Abstract
Background
Patellofemoral joint osteoarthritis is a highly prevalent condition and an important source of pain and disability. Nonetheless, biomechanical risk factors associated with patellofemoral joint osteoarthritis remain unclear. The purpose of this study was to compare biomechanical factors that are associated with patellofemoral joint loading during walking between individuals with isolated patellofemoral joint osteoarthritis and no osteoarthritis.
Methods
MR images of the knee were obtained using a 3D fast-spin echo sequence to identify patellofemoral joint cartilage lesions (patellofemoral joint osteoarthritis group). Thirty-five subjects with isolated patellofemoral joint osteoarthritis (29 females) and 35 control subjects (21 females) walked at a self-selected speed and as fast as possible. Peak knee flexion moment, flexion moment impulse and peak patellofemoral joint stress during the first and second halves of the stance phase were compared between groups.
Findings
When compared to the controls, individuals with patellofemoral joint osteoarthritis demonstrated significantly higher peak knee flexion moment (P =.03, Eta2 =.07), higher knee flexion moment impulse (P =.03, Eta2 =.07) and higher peak patellofemoral joint stress (P =.01, Eta2 =.10) during the second half of the stance phase. No significant group difference was observed during the first half of the stance phase.
Interpretation
Findings of this study suggest that increased mechanical loading (i.e. knee flexion moment, impulse and patellofemoral joint stress) during the second half of the stance phase is associated with patellofemoral joint osteoarthritis. Prevention and rehabilitation programs for patellofemoral joint osteoarthritis may focus on reducing the loading on the patellofemoral joint, specifically during late stance.
Keywords: patellofemoral joint, osteoarthritis, gait, stress, kinetics
1. Introduction
Patellofemoral joint (PFJ) osteoarthritis (OA) is a highly prevalent knee disease. Based on the findings of radiographic[1] and magnetic resonance (MR) imaging[2] studies, 64% of adults over 50 years have PFJ OA with one third of them having isolated PFJ OA. This suggests that the prevalence of PFJ OA is as high, if not higher than, tibiofemoral joint OA [1–3]. Moreover, PFJ OA has been found to be an important source of pain and dysfunction in the knee joint [4–6]. While a large body of literature has been established with regards to biomechanical risk factors associated with tibiofemoral joint OA, there is a substantial paucity of data on the biomechanical characteristics of individuals with PFJ OA.
Articular cartilage lesions are a hallmark sign of OA and can result from mechanical overload [7–9]. Several biomechanical factors can provide direct or indirect estimations of mechanical loading of the articular cartilage of PFJ during functional activities. For example, PFJ stress represents the compressive (joint reaction) force applied to the PFJ per unit area. An increased PFJ stress indicates a higher mechanical loading on the PFJ. Additionally, increased knee flexion moments can result in higher PFJ reaction forces at a given knee angle and thus, may lead to increased PFJ stress [10, 11]. Taken together, biomechanical factors, such as PFJ stress, knee flexion moment, knee flexion moment impulse, may be potential risk factors associated with PFJ OA.
A few recent studies investigated PFJ loading during functional activities in individuals with and without PFJ OA [12–14]. Farrokhi et al.[14] reported a higher knee flexion moment during single-leg stance of gait in individuals with combined tibiofemoral joint and PFJ OA compared to individuals with isolated tibiofemoral joint OA. On the contrary, Crossley et al.[12] reported that people with isolated PFJ OA walked with similar vasti muscle forces and Fok et al.[13] reported that individuals with isolated PFJ OA or combined PFJ and tibiofemoral joint OA ascended and descended stairs with lower knee flexion moments and PFJ reaction forces when compared to asymptomatic controls. The conflicting results may be due to the differences in methodology and subject selection among these studies and highlight the need of further research in this area. It is important to note that neither of these previous studies evaluated PFJ OA based on the presence of cartilage lesions seen on MR imaging, but rather on indirect signs of cartilage wear, such as joint space narrowing on radiographs which is a later stage finding. Since OA is characterized by articular cartilage lesions, these previous studies may not be sensitive enough to identify risk factors associated with PFJ OA especially those with early stage disease. Moreover, in order to better identify biomechanical risk factors associated with PFJ OA, it would be important to exclude individuals with tibiofemoral joint OA since they have been reported to demonstrate altered knee flexion moment during walking [15].
The purpose of this study was therefore to compare biomechanical factors that are associated with PFJ loading (i.e. knee flexion moment, knee flexion moment impulse and PFJ stress) during walking between individuals with no OA and isolated PFJ OA (as defined by articular cartilage lesions on MR imaging). Because increased PFJ loading may lead to mechanical damages on PFJ cartilage, which is used to define OA in this study, we hypothesized that individuals with PFJ OA would exhibit higher knee flexion moment, knee flexion moment impulse and PFJ stress during walking.
2. Methods
2.1 Subjects
A total of 112 subjects above 35 years with and without knee OA symptoms were recruited from the community as a part of a longitudinal study on knee OA. The exclusion criteria were (1) history of lower extremity or spine surgery, (2) self-reported inflammatory arthritis, (3) any conditions that limits the ability to walk (without assistant device) and (4) contraindications to MR imaging. For the purpose of OA classification, all subjects underwent knee MR imaging using a 3.0-Tesla GE MR 750w Scanner (General Electric, Milwaukee, WI, USA) and an 8-channel transmit-receive knee coil (Invivo, Orlando, FL, USA). A high-resolution 3D fast spin-echo CUBE sequence (repetition time/echo time = 1500/26.69 ms, field of view = 16 cm, matrix = 384 × 384, slice thickness = 0.5 mm, echo train length = 32, bandwidth = 37.5 kHz, number of excitations = 0.5, acquisition time = 10.5 min) was acquired to evaluate cartilage health.
Articular cartilage lesions of the PFJ (patella, trochlea) and tibiofemoral joint (medial and lateral tibia, medial and lateral femoral condyle) were graded by an experienced board certified radiologist using the modified Whole Organ Magnetic Resonance Imaging Score (WORMS) [16–19]. Cartilage lesions were graded as follows: 0 = normal thickness, 1 = normal thickness, increased signal intensity, 2 = partial thickness focal lesion less than 1 cm of greatest width, 2.5 = full thickness focal lesion less than 1 cm of greatest width, 3 = multiple areas partial lesion less than 1 cm of greatest width, or grade 2 lesion wider than 1 cm but less than 75% of the region, 4 = diffuse partial thickness loss greater than 75% of the region, 5 = multiple areas of full thickness lesion greater than 1 cm but less than 75% of the region, and 6 = diffuse full thickness loss greater than 75% of the region [19]. PFJ OA was defined if the patella or trochlea presented cartilage lesions in WORMS ≥ 2; TFJ OA was defined when the medial or lateral tibia, or medial or lateral femoral condyle presented cartilage lesions in WORMS ≥ 2 [2].
The 112 recruited subjects were then stratified into: no OA (n=46), isolated PFJ OA (n=35), isolated tibiofemoral joint OA (n=9) and mixed PFJ and tibiofemoral joint OA (n=22). To avoid potential influence of tibiofemoral joint OA on gait characteristics, 35 subjects with isolated PFJ OA and 35 age- and BMI-matched controls with no OA were included in this study. Prior to data collection, all subjects signed a written informed consent approved by the Committee of Human Research at the University of California, San Francisco. All participants completed the Knee injury and Osteoarthritis Outcome Score (KOOS) survey (100 = no symptom, 0 = maximum symptom) and the short-form International Physical Activity Questionnaire (IPAQ). In addition, all participants also completed six-minute-walk, time-up-and-go, and stair-climbing tests to determine overall functional capacity.
2.2 Gait Analysis
Three-dimensional lower extremity kinematics were recorded using a 10-camera motion capture system (VICON, Oxford Metrics, UK) at a sampling rate of 250 Hz. Ground reaction force data were obtained using two embedded force platforms (AMTI, Watertown, MA, USA) at a sampling rate of 1000 Hz. Marker and ground reaction force data were collected and synchronized using motion capture software (Nexus, Oxford Metrics, UK).
Prior to the walking test, retro-reflective (14 mm spheres) anatomical markers were placed on the following bony landmarks: L5/S1 junction, bilateral iliac crests, anterior superior iliac spines, greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, and 1st and 5th metatarsal heads. Additionally, tracking marker clusters mounted on semi-rigid plastic plates were placed bilaterally on the lateral surfaces of the subject’s thighs, shanks, and heel counters of the shoes. A standing calibration trial was obtained to define the segment coordinate systems and joint axes. After the calibration trial, anatomical markers were removed, except for those on L5-S1 junction, iliac crests, and anterior superior iliac spine, which served as tracking markers for the pelvis. The tracking markers remained on the subject throughout the entire data collection session.
Subjects were instructed to walk at two different speeds: 1) self-selected speed (purposeful walk, described to subjects as “you have some place to be, but you are not late”) (Free-Walk) and 2) as fast as possible (Fast-Walk). Five successful trials were obtained for each walking condition. A successful trial was defined when the foot of the tested limb fell within borders of either of the force platforms from initial contact to toe-off and the speed was within ± 5% of the first successful trial.
2.3 Data Process
Kinematic and kinetic data were computed using Visual3D (C-Motion, Germantown, MD, USA) and MATLAB software (Mathworks Inc., MA, USA). Marker trajectory data were low-pass filtered using a 4th-order Butterworth filter with a cutoff frequency at 6 Hz. Joint axes were defined by the anatomical markers placed during the standing calibration trial. Hip joint center was defined as one-fourth the distance between the markers on bilateral greater trochanters. Knee joint center was defined as the midpoint of the distance between the markers on the medial and lateral epicondyle of the femur in a plane defined by the hip joint center, knee joint center, and the marker placed on greater trochanter. Ankle joint center was defined as the midpoint of the distance between the markers on the medial and lateral malleoli. Joint kinematics were calculated using Cardan rotation sequence in an order of flexion/extension, abduction/adduction and internal/external rotation and were not normalized to the standing calibration position. Net joint moments were reported as external moments and normalized to each subject’s body mass (kg) and height (m). Knee flexion moment impulse was calculated as the integral of knee flexion moment (Nm/kg×m) with respect to time (ms).
PFJ stress was computed using a previously described sagittal plane biomechanical model [20, 21]. This model used subject-specific knee flexion angles and net joint moments (obtained from inverse dynamics), and data from literature (i.e. quadriceps effective lever arm, ratio between quadriceps force and PFJ reaction force, and PFJ contact area) to estimate PFJ reaction force and contact area.
First, the model calculated the quadriceps effective lever arm as a function of knee flexion angle using cadaveric data reported by Eijden et al. [22]. Second, the quadriceps force was computed by dividing the knee flexion moment by the effective lever arm. Third, PFJ reaction force was estimated by multiplying the quadriceps force with a ratio reported by van Eijden et al.[23] that defines the relationship between quadriceps force and PFJ reaction force as a function of knee flexion angle. Fourth, PFJ contact area was estimated based on cadaveric data reported by Powers et al. [24]. A second-order polynomial curve was fitted to discrete data of PFJ contact area at seven knee flexion angles (0, 15, 30, 45, 60, 75 and 90 degrees). PFJ contact area was then calculated as a function of knee flexion angle during the stance phase. Lastly, PFJ reaction force was divided by PFJ contact area to estimate PFJ stress during the stance phase of walking.
Peak knee flexion moment, knee flexion moment impulse and peak PFJ stress during the first and second halves of the stance phase were recorded. The stance phase was defined when the vertical ground reaction force exceeded 20 N. Averaged data from five successful trials were used for statistical analyses.
2.4 Statistical Analysis
Independent t-tests were performed to compare walking speed between the control and PFJ OA groups during Free- and Fast-Walk conditions. Separate 2-by-2 (group by walking condition) mixed-design analysis of variance (ANOVA) with a repeated factor of walking condition, and a covariate of gender was used to examine group (PFJ OA vs. control) effects on each variables of interest (knee flexion moment, knee flexion moment impulse and PFJ stress during the first and second halves of stance). When there was a significant group difference in PFJ stress, a 2-way ANOVA was used to identify factors (knee flexion angle and moment) that might contribute to the differences in PFJ stress. Statistical analysis was performed using SPSS software (IBM SPSS 22.0.0) with a significance level set at 0.05. The effect size was calculated using Eta squared.
3. Results
Demographic, pain, and functional activity level data of the PFJ OA and control groups are presented in Table 1. The WORMS grades of the more affected cartilage (patella or trochlea) of the PFJ OA group were: WORMS 2: n = 11, 2.5: n = 0, 3: n = 18, 4: n = 3, 5: n = 3. No significant difference in walking speed was observed between control and PFJ OA groups during Free-Walk [Mean (SD), Control: PFJ OA = 1.54 (0.16): 1.50 (0.27) m/sec, P = .50] and Fast-Walk [Mean (SD), Control: PFJ OA = 1.91 (0.24): 1.95 (0.30) m/sec, P = .62] conditions.
Table 1.
Mean (SD) of demographic, pain, and functional data for the control and patellofemoral join osteoarthritis (PFJ OA) groups.
| Control | PFJ OA | P value | |
|---|---|---|---|
| Demographics | |||
| Gender (Male:Female) | 14:21 | 6:29 | 0.03 |
| Age (years) | 51.4 (9.4) | 53.7 (10.0) | 0.33 |
| Height (m) | 1.61 (0.08) | 1.65 (0.08) | 0.08 |
| Mass (kg) | 63.8 (10.97) | 65.1 (9.6) | 0.61 |
| BMI (kg/m2) | 24.4 (3.3) | 24.0 (3.2) | 0.55 |
| Pain and Function | |||
| KOOS: Pain (%) | 89.5 (12.6) | 87.3 (13.6) | 0.50 |
| KOOS: Activities of Daily Living (%) | 94.1 (9.7) | 93.6 (8.1) | 0.80 |
| IPAQ: Walking (MET/week) | 2427.4 (4818.9) | 2472.6 (5394.1) | 0.97 |
| Timed Up and Go test (sec) | 6.0 (0.9) | 5.8 (0.7) | 0.47 |
| Timed stairs (sec) | 11.6 (1.8) | 12.2 (1.9) | 0.21 |
| 6 Minute Walk Test (m) | 627.7 (87.8) | 640.7 (81.8) | 0.53 |
KOOS (Knee injury and Osteoarthritis Outcome Score): 100 = no symptom, 0 = maximum symptom
IPAQ (International Physical Activity Questionnaire)
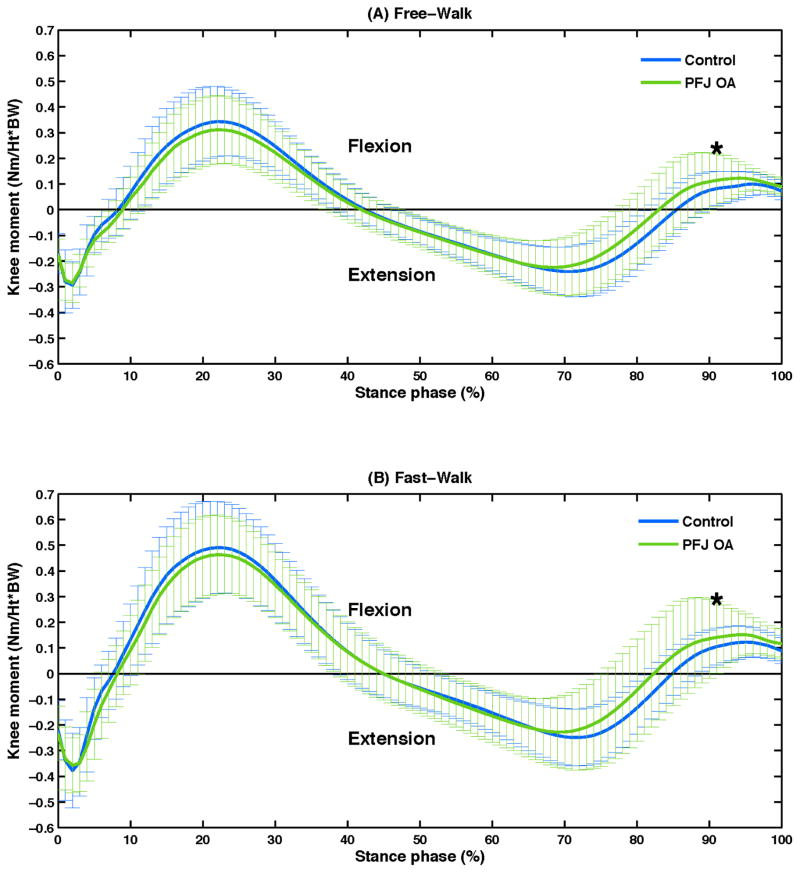
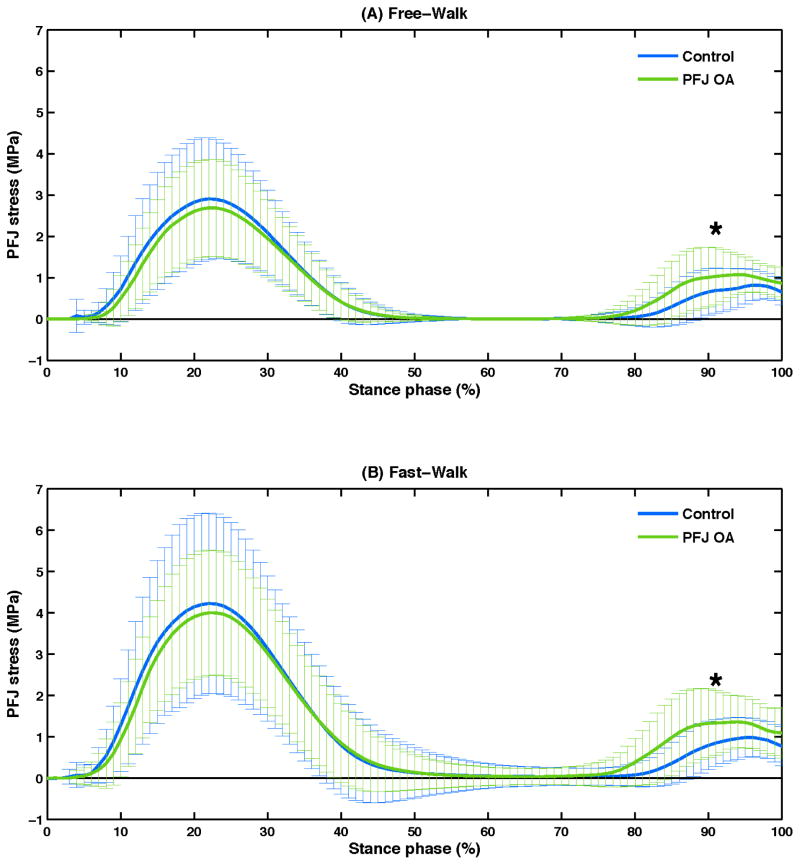
Time-series curves of knee joint moments and PFJ stress during the stance phase are presented in Figure 1 and 2. Significant group differences in peak knee flexion moment (P = .03), knee flexion moment impulse (P = .03) and peak PFJ stress (P = .01) were observed during the second half of the stance phase (Table 2). When compared to the control group, PFJ OA group demonstrated higher peak knee flexion moment, higher knee flexion moment impulse, and higher peak PFJ stress during both Free- and Fast-Walk conditions. No significant group difference was observed for peak knee flexion moment (P = .71), knee flexion moment impulse (P = .34) and peak PFJ stress (P = .80) during the first half of the stance phase (Table 2). Moreover, no significant speed-by-group interaction effects were revealed by ANOVA.
Figure 1.
Mean (SD, indicated by the vertical lines) of knee moments during the stance phase for the control and patellofemoral joint osteoarthritis (PFJ OA) groups during Free-Walk (A) and Fast-Walk (B) conditions.
Figure 2.
Mean (SD, indicated by the vertical lines) of patellofemoral joint (PFJ) stress during the stance phase for the control and PFJ osteoarthritis (PFJ OA) groups during Free-Walk (A) and Fast-Walk (B) conditions.
Table 2.
Mean (SD) of patellofemoral joint (PFJ) loading during Free- and Fast-Walk conditions for the control and patellofemoral join osteoarthritis (PFJ OA) groups.
| Free-Walk | Fast-Walk | P value* Partial Eta2† |
|||
|---|---|---|---|---|---|
| Control | PFJ OA | Control | PFJ OA | ||
| Knee flexion moment 1st peak (Nm/kg×m) | 0.35 (0.13) | 0.32 (0.14) | 0.51 (0.18) | 0.49 (0.16) | 0.71 0.02 |
| Knee flexion moment impulse 1st half (Nm-ms/kg×m) | 42.0 (19.8) | 36.2 (15.9) | 55.3 (25.0) | 49.2 (19.3) | 0.34 0.01 |
| PFJ stress 1st peak (MPa) | 2.98 (1.46) | 2.79 (1.20) | 4.36 (2.24) | 4.20 (1.55) | 0.80 0.01 |
| Knee flexion moment 2nd peak (Nm/kg×m) | 0.12 (0.05) | 0.16 (0.06) | 0.15 (0.07) | 0.20 (0.08) |
0.03 0.07 |
| Knee flexion moment impulse 2nd half (Nm-ms/kg×m) | 7.8 (5.7) | 11.9 (7.6) | 8.8 (7.2) | 13.8 (10.4) |
0.03 0.07 |
| PFJ stress 2nd peak (MPa) | 1.01 (0.46) | 1.33 (0.57) | 1.22 (0.62) | 1.67 (0.75) |
0.01 0.10 |
Between-subject (control vs. PFJ OA) effect revealed by 2-by-2 (group-by-condition) mixed-model analysis of variance (ANOVA) with a covariate of gender. Bold numbers indicates significant group differences (P value < .05).
Partial Eta2 is computed as the sums of squares of the group effect divided by sums of squares of the error and group effect
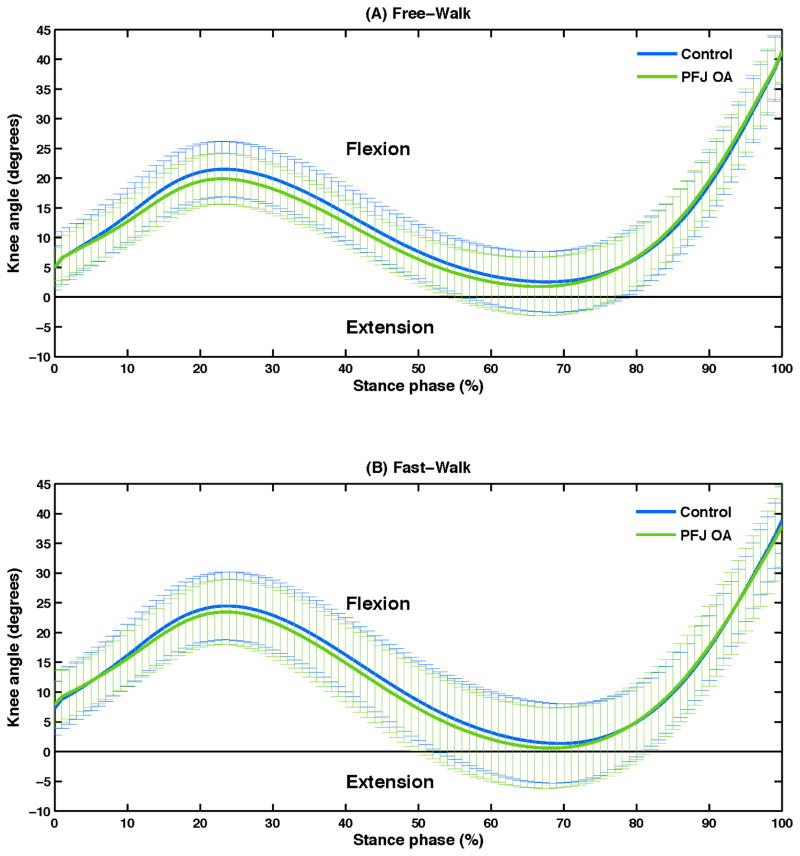
Post-hoc analyses examining knee flexion angle and moment at the time of second peak PFJ stress revealed significant group differences. The PFJ OA group exhibited significantly lower knee flexion angle [Free-Walk, control: PFJ OA = 30.9 (7.9): 27.1 (6.4) degrees; Fast-Walk, control: PFJ OA = 27.7 (8.0): 23.9 (7.7) degrees, P = .045] (Figure 3) and higher flexion moment [Free-Walk, control: PFJ OA = 0.19 (0.08): 0.26 (0.10) Nm/kg×m; Fast-Walk, control: PFJ OA = 0.24 (0.11): 0.32 (0.14) Nm/kg×m, P = .016] when compared to the control group.
Figure 3.
Mean (SD, indicated by the vertical lines) of knee angles during the stance phase for the control and patellofemoral joint osteoarthritis (PFJ OA) groups during Free-Walk (A) and Fast-Walk (B) conditions.
4. Discussion
This study intended to compare PFJ loading between individuals with and without PFJ OA (as defined by articular cartilage lesions) during walking. Findings of this study support the hypothesis that individuals with PFJ OA exhibit higher loadings at the PFJ when compared to the controls. Specifically, significantly higher knee flexion moments, knee flexion moment impulse, and PFJ stress were observed during the second half of the stance phase in the PFJ OA group. On average, individuals with PFJ OA exhibited 33% higher peak knee flexion moments, 53% – 57% greater knee flexion moment impulse, and 32% – 37% higher peak PFJ stress during the second half of the stance phase during self-selected and fast walking. On the contrary, the two groups did not present significant differences in PFJ loading during the first half of the stance phase.
Results of this study provide information as to kinetic gait characteristics associated with PFJ OA. Given that none of the subjects had tibiofemoral joint OA at the time of testing, we believe that the observed gait characteristics are uniquely associated with the presence of PFJ OA. Based on the results of MR grading, 29 out of 35 PFJ OA subjects had WORMS between 2 and 3 on PFJ cartilage lesion, indicating that the majority of the PFJ OA participants were at early stage of PFJ OA. This may explain why the PFJ OA cohort in this study did not present significant differences in pain, function and self-selected walking speed compared to the control group. Together, it suggests that findings of this study may better reflect gait characteristics of individuals with early stage PFJ OA who are at high level of function and low level of pain. Since non-surgical interventions of OA are more effective during early stage disease, knowledge gained from this study are critical and can be used to inform clinical management of early stage PFJ OA.
Consistent with Farrokhi et al.[14], the observed difference in knee flexion moment during the second half of the stance phase between individuals with and without PFJ OA was small but significant. Although the second peak of knee flexion moment was around 35% – 50% of the magnitude of the first peak, given the highly repetitive nature of walking during daily living, a small increase in knee flexion moment over each step can result in a large increase in the accumulative loading on the PFJ. It must be kept in mind that the PFJ OA participants in this study were at early stage OA and presented a comparable activity level in walking and daily living as the controls (Table 1). As such, they may be highly susceptible to cartilage lesions due to a small increase in PFJ loading. The overall group differences in knee flexion moment are 0.04 and 0.05 Nm/kg×m during free- and fast-walk, respectively. A similar magnitude of reduction in knee moment in the frontal plane (knee adduction moment) during walking was shown to be associated with significant improvements in pain and function in tibiofemoral OA cohort [25]. Taken together, we believe the observed difference in knee flexion moment in this study is clinically meaningful.
Results of this study also revealed that individuals with PFJ OA demonstrated higher knee flexion moment impulse during the second half of stance phase. Previous studies investigating biomechanical risk factors of tibiofemoral joint OA suggest that moment impulse may be a more sensitive predictor of disease severity and progression than peak moment, since it takes into account both duration and magnitude of loading [26, 27]. In this study, significant group differences were observed for both peak knee flexion moment and flexion moment impulse. However, a larger percentage of difference was shown in the moment impulse (53% – 57%) than peak moment (33%) between groups. Post-hoc analysis showed no significant difference in the duration of knee flexion moment during the second half of stance between groups. This indicates that the greater knee flexion moment impulse observed in the PFJ OA group was primarily resulted from higher knee flexion moment over similar duration of loading.
PFJ OA participants in this study also demonstrated a higher PFJ stress during the second half of stance compared to the controls. Using a similar biomechanical model, Brechter et al.[28] reported a higher PFJ stress during walking in individuals with patellofemoral pain, which has been proposed to be a precursor of PFJ OA [29]. An elevated PFJ stress can lead to cartilage wear and tear and thus, irreversible cartilage lesions. In this study, the PFJ OA subjects displayed a lower knee flexion angle and a higher knee flexion moment at the time of PFJ stress second peak. Although a lower knee flexion angle is typically accompanied with a lower knee flexion moment, post-hoc analysis revealed a greater ground reaction force in the PFJ OA group, which could explain the co-occurrence of lower knee flexion angle and higher knee flexion moment. A lower knee flexion angle is associated with a smaller PFJ contact area [30, 31], while a higher knee flexion moment can result in a higher PFJ reaction force [10, 11, 32]. Given that stress is calculated as force per unit area, these altered joint mechanics could result in a higher PFJ stress.
Findings of this study are in disagreement with previous studies by Crossley et al.[12] and Fok et al.[13] who reported that individuals with PFJ OA presented a similar or lower loading at the PFJ during walking and stair ambulation. Several factors may contribute to the discrepancy in findings. First, participants with PFJ OA in the previous studies had at least a moderate level of pain (VAS ≥ 4) during functional activities [12, 13]. Thus, the observed biomechanical characteristics might present a pain-protective mechanism, which has been reported in individuals with patellofemoral pain [33, 34], rather than a contributing mechanism of PFJ OA. The PFJ OA participants in this study displayed a lower level of pain (KOOS Pain: 87) than those in the previous studies (KOOS Pain: 61–65) [12, 13] and did not present significant difference in pain compared to the controls. As such, it is plausible to suggest that findings of the current study might be less influenced by pain and better reflect risk factors associated with PFJ OA.
Second, the presence of PFJ OA was identified differently in the previous studies compared to the current investigation. Specifically, we used MRI-evaluated cartilage lesions to define PFJ OA, whereas the previous studies relied on radiographic evidence of OA for group delineation [12, 13]. Given that early stage cartilage lesions are not detectable on radiographic images [35–38], subjects with early stage PFJ OA might not be included in the OA group but allocated to the control group in the previous studies. This current study may provide more sensitive comparison of gait characteristics between individuals with and without PFJ OA.
Lastly, PFJ loading was assessed during the first and second halves of the stance phase in this study, whereas previous studies reported only one peak value during the entire stance phase [12, 13]. Two distinct peaks are often observed in knee kinetics during the stance phase of gait cycle [20, 28, 39]. While this current and the previous studies did not observe difference in the first peak between PFJ OA and control groups, we found a significantly higher PFJ loading during the second peak. Findings of the current study may provide more thorough information as to biomechanical characteristics of individuals with PFJ OA during different phases of the gait cycle.
Based on our findings, rehabilitation and prevention programs for PFJ OA may focus on reducing the second peak of PFJ loading during gait. This peak typically occurs during the second period of double limb support in which the body weight is being transferred from one limb to the other. During this phase, the ankle continues to push off the ground while the knee flexion angle increases. As a result, the ground reaction force passes posteriorly to the knee joint center and leads to knee flexion moment. Recent studies showed that a slightly more forward-lean trunk posture is related to lower knee flexion moment and PFJ stress during walking and running [11, 40]. Moreover, increasing step rate and decreasing step length have been shown to reduce PFJ stress and knee flexion moment during running [32, 41] Future studies are warranted to investigate the effects of these gait modifications (i.e. increased trunk lean, increased step rate, decreased step length) on PFJ loading during late stance phase of gait and in symptomatic individuals with PFJ OA.
Several limitations should be considered when interpreting the findings of the current study. First, due to the cross-sectional design, causal relationship between increased PFJ loading and PFJ OA cannot be drawn. Longitudinal studies are needed to elucidate this premise. Second, a 2-dimensional model was used to estimate PFJ stress. Joint motions and forces in the frontal and transverse planes were not taken into account. Third, PFJ contact area was estimated based on the results of cadaveric studies and may not represent the contact area of participants in the current study. However, to our knowledge, no study has examined the differences in contact area in individuals with and without PFJ OA. Lastly, there were a higher percentage of females in the PFJ OA group. Although gender has been considered as a covariate in the statistical analyses, it is important to keep in mind that the observed gait pattern may better reflect the gait characteristics of females with PFJ OA. There fore, caution should be taken when generalizing the results to male population.
5. Conclusion
Results of this current study revealed that individuals with PFJ OA presented with higher PFJ loading (i.e. knee flexion moment, knee flexion moment impulse and PFJ stress) during the late stance of walking when compared to controls. Further, this behavior was noted during two gait speeds. However, we did not observe significant differences in PFJ loading during the first half of the stance phase. Findings of this study suggest that increased PFJ loading during the second half of the stance phase may be an important factor associated with PFJ OA. Prevention and rehabilitation programs for PFJ OA may focus on reducing the mechanical loading on the PFJ, specifically during late stance.
Highlights.
People with patellofemoral osteoarthritis show higher flexion moment second peak
They also show higher flexion impulse and patellofemoral stress during late stance
The increased mechanical loading may be related to patellofemoral cartilage lesions
Acknowledgments
The project described was supported by Grant Number R01AR062370 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors would like to thank Danielle C Kassimatis for her assistance with data analysis and management.
Footnotes
Conflict of interests
None of the authors have any financial and personal relationships with other people or organizations that could potentially and inappropriately influence (bias) this work and conclusions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duncan RC, Hay EM, Saklatvala J, Croft PR. Prevalence of radiographic osteoarthritis--it all depends on your point of view. Rheumatology (Oxford) 2006;45:757–760. doi: 10.1093/rheumatology/kei270. [DOI] [PubMed] [Google Scholar]
- 2.Stefanik JJ, Niu J, Gross KD, Roemer FW, Guermazi A, Felson DT. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage. 2013;21:695–699. doi: 10.1016/j.joca.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51:844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornaat PR, Bloem JL, Ceulemans RYT, Riyazi N, Rosendaal FR, Nelissen RG, Carter WO, Hellio Le Graverand M-P, Kloppenburg M. Osteoarthritis of the knee: Association between clinical features and MR imaging findings. Radiology. 2006;239:811–817. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis Cartilage. 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 6.Duncan R, Peat G, Thomas E, Wood L, Hay E, Croft P. Does isolated patellofemoral osteoarthritis matter? Osteoarthritis Cartilage. 2009;17:1151–1155. doi: 10.1016/j.joca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 8.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 9.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–1774. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 10.Besier TF, Gold GE, Beaupré GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Excerc. 2005;37:1924–1930. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]
- 11.Teng H-L, Powers CM. Sagittal plane trunk posture influences patellofemoral joint stress during running. J Orthop Sports Phys Ther. 2014;44:785–792. doi: 10.2519/jospt.2014.5249. [DOI] [PubMed] [Google Scholar]
- 12.Crossley KM, Dorn TW, Ozturk H, van den Noort J, Schache AG, Pandy MG. Altered hip muscle forces during gait in people with patellofemoral osteoarthritis. Osteoarthritis Cartilage. 2012;20:1243–1249. doi: 10.1016/j.joca.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Fok LA, Schache AG, Crossley KM, Lin Y-C, Pandy MG. Patellofemoral joint loading during stair ambulation in people with patellofemoral osteoarthritis. Arthritis Rheum. 2013;65:2059–2069. doi: 10.1002/art.38025. [DOI] [PubMed] [Google Scholar]
- 14.Farrokhi S, O’Connell M, Fitzgerald GK. Altered gait biomechanics and increased knee-specific impairments in patients with coexisting tibiofemoral and patellofemoral osteoarthritis. Gait Posture. 2014 doi: 10.1016/j.gaitpost.2014.08.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.08.009. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the Osteoarthritis Initiative. Radiology. 2010;254:509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizai H, Virayavanich W, Joseph GB, Nardo L, Liu F, Liebl H, Nevitt MC, Lynch JA, McCulloch CE, Link TM. Cartilage lesion score: Comparison of a quantitative assessment score with established semiquantitative MR scoring systems. Radiology. 2014;271:479–487. doi: 10.1148/radiol.13122056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza RB, Feeley BT, Zarins ZA, Link TM, Li X, Majumdar S. T1rho MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee. 2013;20:113–119. doi: 10.1016/j.knee.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Ho K-Y, Blanchette MG, Powers CM. The influence of heel height on patellofemoral joint kinetics during walking. Gait Posture. 2012;36:271–275. doi: 10.1016/j.gaitpost.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Chinkulprasert C, Vachalathiti R, Powers CM. Patellofemoral joint forces and stress during forward step-up, lateral step-up, and forward step-down exercises. J Orthop Sports Phys Ther. 2011;41:241–248. doi: 10.2519/jospt.2011.3408. [DOI] [PubMed] [Google Scholar]
- 22.van Eijden TM, Kouwenhoven EK, Verburg J, Weijs WA. A mathematical model of the patellofemoral joint. J Biomech. 1986;19:219–229. doi: 10.1016/0021-9290(86)90154-5. [DOI] [PubMed] [Google Scholar]
- 23.van Eijden TM, Weijs WA, Kouwenhoven EK, Verburg J. Forces acting on the patella during maximal voluntary contraction of the quadriceps femoris muscle at different knee flexion/extension angles. Acta Anat (Basel) 1987;129:310–314. doi: 10.1159/000146421. [DOI] [PubMed] [Google Scholar]
- 24.Powers CM, Lilley JC, Lee TQ. The effects of axial and multi-plane loading of the extensor mechanism on the patellofemoral joint. Clin Biomech. 1998;13:616–624. doi: 10.1016/s0268-0033(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 25.Shull PB, Silder A, Shultz R, Dragoo JL, Besier TF, Delp SL, Cutkosky MR. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020–1025. doi: 10.1002/jor.22340. [DOI] [PubMed] [Google Scholar]
- 26.Bennell KL, Creaby MW, Wrigley TV, Bowles KA, Hinman BS, Cicuttini F, Hunter DJ. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis. 2010;69:1151–1154. doi: 10.1136/ard.2009.118182. [DOI] [PubMed] [Google Scholar]
- 27.Kean CO, Hinman RS, Bowles KA, Cicuttini F, Davies-Tuck M, Bennell KL. Comparison of peak knee adduction moment and knee adduction moment impulse in distinguishing between severities of knee osteoarthritis. Clin Biomech. 2012;27:520–523. doi: 10.1016/j.clinbiomech.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Brechter JH, Powers CM. Patellofemoral stress during walking in persons with and without patellofemoral pain. Med Sci Sports Excerc. 2002;34:1582–1593. doi: 10.1097/00005768-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Hinman RS, Lentzos J, Vicenzino B, Crossley KM. Is patellofemoral osteoarthritis common in middle-aged people with chronic patellofemoral pain? Arthritis Care Res. 2014;66:1252–1257. doi: 10.1002/acr.22274. [DOI] [PubMed] [Google Scholar]
- 30.Powers CM, Shellock FG, Pfaff M. Quantification of patellar tracking using kinematic MRI. J Magn Reson Imaging. 1998;8:724–732. doi: 10.1002/jmri.1880080332. [DOI] [PubMed] [Google Scholar]
- 31.Salsich GB, Ward SR, Terk MR, Powers CM. In vivo assessment of patellofemoral joint contact area in individuals who are pain free. Clin Orthop Relat Res. 2003;417:277–284. doi: 10.1097/01.blo.0000093024.56370.79. [DOI] [PubMed] [Google Scholar]
- 32.Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heiderscheit BC. Increasing running step rate reduces patellofemoral joint forces. Med Sci Sports Excerc. 2014;46:557–564. doi: 10.1249/MSS.0b013e3182a78c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salsich GB, Brechter JH, PowerS CM. Lower extremity kinetics during stair ambulation in patients with and without patellofemoral pain. Clin Biomech. 2001;16:906–912. doi: 10.1016/s0268-0033(01)00085-7. [DOI] [PubMed] [Google Scholar]
- 34.Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture. 2002;16:115–123. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 35.Schiphof D, Oei EHG, Hofman A, Waarsing JH, Weinans H, Bierma-Zeinstra SMA. Sensitivity and associations with pain and body weight of an MRI definition of knee osteoarthritis compared with radiographic Kellgren and Lawrence criteria: a population-based study in middle-aged females. Osteoarthritis Cartilage. 2014;22:440–446. doi: 10.1016/j.joca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Palmer AJR, Brown CP, McNally EG, Price AJ, Tracey I, Jezzard P, Carr AJ, Glyn-Jones S. Non-invasive imaging of cartilage in early osteoarthritis. Bone Joint J. 2013;95-B:738–746. doi: 10.1302/0301-620X.95B6.31414. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi D, Guermazi A, Kwoh CK. Clinical and translational potential of MRI evaluation in knee osteoarthritis. Curr Rheumatol Rep. 2013;16:391. doi: 10.1007/s11926-013-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guermazi A, Roemer FW, Felson DT, Brandt KD. Motion for debate: Osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum. 2013;65:2748–2758. doi: 10.1002/art.38086. [DOI] [PubMed] [Google Scholar]
- 39.Landry SC, McKean KA, Hubley-Kozey CL, Stanish WD, Deluzio KJ. Knee biomechanics of moderate OA patients measured during gait at a self-selected and fast walking speed. J Biomech. 2007;40:1754–1761. doi: 10.1016/j.jbiomech.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Leteneur S, Simoneau E, Gillet C, Dessery Y, Barbier F. Trunk’s natural inclination influences stance limb kinetics, but not body kinematics, during gait initiation in able men. PLoS One. 2013;8:e55256. doi: 10.1371/journal.pone.0055256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willson JD, Ratcliff OM, Meardon SA, Willy RW. Influence of step length and landing pattern on patellofemoral joint kinetics during running. Scand J Med Sci Sports. 2015 doi: 10.1111/sms.12383. Epub ahead of print. [DOI] [PubMed] [Google Scholar]