks are neither performed nor observed. Additionally, a CPC score of 3 can include everything from an alert, interactive patient to a minimally conscious patient, thus lacking texture. Patients with a CPC of 3 are sometimes discharged to home, or to hospice or long term acute care for the most severely injured.9 Even patients considered to have a good outcome based on CPC of 1, on deeper inspection may have significant limitations in memory and executive function and commonly have mild cognitive impairment.10 These impairments are not without significant consequences including lower functioning in society, low quality of life and high caregiver strain.11 More detailed outcome measures could better identify and differentiate neurocognitive impairments to improve the lexicon for research, allow for the comparison of clinical outcomes and guide appropriate follow up therapy and support.
Recent work in neuropsychological testing has focused on the development and validation of standardized, efficient, and generalizable computer measures. Standard neuropsychological testing can readily detect cognitive impairment. However, it requires several hours to complete and specialized training for the tester. The Computer Assessment of Mild Cognitive Impairment (CAMCI) is a self-administered, computerized neurocognitive test, requiring 25 - 35 minutes, that scores itself automatically and does not require specialized supervision.12 The CAMCI was designed to detect mild cognitive impairment preceding dementia and measures accuracy and reaction time for multiple domains including attention, memory (verbal, visual, working, recognition, prospective, and incidental recall) and executive function. Although, the CAMCI has been shown as a sensitive and specific measure of mild cognitive impairment in the elderly population, it has not been used to assess post cardiac arrest patients.12 This study examined whether CAMCI testing is feasible in cardiac arrest survivors. Our criterion for utility was whether CAMCI could detect deficits in patients who appeared well with global measures (CPC or mRS), and whether CAMCI could distinguish degrees of brain injury. To test the latter, we hypothesized that CAMCI scores would be lower in patients who were comatose on hospital arrival (moderate brain injury) relative to patients who were awake on arrival (none or minimal brain injury).
Methods
This was a prospective convenience sample of subjects who awoke after resuscitation from cardiac arrest between 4/1/2010 and 7/31/2013. Subjects recruited were treated after an in hospital cardiac arrest (IHCA) or out of hospital cardiac arrest (OHCA) in a single tertiary care facility. Inclusion criteria were the successful resuscitation from cardiac arrest, ability to follow commands, and completion of the CAMCI test. Subjects with uncorrectable audio and/or visual impairment, inadequate comprehension of English to understand the instructions, a physical disability that would prohibit the use of a touchscreen, dementia or who were not independently living in the community at baseline (i.e., nursing home or acute care facility residents) were excluded. Demographic information including: age, pre-arrest Charlson comorbidity index, location of arrest, primary rhythm of arrest, use of therapeutic hypothermia (TH), SOFA Cardiac and Pulmonary scores, coronary angiography, and neurologic outcome using Cerebral Performance Category (CPC) and Modified Rankin Scale (mRS) were abstracted from the chart.13 The Pittsburgh Cardiac Arrest Category (PCAC), a validated illness severity score in this population, was recorded on hospital arrival.14, 15 Comatose (defined as not following commands) subjects were treated with a standardized post-arrest care bundle, including TH and coronary angiography as appropriate.3,16
After awakening (defined as following commands), post-cardiac arrest subjects in our facility received neurocognitive testing using the CAMCI. This testing was obtained following discharge from the intensive care unit and prior to hospital discharge. The test was administered on a laptop computer in the subject's hospital room. In order to minimize disruptions and distractions a sign was placed outside the door advising hospital staff and visitors to refrain from entry while testing was in progress, the television was turned off, and present visitors were asked to leave the room or remain silent for the duration of the testing. The test administrator provided the subject with instruction on the use of the device and was present for the duration of the test, however he/she was also quiet while the patient was actively testing. At the start of the assessment, subjects were prompted to provide information about their age, education level, alcohol use, memory decline, anxiety/depression, and driving, computer, and ATM experience. The CAMCI includes eight subtasks that facilitate testing of multiple cognitive domains including: attention, verbal memory, visual memory, working memory, recognition memory, prospective memory, incidental recall, and executive function.17 It also includes a virtual road trip to the grocery store with stops at a post office and an ATM machine, all of which require the functional use of each of the cognitive domains measured by the CAMCI. At the end of the assessment the CAMCI uses age and education adjusted normative data to calculate a percentile score by averaging the weighted Z scores of 12 variables of accuracy, then converting them to a percentile. The “Risk level” for mild cognitive injury is derived from the percentile score (0-9th % = High risk, 10-20th % = Moderately high risk, 21-30th % = Moderate risk, 31-40th % = Moderately low risk, 41-100th % = Low risk). As this is the first use of the CAMCI in this population, we present data from healthy elderly subjects for reference in the results.18
Neurologic outcome was assessed using the CPC and mRS. As in our prior work, medical charts at the time of hospital discharge were reviewed using a standard written template to determine the CPC and mRS.9 A good neurologic outcome was defined as a CPC of <3 and mRS <3. CAMCI scores and accuracy were compared between subjects who were initially comatose and not comatose following resuscitation using the Mann-Whitney test in the subtest categories of memory (word recognition & recall, functional memory, and recurring pictures), attention (digit span forward), and executive function (digit span reverse, go/no-go decision-making, intersections, and ATM use). Demographic data were compared using a Chi square, t-test or Fisher's Exact test. Analyses were completed using Stata 11.2 (College Station, TX).
Results
Of the 219 subjects who awoke following resuscitation during this time epoch, 91 were comatose and 128 were awake on initial examination. From this cohort, 44 initially comatose and 28 initially awake subjects were recruited. All subjects were out of the intensive care unity and had a Glasgow Coma Scale of 15 before testing. Of these, 60% were male with a mean age of 54 (SD 14) years. [Table 1] The awake cohort had higher Charlson Comorbidity Scores than the comatose cohort. Ventricular fibrillation was the most common primary rhythm of arrest and the majority of subjects experienced OHCA. Of the 44 comatose subjects, 40 received TH. One subject who rapidly awoke (i.e., not initially comatose) also received TH. None of the subjects demonstrated cerebral edema on initial CT of the brain. Two subjects demonstrated malignant EEG patterns during their hospital course. Both had reactive EEG patterns underneath and were discharged to acute rehabilitation. Although 66% of subjects experienced a good neurologic outcome based on CPC criteria, only 53% had a good outcome based on mRS criteria. The median length of stay in the hospital for the entire cohort was 12 days (IQR 8-20). Table 2 shows self-reported characteristics of the subjects at the time of CAMCI administration. Comatose and awake subjects did not differ in any of these areas. The awake cohort completed CAMCI testing earlier in their hospital course than the comatose cohort (p=0.003). [Table 1] Time from resuscitation to CAMCI testing was not associated with total CAMCI score in this cohort (Pearson's r2 value -0.1941, p=0.20).
Table 1. Demographic composition of the cohort.
| Cohort (N=72) | Not Comatose (N=28) | Comatose (N=44) | p | |
|---|---|---|---|---|
|
| ||||
| Age, years | 54.4 (SD 14.4) | 58 (SD 10.8) | 52 (SD 16) | 0.08 |
|
| ||||
| Male | 43 (60%) | 16 (57%) | 27 (61%) | 0.72 |
|
| ||||
| OHCA | 55 (76%) | 18 (64%) | 37 (84%) | 0.055 |
|
| ||||
| Charlson Comorbidity Index (Not age adjusted) | 1 (IQR 0, 2) | 1 (IQR 0, 3) | 0 (IQR 0, 1) | 0.07 |
|
| ||||
| Age-Adjusted Charlson Comorbidity Index | 2 (IQR 0, 4) | 3 (IQR 2,5) | 2 (IQR 0, 3) | 0.02 |
|
| ||||
| Rhythm | ||||
| VF/VT | 52 (72%) | 19 (68%) | 33 (75%) | 0.77 |
| PEA | 10 (14%) | 4 (14%) | 6 (14%) | |
| Asystole | 7 (10%) | 4 (14%) | 3 (7%) | |
| Unknown | 3 (4%) | 1 (4%) | 2 (4%) | |
|
| ||||
| Cardiac Catheterization | 48 (67%) | 19 (68%) | 29 (66%) | 0.87 |
|
| ||||
| Therapeutic Hypothermia | 41 (57%) | 1 (4%) | 40 (91%) | <0.01 |
|
| ||||
| Pittsburgh Cardiac Arrest Category | <0.01 | |||
| I | 28 (41%) | 28 (100%) | 0 (0%) | |
| II | 27 (40%) | 0 (0%) | 27 (61%) | |
| III | 7 (10%) | 0 (0%) | 7 (16%) | |
| IV | 6 (9%) | 0 (0%) | 6 (14%) | |
|
| ||||
| SOFA Cardiovascular | 0 (IQR 0, 1) | 1 (IQR 0, 4) | 0 (IQR 0, 0) | 0.05 |
|
| ||||
| SOFA Pulmonary | 2 (IQR 1, 3) | 1 (IQR 0, 3) | 2 (IQR 2, 3) | 0.24 |
|
| ||||
| Discharge Disposition | 0.32 | |||
| Home | 48 (67%) | 19 (68%) | 29 (66%) | |
| Acute Rehab | 10 (14%) | 2 (7%) | 8 (18%) | |
| SNF | 12 (17%) | 5 (18%) | 7 (16%) | |
| LTAC | 1 (1%) | 1 (4%) | 0 (0%) | |
| Died | 1 (1%) | 1 (4%) | 0 (0%) | |
|
| ||||
| CPC | 0.36 | |||
| 1 | 25 (35%) | 12 (43%) | 13 (30%) | |
| 2 | 22 (31%) | 7 (25%) | 15 (34%) | |
| 3 | 24 (33%) | 8 (28%) | 16 (36%) | |
| 4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 5 | 1 (1%) | 1 (4%) | 0 (0%) | |
|
| ||||
| mRS | 0.57 | |||
| 0 | 8 (11%) | 3 (11%) | 5 (11%) | |
| 1 | 13 (18%) | 7 (25%) | 6 (14%) | |
| 2 | 17 (24%) | 7 (25%) | 10 (23%) | |
| 3 | 16 (22%) | 5 (18%) | 11 (25%) | |
| 4 | 17 (24%) | 5 (18%) | 12 (27%) | |
| 5 | 1 (1%) | 1 (4%) | 0 (0%) | |
|
| ||||
| Hospital length of stay, days | 12 (IQR 8, 20) | 8.5 (IQR 6, 17) | 12.5 (IQR 12, 21) | 0.23 |
|
| ||||
| Arrest to following commands, days | 1 (IQR 0, 2) | 0 (IQR 0, 1) | 2 (IQR 1, 2) | 1.00 |
|
| ||||
| Arrest to CAMCI test, days | 8 (IQR 5, 12) | 4.5 (IQR 3, 8) | 10 (IQR 7, 15) | 0.003 |
|
| ||||
| Awake to CAMCI test, days | 6 (IQR 4, 10) | 4 (IQR 2, 8) | 7 (IQR 5,11) | 0.04 |
Note. IQR = interquartile range, SD = standard deviation.
Table 2. Self-reported characteristics at CAMCI administration.
| Cohort (N=72) | Not Comatose (N=28) | Comatose (N=44) | p | |
|---|---|---|---|---|
| Anxiety, n (%) | 48 (67) | 16 (57) | 32 (73) | 0.17 |
| Alcohol Use, n (%) | 13 (18) | 4 (14) | 9 (20) | 0.51 |
| Depression, n (%) | 30 (42) | 12 (43) | 18 (41) | 0.87 |
| Memory Decline, n (%) | 23 (32) | 8 (29) | 15 (34) | 0.62 |
| Currently Drive, n (%) | 61 (85) | 23 (82) | 38 (86) | 0.63 |
| Ever Drive, n (%) | 71 (99) | 27 (96) | 44 (100) | 0.21 |
| Computer Use, n (%) | 54 (75) | 21 (75) | 33 (75) | 1.00 |
| ATM Use, n (%) | 55 (76) | 22 (79) | 33 (75) | 0.73 |
Table 3 presents overall CAMCI scores and the accuracy in the different neurocognitive areas. Comatose and not comatose subjects did not differ in their overall scores (p=0.33), or in any of the subtest areas (p values ranged from 0.06 – 0.79). Compared to the older adult reference group,18 for which all subtest areas were Low Risk for mild cognitive injury, the not comatose cohort had 1 subtest for which there was a Moderate Risk (Nonverbal Accuracy), and 2 for which there was a Moderately Low Risk (Verbal Accuracy and Executive Accuracy). The Comatose cohort had 4 subtests which were deemed Moderately Low Risk (Verbal Accuracy, Attention Accuracy, Executive Accuracy and Nonverbal Accuracy). Table 4 shows data from the individual subtests in the areas of memory, attention, and executive function. Subjects showed deficits in all subtests except attention and verbal recognition. The most pronounced deficit was seen in word recall with subjects scoring on average below 50%. There were no significant differences between any of the subtest scores in any of the three categories between comatose and not comatose subjects.
Table 3.
Overall CAMCI score and accuracy by comatose and awake groups. Reference from Tierney et al. also included.
| Reference (N=72) | Cohort (N=72) | Not Comatose (N=28) | Comatose (N=44) | p | |
|---|---|---|---|---|---|
| CAMCI Score, raw (max 100) | 41 (SD 21) | 37 (IQR 19, 53) | 40 (IQR 20.5, 53) | 34 (IQR 15, 53) | 0.33 |
| Verbal Accuracy Percentile | 50 (SD 35) | 38 (SD 33) | 40 (SD 31) | 37 (SD 34) | 0.79 |
| Functional Accuracy Percentile | 43 (SD 32) | 48 (SD 32) | 52 (SD 30) | 45 (SD 32) | 0.79 |
| Attention Accuracy Percentile | 56 (SD 28) | 44 (SD 33) | 54 (SD 29) | 39 (SD 33) | 0.06 |
| Executive Accuracy Percentile | 42 (SD 25) | 36 (SD 24) | 38 (SD 24) | 34 (SD 25) | 0.33 |
| Nonverbal Accuracy Percentile | 42 (SD 30) | 33 (SD 27) | 28 (SD 28) | 36 (SD 27) | 0.33 |
Note. IQR = interquartile range, SD = standard deviation.
Table 4.
Memory, attention, and executive function testing results by comatose and awake groups.
| Cohort (N=72) | Not Comatose (N=28) | Comatose (N=44) | p | |
|---|---|---|---|---|
| Memory | ||||
| Verbal recognition, correct of 6 | 6 (IQR 5, 6) | 6 (IQR 5, 6) | 6 (IQR 5, 6) | 1.00 |
| Word recall, correct of 5 | 2 (IQR 1, 4) | 2 (IQR 2, 4) | 2 (IQR 1, 4) | 0.65 |
| Functional memory, errands of 6 | 4 (IQR 3, 5.5) | 5 (IQR 4, 6) | 4 (IQR 3, 5) | 0.25 |
| Recurring Pictures, target correct (%) | 80 (IQR 70, 90) | 82.5 (IQR 75, 90) | 80 (IQR 67.5, 90) | 0.57 |
| Recurring Pictures, non-target correct (%) | 79 (IQR 64, 87.5) | 73 (IQR 59, 86) | 79 (IQR 66, 89) | 0.41 |
| Attention | ||||
| Digit span forward, max of 6 | 6 (IQR 5, 6) | 6 (IQR 5.5, 6) | 6 (IQR 5, 6) | 0.42 |
| Executive Function | ||||
| Digit span reverse, max of 5 | 4 (IQR 3, 5) | 4 (IQR 3, 5) | 3.5 (IQR 3, 4) | 0.23 |
| Go/no-go rule 1, out of 10 | 9 (IQR 8, 10) | 9 (IQR 8.5, 10) | 9 (IQR 8, 10) | 0.79 |
| Go/no-go rule 2, out of 10 | 9.5 (IQR 6.5, 10) | 10 (IQR 8, 10) | 9 (IQR 6, 10) | 0.33 |
| Intersections, out of 18 | 16 (IQR 14, 17) | 16 (IQR 13.5, 16.5) | 16 (IQR 14, 18) | 0.31 |
| ATM, out of 7 | 6.5 (IQR 5, 7) | 7 (IQR 6, 7) | 6 (IQR 3, 7) | 0.33 |
Note. Lower scores indicate greater impairment. IQR = interquartile range.
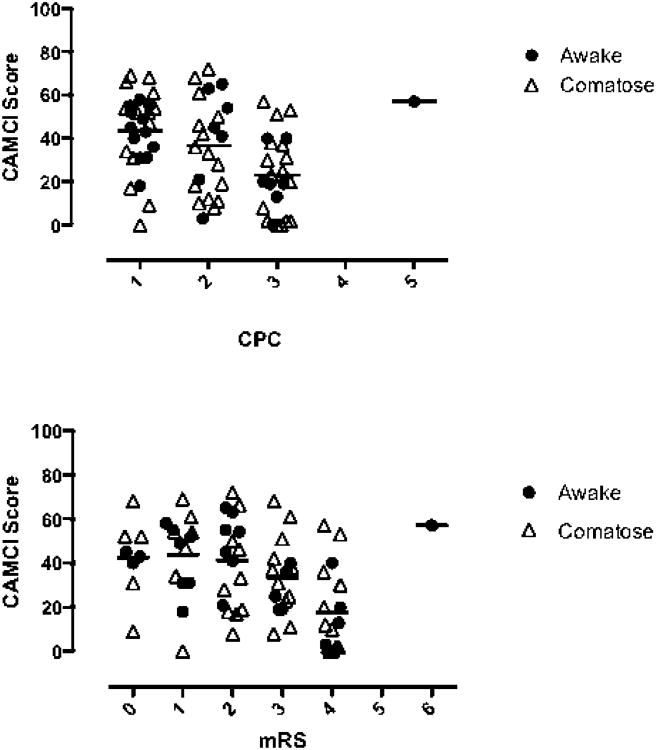
There was a range of CAMCI scores for each level of CPC and mRS. Most subjects with “normal” CPC (1) or mRS (0-1) had measurable deficits on CAMCI. The mean values of CAMCI differed between CPC and mRS categories (p<0.001 for both). Variation between CAMCI assessment for mild cognitive injury and both chart review CPC and mRS are shown in the figure.
Figure.
Top panel: chart review CPC and CAMCI score. Bottom Panel: chart review mRS and CAMCI score. Bars delineate mean for the group. Grey circle indicates comatose on hospital arrival, black circle indicates not comatose on hospital arrival.
Discussion
This study has demonstrated that it is feasible to obtain CAMCI testing in a cohort of subjects successfully resuscitated from cardiac arrest. It also suggests cognitive impairment is present in the acute period following resuscitation from cardiac arrest. While these data did not detect a difference in CAMCI scores between subjects who were awake (following commands) versus those who were comatose (not following commands) immediately after resuscitation, it is likely underpowered to detect such a difference. Prior literature reports that only about half of survivors of OHCA have cognitive impairment, with memory most frequently affected, followed by attention and executive function, but that the incidence varies widely according to the measure utilized.5 In this study, almost all subjects demonstrated impairment in the domains of memory, attention and executive function after resuscitation from cardiac arrest, all of which have been demonstrated by previous studies. 6-8, 19
Cognitive findings were demonstrated in not only the initially comatose cohort, but also the initially not comatose cohort. Although this could suggest that the care bundle that includes TH for comatose patients is very effective at returning them to baseline, our sample size is small and consequently may be unable to detect a difference between these cohorts. It is important to note that the majority of comatose subjects had a PCAC of II or III (77%) and an initial rhythm of ventricular fibrillation (75%), which have been associated with higher likelihood of survival and found to be predictors of discharge to home.14 This is corroborated by the high rates of good outcome using either CPC or discharge disposition definitions.
All subjects were initially admitted to the ICU. While the CAMCI test was completed after discharge from the ICU, it is possible that cognitive dysfunction as part of a recently described “post-intensive care syndrome” (PICS) from the ICU admission contributed to the cognitive impairment found in this cohort.20-22 Also, intermittent analgesic use is common during the hospitalization phase of the post-arrest patient and may have affected their performance. Similarly, functional and cognitive outcomes change over time.23 This work represents a single time point for evaluation. Future studies examining CAMCI testing over time or in patients admitted to the ICU for reasons other than cardiac arrest would provide important benchmarking to further describe PICS.
These data demonstrate that global measures of outcome such as CPC and mRS do not fully characterize cognitive injury patterns that are common in this population, specifically, executive function and recall. While CAMCI scores vary by CPC and mRS, even those subjects with “good” outcomes (CPC<3 and mRS<3) demonstrated significant deficits. Although detailed neurocognitive testing may not be feasible in all institutions, testing these domains prior to making decisions about placement in rehabilitation programs and discharge appears prudent. Developing an impairment measure tailored to this population that evaluates functional, cognitive, and executive deficits in a timely fashion remains a goal for the resuscitation community.
There are several limitations that deserve mention. First, the sample size is small and limits our ability to detect differences between groups. Importantly, only those patients able and willing to complete CAMCI testing were provided the test, potentially creating a sampling bias. The presumed etiology of arrest is not recorded in our database, precluding our ability to evaluate subgroup analyses based on etiology of arrest. In addition, more severely injured comatose patients did not attempt the test. A previous study showed good outcomes by CPC and mRS in 20-22% of patients.9 As the majority of subjects in our study had a good outcome by CPC and mRS (66% and 53%) and had discharge disposition to home or acute rehab (81%), it is likely that our results reflect a more robust sample of cardiac arrest survivors. Thus, the generalizability of our results may be limited and cognitive impairments are possibly worse than described here for many individuals. Another limitation is that in addition to the possible PICS mentioned above, subjects' performance may have been hindered by sedatives or analgesic drugs, as well as by fatigue, which is commonly associated with cognitive deficits.24 These are confounding factors that could be controlled for in future studies. The time course in cognitive recovery is unclear as some studies have demonstrated improvement over time while others have not.25-29 Future studies with multiple measurements using CAMCI at set intervals both during hospitalization and over time following discharge could reveal the potential for CAMCI in this regard.
Conclusion
In-hospital CAMCI testing is feasible and suggests neurocognitive deficits in post cardiac arrest patients are common. CAMCI testing may provide a more textured assessment than CPC and mRS. Impairment in memory, attention, and executive function is shown in many patients successfully resuscitated from cardiac arrest, including those who appear to be globally intact. Outcome evaluations should test for deficits in memory, attention, and executive function as better identification and delineation of these deficits at the time of hospital discharge may provide an opportunity for functional rehabilitation.
Acknowledgments
The authors would like to thank Mr. Patrick Morgan for test administration and data abstraction.
Dr. Rittenberger is supported by Grant Number 1 KL2 RR024154-02 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Rittenberger is also supported by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship.
Appendix
The Post Cardiac Arrest Service researchers are:
Jon C. Rittenberger, MD, MS
Clifton W. Callaway, MD, PhD
Francis X. Guyette, MD, MPH
Ankur A. Doshi, MD
Cameron Dezfulian, MD
Joshua C. Reynolds, MD, MS
Adam Frisch, MD, MS
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional Variation in Out-of-Hospital Cardiac Arrest Incidence and Outcome. JAMA. 2008;300(12):1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raina KD, Callaway C, Rittenberger JC, Holm MB. Neurological and functional status following cardiac arrest: Method and tool utility. Resuscitation. 2008;79(2):249–56. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittenberger JC, Guyette FX, Tisherman SA, et al. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79(2):198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen N, Wetterslev J, Cronbert T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 5.Moualert VR, Verbundt JA, van Heugten CM, Wade DT. Cognitive impairments I survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Wilson BA. Cognitive functioning of adult survivors of cerebral hypoxia. Brain injury. 1996;10(12):863–874. doi: 10.1080/026990596123846. [DOI] [PubMed] [Google Scholar]
- 7.Nunes B, Pais J, Garcia R, Magalhaes Z, Granja C, Silva MC. Cardiac arrest: longterm cognitive and imaging analysis. Resuscitation. 2003;57(3):287–97. doi: 10.1016/s0300-9572(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 8.van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J. 2004;148(3):416–21. doi: 10.1016/j.ahj.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Rittenberger JC, Raina K, Holm MB, et al. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–40. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronberg T, Lilja G, Rundgren M, et al. Long-term neurological outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2009;80:1119–1123. doi: 10.1016/j.resuscitation.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Wachelder EM, Moulaert VRMP, van Heugten C, et al. Life after survival: Long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80:517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Saxton J, Morrow L, Eschman A et al. Computer Assessment of Mild Cognitive Impairment. Postgraduate Medicine. 2009;121(2):177–85. doi: 10.3810/pgm.2009.03.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, et al. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chron Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82(11):1399–404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppler PJ, Calderon L, Sabedra A, Doshi A, Callaway CW, Rittenberger JC, Dezfulian C. Validation of the Pittsburgh cardiac arrest category. Resuscitation. 2015 Jan 27; doi: 10.1016/j.resuscitation.2015.01.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds JC, Callaway CW, El Khoudary SR, et al. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. Journ Intens Care Med. 2009;24(3):179–86. doi: 10.1177/0885066609332725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker JT, Dew MA, Aizenstein HJ, et al. Concurrent validity of a computer-based cognitive screening tool for use in adults with HIV disease. Aids Patient Care and STDS. 2011;25(6):351–7. doi: 10.1089/apc.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney MC, Naglie G, Upshur R, et al. Feasibility and validity of the self-administered computerized assessment of mild cognitive impairment with older primary care patients. Alzheimer Dis Assoc Disord. 2014 May 24; doi: 10.1097/WAD.0000000000000036. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. The neurological and cognitive sequelae of cardiac arrest. Neurology. 2004;63(10):1774–8. doi: 10.1212/01.wnl.0000144189.83077.8e. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JE, Hopkins RO, Louis D, Iwashyna TJ. Post-intensive Care Syndrome. [Accessed Oct 14, 2014];SCCM. 2013 http://www.myicucare.org/Adult-Support/Pages/Post-intensive-Care-Syndrome.aspx.
- 21.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):5029. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 22.Wergin R, Modrykamien A. Cognitive impairment in ICU survivors: assessment and therapy. Cleve Clin J Med. 2012;79(10):705–12. doi: 10.3949/ccjm.79a.12038. [DOI] [PubMed] [Google Scholar]
- 23.Raina KD, Rittenberger JC, Callaway C, Holm MB. Functional Outcomes: one year after a cardiac arrest. Neurocrit Care. 2015 doi: 10.1155/2015/283608. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases. 1994;18:S79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 25.Sauve MJ, Doolittle N, Walker JA, et al. Factors associated with cognitive recovery after cardiopulmonary resuscitation. Am J Crit Care. 1996;5(2):127–9. [PubMed] [Google Scholar]
- 26.Sauve MJ, Walker JA, Massa SM, et al. Patterns of cognitive recovery in sudden cardiac arrest survivors: the pilot study. Heart Lung. 1996;25(3):172–81. doi: 10.1016/s0147-9563(96)80027-6. [DOI] [PubMed] [Google Scholar]
- 27.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269(2):237–42. [PubMed] [Google Scholar]
- 28.Dougherty CM. Longitudinal recovery following sudden cardiac arrest and internal cardioverter defibrillator implantation: survivors and their families. Am J Crit Care. 1994;3(2):145–54. [PubMed] [Google Scholar]
- 29.Drysdale EE, Grubb NR, Fox KA, O'Carroll RE. Chronicity of memory impairment in long-term out-of-hospital cardiac arrest survivors. Resuscitation. 2000;47(1):27–32. doi: 10.1016/s0300-9572(00)00194-5. [DOI] [PubMed] [Google Scholar]