Abstract
All forms of diabetes share the common etiology of insufficient pancreatic β-cell function to meet peripheral insulin demand. In pancreatic β-cells, mitochondria serve to integrate the metabolism of exogenous nutrients into energy output, which ultimately leads to insulin release. As such, mitochondrial dysfunction underlies β-cell failure and the development of diabetes. Mitochondrial regulation of β-cell function occurs through many diverse pathways, including metabolic coupling, generation of reactive oxygen species, maintenance of mitochondrial mass, and through interaction with other cellular organelles. In this chapter, we will focus on the importance of enzymatic regulators of mitochondrial fuel metabolism and control of mitochondrial mass to pancreatic β-cell function, describing how defects in these pathways ultimately lead to diabetes. Furthermore, we will examine the factors responsible for mitochondrial biogenesis and degradation and their roles in the balance of mitochondrial mass in β-cells. Clarifying the causes of β-cell mitochondrial dysfunction may inform new approaches to treat the underlying etiologies of diabetes.
Keywords: islet, diabetes, mitochondria, mtDNA, mitophagy, metabolism
1. Introduction
“Lex II: Mutationem motus proportionalem esse vi motrici impressae, et fieri secundum lineam rectam qua vis illa imprimitur.”
“Law II: The alteration of motion is ever proportional to the motive force impress’d; and is made in the direction of the right line in which that force is impress’d.”
- Second Law of Motion, Sir Isaac Newton, Philosophiæ Naturalis Principia Mathematica, 1687 (English translation by Andrew Motte, 1729).
Perhaps one of the most impactful scientific works ever written; the Principia outlined the guiding characteristics and seminal definitions of modern physics and astronomy. A foundation for classical mechanics, Newton’s Second Law of Motion illustrates that the net force of an object’s movement is derived from its linear momentum, which is a product of the mass and velocity of an object (p=mv). The impact of this work has reached beyond the physical world to other fields, including the metaphysical, and it is useful to illustrate biological concepts.
In this sense, Newton’s Second Law can be applied liberally to describe how pancreatic β-cell health and function alters the balance of glucose homeostasis from the non-diabetic to diabetic state. All forms of diabetes share the common defect of inadequate β-cell function to meet peripheral insulin demand. Pancreatic β-cells secrete insulin following post-prandial glucose influx through a coordinated process requiring mitochondrial ATP generation necessary for closure of the ATP sensitive KATP channel, ultimately leading to Ca2+ influx and insulin exocytosis (Satin, 2000). Furthermore, β-cells rely heavily upon their mitochondria to generate the necessary currency (ATP) for nearly all key cellular processes, not limited to, but including insulin transcription, translation, packaging, and secretion (Wiederkehr and Wollheim, 2006). Therefore, mitochondria maintain the energy output (or momentum) needed for normal insulin release and glucose control, with defects in mitochondrial bioenergetics or metabolic coordination underlying the eventual development of diabetes.
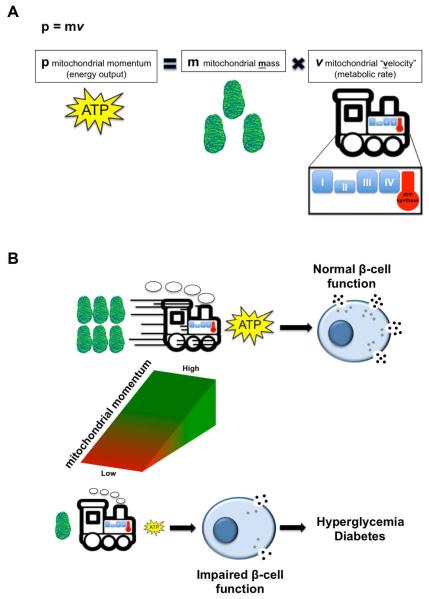
In a Newtonian context, mitochondrial momentum would therefore be estimated as a product of mitochondrial mass and velocity (Figure 1A). As would be expected, a decline in either variable would reduce β-cell function and insulin release due to a decline in mitochondrial momentum (Figure 1B). Mitochondrial “velocity” represents mitochondrial respiration or metabolism, while maintenance of mitochondrial mass is a balance of mitochondrial biogenesis and turnover. We hypothesize that in β-cells, defects in either mitochondrial metabolism or mass can disrupt the delicate balance required to maintain mitochondrial momentum, resulting in hyperglycemia and diabetes.
Figure 1. Mitochondrial momentum is necessary for maintenance of insulin secretion and glucose control.
(A) Schematic model of key contributors to mitochondrial momentum, adapted from Newton’s Second Law. (B) The outcomes of mitochondrial momentum resulting in normal β-cell function (with adequate mitochondrial mass and velocity). Reduced mitochondrial mass or velocity leads to poor mitochondrial momentum and insulin secretion, ultimately resulting in hyperglycemia and diabetes.
In this chapter, we will examine this hypothesis, by first discussing the importance of mitochondrial metabolism to pancreatic β-cell function and describing how defects in fuel sensing and glucose oxidation occur in type 2 diabetes mellitus (T2DM). Second, we will examine the nuclear and mitochondrial specific factors responsible for mitochondrial biogenesis and maintenance of mitochondrial DNA (mtDNA) and the role that these factors play in β-cell function and glucose control. Lastly, we will discuss the relevance of the mitochondrial quality control machinery, mediated by mitochondrial dynamics and mitophagy, in coordinating mitochondrial mass and function in β-cells. Through the examination of β-cell mitochondrial momentum, we will evaluate recent mitochondrially focused studies (including focused examinations of mitochondrial biogenesis, mitophagy, and mitohormesis) from a new and previously unappreciated perspective. A deeper mechanistic understanding of the loss of mitochondrial momentum in diabetes may inspire new therapies to improve β-cell function and insulin secretion.
2. Mitochondrial “velocity”: fuel metabolism in the control of insulin release
Insulin secretion is modulated by the metabolism of the three principal fuel sources: carbohydrates, proteins, and lipids. Several vital metabolic enzymes couple glucose availability to insulin secretion to regulate glucose homeostasis. Key among these, glucokinase (GCK) is an important pancreatic β-cell glucose sensor that responds to changes in ambient glucose concentration and determines the rate of glucose oxidation, thus leading to an activation of insulin release (Matschinsky et al., 1993). Both gain and loss-of-function studies affirm that GCK enzymatic activity ultimately correlates with downstream mitochondrial metabolic function and glucose-stimulated insulin release (Matschinsky, 1996).
Regulators of amino acid-stimulated insulin secretion (AASIS) also play an important role in connecting metabolite availability via the mitochondrial TCA cycle to insulin release. Defects in AASIS have been primarily observed in monogenic hyperinsulinism/hypoglycemia syndromes, illustrating the importance of amino acid stimulation of insulin secretion in humans (Chandran et al., 2014). While these gain-of-function mutations lead to enhanced insulin secretion, they also demonstrate the importance of mitochondrial “velocity” in control of insulin release and diabetes.
2.1 Glucose metabolism in human islets
Islet glucose metabolism is strongly activated following meal intake and leads to both increased β-cell insulin secretion and suppressed α-cell glucagon secretion to return blood glucose levels back to baseline. In diabetes, both glucose stimulated insulin secretion (GSIS) and glucagon suppression are impaired. To evaluate the contribution of glucose metabolism in T2DM, U-13C-glucose stable isotope tracing in human islets demonstrates an alteration of glucose metabolism in T2DM which correlates with β-cell dysfunction and impairment of glucagon suppression (Li et al., 2013). Islets from T2DM donors demonstrate a lack of glucose-stimulated oxygen consumption, paralleling the defects in GSIS, suggesting that a defect in β-cell mitochondrial function is a major cause of impaired GSIS in T2DM. Furthermore, pharmacologic activation of GCK can partially correct the impairments of both mitochondrial metabolism and GSIS in T2DM human islets (Doliba et al., 2012). Additional analysis of T2DM islet samples demonstrated that islet donors with milder disease (glucose controlled with diet or metformin monotherapy alone) maintain relatively normal GSIS, mitochondrial function, as well as amino acid metabolites and glucose-mediated glucagon suppression. On the other hand, islets from T2DM donors with more advanced disease (including loss of GSIS and glucagon suppression) displayed a dramatically altered metabolic profile with large accumulations of alanine, glutamate, glutamine, glycine and serine (a near tripling of the sum of this group of AAs), indicating a generalized defect of amino acid metabolism. Interestingly, concentrations of the neurotransmitter γ-aminobutyric acid (GABA), a glutamate metabolite synthesized in β-cells, were reduced by nearly 80% in the advanced disease subgroup. Reduced GABA metabolism due to decreased TCA cycle flux could potentially lead to lower levels of downstream α-cell inhibitory neurotransmitters, such as γ-hydroxybutyrate (GHB). Reduced GABA and GHB levels may explain the loss of glucagon suppression within T2DM islets, similar to findings observed with pharmacologic inhibitors of the GABA pathway (Molven et al., 2004). Taken together, these studies highlight mitochondrially mediated metabolic imbalances downstream of glucose within T2DM islets, resulting in dysregulation of both insulin and glucagon secretion.
2.2 Glutaminolysis and insulin secretion
While glucose is the major physiological regulator of insulin secretion, amino acids (including glutamine, leucine, and alanine) also promote insulin release from pancreatic β-cells (Fajans et al., 1967; Newsholme et al., 2007). Glutamine is consumed at high rates in pancreatic islets (Dixon et al., 2003) and is utilized for nucleic acid synthesis and protein synthesis, as well as a mitochondrial metabolic substrate to promote insulin secretion under basal (or low glucose) conditions (Gao et al., 1999). Glutaminolysis, the conversion of glutamine to α-ketoglutarate (α-KG) that occurs within the mitochondria, is a well known regulator of AASIS (Sener and Malaisse, 1980). The key enzymes in glutaminolysis, including phosphate-dependent glutaminase and the mitochondrial matrix protein glutamate dehydrogenase (GDH), serve as intracellular energy sensors of changes of fuel supply (mainly glucose) to switch glutamine oxidation on or off in the β-cell (Gao et al., 1999; Li et al., 2003). Glutamine oxidation through GDH is activated by ADP and leucine (during prolonged starvation or protein intake alone) or inhibited by GTP and ATP (following post-prandial glucose uptake) to catalyze the reversible oxidative deamination of L-glutamate to α-KG (Li et al., 2011b). Thus, glucose oxidation and glutaminolysis exist in a balance mediated by GDH to regulate insulin release in both basal and stimulated conditions.
Activating mutations of GDH have been identified in children with hyperinsulinism and hyperammonemia (HI/HA) syndrome, with mutations leading to a lack of sensitivity to GTP allosteric inhibition (Gloyn, 2003). GDH-linked hyperinsulinism (GDH-HI) patients are sensitive to protein feeding, with notable hyperinsulinemic hypoglycemia following protein rich meals (Hsu et al., 2001; Kelly et al., 2001). Transgenic mice with β-cell specific expression of the activating H454Y GDH mutation also develop hypoglycemia similar to GDH-HI patients, with enhanced insulin release following amino acid exposure in vivo or in isolated islets (Li et al., 2011a; Li et al., 2006). Furthermore, stable isotopic labeling and GDH flux analysis reveals that H454Y GDH islets have increased enzymatic flux correlating with loss of allosteric inhibition of GDH (Li et al., 2006).
Mitochondrial GTP (mtGTP) serves as a major regulator of GSIS (Kibbey et al., 2007), in addition to its role as an allosteric inhibitor of GDH. Levels of mtGTP, produced by the GTP-specific isoform of succinyl-CoA synthetase (SCS), directly reflect the flux rate of TCA cycle and glucose oxidation in β-cells. Suppression of GTP production by siRNA knockdown of GTP-specific SCS leads to impaired insulin release, mitochondrial oxygen consumption, and cytosolic Ca2+ influx in response to glucose (Kibbey et al., 2007). Mitochondrial GTP drives KATP channel independent, non-canonical insulin secretion through anapleurotic phosphoenolpyruvate cycling (Stark et al., 2009). In hypoglycemic hypoglucagonemic H454Y GDH transgenic mice, glucagon secretion is restored following pharmacologic GDH inhibition, which suggests that allosteric mtGTP-inhibition of GDH may also have paracrine effects on α-cells (Kibbey et al., 2014). These observations not only implicate both GDH and mtGTP in control of AASIS and hyperinsulinism, but also connect GDH and mtGTP to the maintenance of both α and β-cell function.
2.3 Cross-talk between amino acid and fatty acid metabolism at the mitochondria: implications for insulin release
The observation of hyperinsulinemia due to short-chain L-3-hydroxyacyl-CoA dehydrogenase (SCHAD) deficiency highlights the importance of fatty acid oxidation enzymes to insulin release (Hussain et al., 2005; Molven et al., 2004). SCHAD is a mitochondrial fatty acid β-oxidation enzyme that catalyzes the β-oxidation cycle for medium and short-chain 3-hydroxy fatty acyl-CoAs (C4 to C10). SCHAD deficiency leads to an accumulation of fatty acid metabolites and ketones, yet the implications of these metabolites on insulin secretion are unclear (Li et al., 2011a; Li et al., 2006). As expected, loss of SCHAD function in mouse models also leads to hypoglycemia as well as fatty acid metabolite accumulation (Stanley et al., 1998). Surprisingly, SCHAD deficiency also leads to amino acid-induced hypoglycemia, similar to what is observed with activating GDH mutations (Zelent et al., 2005). SCHAD loss of function does not lead to enhanced GSIS or increased insulin secretion after treatment with fatty acids. The defects observed in SCHAD knockout islets were primarily secondary to altered enzyme kinetics in GDH. SCHAD knockout islets possess a reduced affinity of GDH for α-KG while leading to increased enzyme efficiency, suggesting that SCHAD modulates GDH substrate binding affinity within its catalytic site. The effects of SCHAD on GDH activity may be secondary to a physical interaction between these two mitochondrial enzymes, as they exist within a protein complex in mitochondria (Li et al., 2010), and highlights a unique connection between two key metabolic enzymes and their respective metabolic pathways in the control of insulin secretion.
It is increasingly evident that metabolism of glucose, proteins, and lipids all play important roles in the regulation of insulin secretion. Through their effects on glutamine metabolism in the mitochondria (in the case of GABA utilization or GDH activity), glucose, amino acid, and fatty acid metabolism are connected in shared pathways of β-cell dysfunction either in states of insulin deficiency or insulin excess (Figure 2). The intersection of the metabolism of these fuel sources and thresholds for metabolite switching within the islets of patients with T2DM remains to be a topic for future investigation. Understanding the mechanisms of mitochondrial “velocity” or fuel metabolism, in both T2DM and HI, will not only help us to identify the disease-causing metabolic pathways, but also could provide new targets for drug therapy applicable for both diseases.
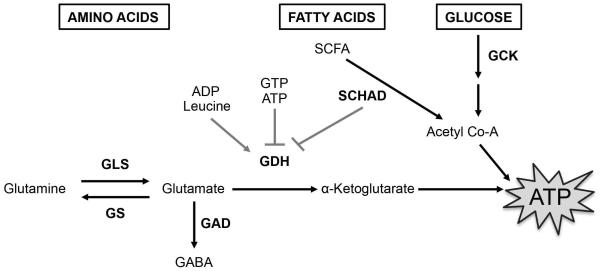
Figure 2. Regulators of β-cell fuel metabolism intersect at the mitochondria to control ATP generation and insulin secretion.
Focused model of metabolism of principal fuel sources in β-cells (black boxes), metabolites (black text), and their key enzymatic regulators (bold black text) vital for fuel metabolism, ATP generation, and insulin secretion. The effects of allosteric activators or inhibitors on GDH activity are indicated with grey lines.
GLS – glutaminase, GS – glutamine synthase, GDH – glutamate dehydrogenase, SCHAD – short-chain L-3-hydroxyacyl-CoA dehydrogenase, GCK – glucokinase, GAD – glutamic acid decarboxylase, SCFA – short-chain fatty acids, GABA – γ-aminobutyric acid
3. Mitochondrial mass: mitochondrial biogenesis and mitochondrial DNA (mtDNA) – a tale of two genomes
Due to the necessity of ATP for both insulin exocytosis and insulin biosynthesis, complex regulatory networks coordinate energy metabolism to meet insulin demand. These networks are also responsible for adaptation of β-cell mitochondria during progressive peripheral insulin resistance to preserve normal glycemic control. This accommodation involves changes in gene expression to increase the levels of mitochondrial proteins (mitochondrial biogenesis), although other mitochondrial maintenance processes, such as mitochondrial dynamics and mitophagy, presumably contribute as well (see Section 4 below).
The assembly of new respiratory complexes is complicated; there are a large number of subunits per complex, and these subunits are encoded on two genomes. The vast majority of mitochondrial proteins are nuclear-encoded and subject to nuclear gene expression regulatory mechanisms. In contrast, the mitochondrial genome, a roughly 16kb circular DNA present in thousands of copies, encodes a small number of oxidative phosphorylation (OXPHOS) subunits and the RNAs required for their expression, with each of these subunits essential for mitochondrial respiration. The abundance of mitochondrial DNA (mtDNA) is tightly regulated, and the relative abundance broadly correlates with respiratory activity across tissues. Mutations in mtDNA sequences occur at an estimated carrier rate of 1/200 live births (Elliott et al., 2008); however, the large number of these genomes can buffer against mitochondrial dysfunction as inherited mitochondrial disorders are more rare, estimated at 1/5000-1/8000 lives births (Thorburn, 2004). The regulation of mitochondrial biogenesis, which is a major contributor to establishing the levels of mtDNA, is presumably an essential factor in coordinating the two genomes to maintain mitochondrial function in the β-cell.
3.1 PGC1α regulatory axis in the β-cell
The best-studied regulator of mitochondrial biogenesis is peroxisome proliferator-activated receptor gamma coactivator 1 or Pgc1α, a transcriptional co-activator that acts with numerous transcription factors to influence the expression of fatty acid metabolism, mitochondrial proteins, and antioxidant defenses (St-Pierre et al., 2006). Pgc1α acts primarily with nuclear respiratory factors 1 and 2 (Nrf1 and Nrf2) to regulate expression of nuclear-encoded components of the electron transport chain (Gugneja and Scarpulla, 1997; Gugneja et al., 1995; Wu et al., 1999). Pgc1α and Nrf1/Nrf2 transcription factors target numerous genes involved in mitochondrial function, with both shared and unique targets between Nrf1 and Nrf2 (Kelly and Scarpulla, 2004). Pgc1α human diabetic muscle cells (Mootha et al., 2003), potentially suggesting Pgc1α-dependent control of glucose uptake and mitochondrial function in T2DM.
The β cell-specific role of Pgc1α is less clear. In humans, decreased expression of PGC1α T2DM islets (Gillberg et al., 2013; Ling et al., 2008) and an increased odds ratio for T2DM with the G482S PGC1α variant have been observed; however, correlative reductions of β-cell function by indirect measures (such as fasting glucose and insulin levels) are not always seen (reviewed in (Mulder and Ling, 2009)). Reduced PGC1α expression in human islets by RNA interference leads to decreased insulin expression and secretion, suggesting that its expression is necessary for β-cell function (Ling et al., 2008). On the other hand, Pgc1α expression is significantly elevated in the islets of diabetic ZDF rats and ob/ob mice (Yoon et al., 2003a). Adenoviral overexpression of Pgc1α in islets or β-cell lines significantly impairs insulin release (Yoon et al., 2003a); however, transgenic Pgc1α overexpression negatively impacts insulin secretion only if overexpression occurs during fetal development (Valtat et al., 2013). Pgc1α overexpression appears to reduce levels of Pdx1, an essential β-cell transcription factor expressed in islet progenitors as well as adult β-cells, but this may not clearly explain the negative developmentally-induced effects of Pgc1α (Valtat et al., 2013; Yoon et al., 2003a). Further study may be necessary to clarify Pgc1α function in the pancreatic β-cell, including investigation of the effects of Pgc1α on mitochondrial respiratory function in β-cell specific knockout models as well as identification of Pgc1α transcriptional targets and binding partners necessary for its action.
Of the two nuclear transcription factors known to collaborate with Pgc1α to regulate expression of mitochondrial proteins, only NRF1 has been associated with diabetes (Cho et al., 2005; Liu et al., 2008). In both Korean and Han Chinese cohorts, two distinct NRF1 haplotypes were found to be either protective or increased risk alleles for T2DM (Cho et al., 2005; Liu et al., 2008). NRF1 function is associated with insulin action and glucose uptake in the muscle of T2DM patients (Patti et al., 2003) and has been recently found to regulate mitochondrial metabolism and GSIS in rodent islets (Zheng et al., 2015). Interestingly, Pdx1 occupies the Nrf1 gene locus in chromatin IP assays in both mouse and human islets (Khoo et al., 2012), suggesting that Pdx1 could regulate Nrf1-dependent expression of mitochondrial proteins. How mitochondrial function is dynamically regulated in the β-cell may in fact lie with the coordinated actions of Pgc1α, Nrf1, and Pdx1.
3.2 Pdx1 and the regulation of mitochondrial function
Islet development and β-cell maintenance is dependent on the homeodomain transcription factor PDX1. Mutations in PDX1 (also known as IPF1) lead to several heritable forms of diabetes, including Maturity Onset of Diabetes in the Young, neonatal diabetes, and early onset T2DM (Hani et al., 1999; Nicolino et al., 2010; Stoffers et al., 1997). Pdx1 loss of function in several rodent models also has been shown to lead to reduced GSIS and mitochondrial function (Brissova et al., 2002; Gauthier et al., 2004; Gauthier et al., 2009). Specifically, the expression a dominant negative form of Pdx1 (Pdx1-DN) in isolated rat islets leads to reduced insulin secretion and mitochondrial respiration as well as altered expression of numerous TCA cycle and OXPHOS enzymes; of the mtDNA-encoded transcripts, mt-ND1 expression was decreased while mt-ATP6 was increased (Gauthier et al., 2004). The mt-ND1 expression closely correlated with mtDNA levels. Additional analysis implicated decreased expression of Tfam, the primary mtDNA packaging and transcription factor (Campbell et al., 2012), to be the potential cause of decreased mtDNA content. By restoring Tfam levels through viral expression, mtDNA abundance, mt-ND1 expression, ATP levels and GSIS were all normalized, indicating that mtDNA depletion was sufficient to cause β-cell dysfunction (Gauthier et al., 2009). β-cell-specific ablation of Tfam is known to lead to a complete loss of mtDNA content and consequentially diminished β-cell mass (Silva et al., 2000), consistent with its well known function to act as a “kill switch” when deleted in respiring cell types (Wang et al., 2001). On the other hand, Tfam expression in Pdx1 haploinsufficient mice is not significantly reduced despite impairments in mitochondrial function and glucose homeostasis (Brissova et al., 2002; Sachdeva et al., 2009). Taken together, these observations suggest that dysregulating the precise control of mitochondrial content, even mildly, can be of major consequence to β-cell function.
3.3 mtDNA mutations and diabetes
Pathogenic mtDNA mutations have been known since 1988, where mutations in the mitochondrial tRNA-Lys were shown to be the cause of the conditions of myoclonic epilepsy and ragged red muscle fibers (MERFF) and Leber’s hereditary optic neuropathy (LHON) (Wallace et al., 1988a; Wallace et al., 1988b). As a highly metabolically active cell type, mtDNA sequence stability is particularly essential for normal β-cell function. This is illustrated by the identification of maternally inherited mitochondrial diabetes and deafness (MIDD), which is associated with the A3243G mutation in mitochondrial tRNA-Leu, a mutation that also causes mitochondrial encephalomyelopathy, lactic acidosis, and stroke-like episodes (MELAS) (van den Ouweland et al., 1992). MIDD generally occurs later in life (4th decade) and, contrary to early suggestions, does occur with other mtDNA mutations, although the most common presenting mutation is the A3243G MELAS mutation (Whittaker et al., 2007). MIDD is usually progressive, with deafness preceding diabetes, and shows reduction in β-cell mass and subsequent insulin deficiency without reduced insulin sensitivity in peripheral tissues. MIDD mutations were among the first evidence of a role for energetic failure in glucose-stimulated insulin secretion.
The integrity and content of mtDNA may play key roles in the development of hyperglycemia. Certainly β-cell function requires mitochondrial activity, as ablation of Tfam and subsequent loss of mtDNA causes hyperglycemia in mice (Silva et al., 2000), although this form of mtDNA depletion does not allow for a nuanced understanding of mitochondrial genome stability in diabetes. On the other hand, more common occurrences of mitochondrial genome deficiencies caused by mtDNA deletions and mtDNA depletion also cause respiratory dysfunction. Mitochondrial genome mutations occur either as single deletions, which usually manifest as de novo mutations, or multiple mtDNA deletions, which usually occur somatically and accumulate with age. Both forms can result in mitochondrial respiratory chain deficiency and disease (Copeland, 2012). In Kearns-Sayre syndrome (KSS), which is associated with a single deletion myopathy, patients can develop impaired GSIS, reduced β-cell mass, and dysmorphic islet architecture (Poulton et al., 1995; Stark and Roden, 2007). The involvement of multiple mtDNA deletions, which accumulate with age in other highly respiring tissues (Damas et al., 2014), in β-cell dysfunction has not been adequately explored. However, islet mtDNA content does decrease with age (Cree et al., 2008), similar to other tissues. When considering age-related accumulation of mtDNA point mutations and deletions in the context of decreasing mtDNA content, the hypothesis that mtDNA stability contributes to age related vulnerability for mitochondrial dysfunction becomes potentially applicable to the β-cell. With the addition of increasing insulin demand by obesity, susceptibility to age-dependent mitochondria genome instability could be a potential contributor to age-related β-cell failure.
3.4 Mitochondrial gene expression
Beyond mtDNA instability, aberrations in mitochondrial gene expression lead to β-cell dysfunction. The mitochondrial methyltransferase Tfb1m regulates mitochondrial gene expression by methylating 12S rRNA and is essential for ribosome subunit assembly (Metodiev et al., 2009). Hypermethylation, either by Tfb1m overexpression or mutation in the nearby ribosomal sequence (position A1555), causes altered mitochondrial ribosome function and OXPHOS defects, increased mitochondrial reactive oxygen species (ROS), activation of proapoptotic transcription factor E2F1, culminating in the progressive loss of critical cells of the inner ear (Raimundo et al., 2012). In T2DM, a common single nucleotide polymorphism (SNP) in the TFB1M locus is associated with an increased risk for diabetes in females and decreased complex I activity and content in human pancreatic islets (Koeck et al., 2011). Islet dysfunction was confirmed in the germ-line deleted Tfb1m+/− mice, as well in islets or β-cell lines following Tfb1m loss of function, suggesting a mitochondrial gene expression defect, potentially through mitochondrial translation (Koeck et al., 2011). More recently, β-cell specific Tfb1m knockout mice were shown to develop impaired insulin production and secretion, as well as reduced β-cell mass (Sharoyko et al., 2014). Concomitant with β-cell dysfunction, β-cell specific Tfb1m knockouts possess increased mitochondrial content with more dysmorphic mitochondria, features commonly found in patients with mitochondrial dysfunction.
3.5 Non-classical regulators of mitochondrial biogenesis
During the development of T2DM, hyperglycemia ensues due to impaired β-cell insulin release to meet the high demands (or insulin resistance) of insulin-responsive peripheral tissues (including skeletal muscle, adipose tissue, and the liver). Important insights into islet mitochondrial dysfunction secondary to peripheral insulin resistance have been gleaned from the muscle IGF-I (insulin-like growth factor 1) receptor-lysine-arginine (MKR) mouse model (Lu et al., 2010). MKR mice express a dominant negative form of the IGF-I receptor specifically in muscle (Fernandez et al., 2001), which results in increased peripheral insulin resistance. MKR mice subsequently develop progressive β-cell mitochondrial structural and functional decline with age culminating in reduced insulin secretion and hyperglycemia (Lu et al., 2010). Additionally, MKR have decreased expression of nuclear encoded mitochondrial metabolic enzymes, mtDNA levels, and mitochondrial gene expression along with increased β-cell oxidative stress (Lu et al., 2010). Interestingly, the precipitating causes of mitochondrial failure are not necessarily clear in this model. Despite the limited change in the expression of mitochondrial genes, mitochondrial protein levels are generally decreased in the MKR islets, so other alterations to the regulation of mitochondrial biogenesis, such as phosphorylation of the protein import pathway, are potential contributors.
Control of β-cell mitochondrial function is tenuous; studies of mitochondrial biogenesis demonstrate that even small changes in mitochondrial respiratory function are important to β-cell function. Numerous factors contribute to vulnerability to respiratory dysfunction in the β-cell, encompassing genetic influences on mitochondrial function, variation in mtDNA copy number, and progressive, age-related changes to mitochondrial biogenesis. It is possible that other genetic risk factors for defective mitochondrial biogenesis in T2DM, in addition to TFB1M, are yet to be identified, as only ~10% of genetic risk for T2DM has been characterized (Vassy et al., 2014). Studies into the component causes of diminished mitochondrial function in β-cells with age and stress will continue to be important to the understanding of the etiology of diabetes.
4. Mitochondrial mass (part 2): mitochondrial dynamics and mitophagy in the regulation of mitochondrial turnover
The contributions of mitochondrial metabolic flux through the mitochondria to nutrient-stimulated insulin release are among the most well-studied facets of β-cell function (reviewed in (Wollheim and Maechler, 2002)). From these studies, it is clear that bioenergetic defects impairing the ATP/ADP ratio ultimately leads to reduced insulin secretion. An area of growing interest is the importance of mitochondrial quality control in the maintenance of mitochondrial function and regulated insulin release. The mitochondrial quality control machinery involves mitochondrial-specific structural proteins, cytosolic factors, and components of the autophagy machinery to maintain a balance of repair or clearance of damaged, unhealthy, or old mitochondria. Specifically, multiple elements control discrete portions of this coordinated process (including mitochondrial fission, fusion, and mitophagy) and loss of any of these functions can lead to impaired insulin release and glucose intolerance.
4.1 Mitochondrial dynamics – mitochondrial fission and fusion
Mitochondrial dynamics involves a network of mitochondria continuously undergoing both fission and fusion. Mitochondrial dynamics has been extensively reviewed elsewhere (Stiles and Shirihai, 2012); however, here we will cover recent developments in this area as well as association to human T2DM pathogenesis. Mitochondrial fusion is regulated by the outer mitochondrial membrane dynamin related protein GTPases mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) (Santel and Fuller, 2001), as well as an inner mitochondrial membrane GTPase protein optic atrophy protein 1 (Opa1) (Misaka et al., 2002). In brief, during mitochondrial fusion, intermixing of membrane proteins, metabolites, and mtDNA aid in preserving functional mitochondria, which may have become depolarized by intracellular or extracellular stress, such as glucolipotoxicity (Molina et al., 2009). Mitochondrial fission is mediated by several proteins, including the transmembrane protein fission 1 (Fis1) (James et al., 2003) and the outer membrane protein mitochondrial fission factor (Mff) (Gandre-Babbe and van der Bliek, 2008), as well as the soluble GTPase dynamin-related protein 1 (Drp1), which is recruited to the mitochondria by Fis1 and Mff to initiate fission events (Yoon et al., 2003b). Mitochondrial fission is essential to isolate dysfunctional or damaged mitochondria, where they can be ultimately sorted to repair mechanisms through the fusion cycle or to degradation via the mitophagy/autophagy machinery (van der Bliek et al., 2013).
Alterations of the balance of mitochondrial dynamics, by gain or loss of function of several of the fission or fusion proteins mentioned above, have been shown to lead to impairments in mitochondrial structure (i.e. fragmentation), function, and/or glucose-stimulated insulin release (Molina et al., 2009; Park et al., 2008; Twig et al., 2008; Zhang et al., 2011). Further, β-cell specific loss of prohibitin 2, a mitochondrial scaffolding protein critical for inner membrane integrity and upstream regulator of Opa1, leads to mitochondrial fragmentation, impaired mitochondrial function, glucose intolerance, reduced insulin release, and increased β-cell apoptosis (Supale et al., 2013). Mitochondrial outer membrane proteins, such as Mfn2 (de Brito and Scorrano, 2008), also mediate mitochondrial-ER interactions and could subsequently regulate mitochondrial function and insulin release through alterations in mitochondrial-ER coupling (Arruda et al., 2014).
Defects in mitochondrial structure, function and dynamics have been explored in animal models of T2DM as well as isolated human T2DM islets. Both diabetic Goto Kakizaki (GK) and Zucker Diabetic Fatty (ZDF) rats develop mitochondrial fragmentation (Dlaskova et al., 2010; Higa et al., 1999). Specifically, ZDF rats also show mitochondrial ultrastructural defects, with swollen mitochondrial membranes and altered cristae structure (Higa et al., 1999). A study of islets from human T2DM donors also suggests that mitochondrial structural defects correlate with reduced mitochondrial function (Anello et al., 2005); however, the mechanisms underlying these structural defects remain to be explored. It is possible that these changes could be secondary to defects in components of the mitochondrial fusion apparatus; for example, SNPs in the human MFN2 locus have been associated with T2DM in a Han Chinese cohort. (Li et al., 2012b). Further study is needed to determine if other T2DM populations have associations with these MFN2 SNPs, or if β-cell mitochondrial dysfunction in T2DM is secondary to defective mitochondrial dynamics induced by glucolipotoxicity.
4.2 Selective mitochondrial turnover by mitophagy
Mitochondrial autophagy or mitophagy is a selective catabolic process critical for the clearance of damaged, depolarized, or aging mitochondria that have been segregated by fission for removal by autophagosomes and lysosomes (Twig et al., 2008). In mammalian cells, several distinct pathways regulate mitophagy. The best understood pathway of mammalian mitophagy is principally regulated by two proteins, the PTEN-induced putative kinase 1 (PINK1) and the E3 ubiquitin ligase Parkin (Jin and Youle, 2012). While erythroid cells also depend on the Bcl-2 family member Nix (or Bnip3L) to induce mitochondrial autophagy (Sandoval et al., 2008; Schweers et al., 2007; Zhang and Ney, 2008), Nix deletion has not been shown to impair β-cell autophagy, insulin secretion or glucose control (Fujimoto et al., 2010), suggesting that Nix does not contribute to β-cell mitophagy.
PINK1 acts as a molecular detector of damaged mitochondria (Narendra et al., 2010; Vives-Bauza et al., 2010). PINK1 is a mitochondrially targeted serine-threonine kinase, which localizes to healthy mitochondria, and is rapidly degraded (Muqit et al., 2006). Following mitochondrial damage or depolarization, full length PINK1 accumulates at the mitochondrial outer membrane, with its kinase domain present facing the cytosol (Narendra et al., 2010). While dependent on mitochondrial PINK1 accumulation, the exact mechanism leading to Parkin recruitment to the mitochondrial surface after mitochondrial depolarization is not known. Parkin localization to the mitochondrial surface subsequently leads to activation of mitophagy (Narendra et al., 2008) by ubiquitinating its target proteins, including mitofusins 1 and 2 (Mfn1 and 2) and porin (Gegg et al., 2010; Geisler et al., 2010), to promote their proteosomal degradation. Additionally, ubiquitination of porin leads to recruitment of cytosolic factors required for recruitment of the autophagy machinery, including sequestosome 1 (p62) and histone deacetylase 6 (HDAC6) (Geisler et al., 2010; Lee et al., 2010). p62 and HDAC6 interaction with autophagosomal proteins, including Beclin and Ambra1, ultimately leads to turnover of Parkin-bound mitochondria through autophagosome-lysosome degradation (Michiorri et al., 2010; Van Humbeeck et al., 2011).
The terminal aspects of mitophagy (i.e. phagophore formation, autophagosome engulfment of mitochondria, autophagosome fusion with autolysosomes) bear many similarities to those of macroautophagy, which is a coordinated cannibalization of cellular organelles and proteins classically in response to nutrient deficiency. β-cell macroautophagy, which has been recently reviewed (Lee, 2014), is vital for the maintenance of normal glucose control through preservation of ER homeostasis (Quan et al., 2012), maintenance of insulin content (Marsh et al., 2007), compensation for peripheral insulin resistance (Ebato et al., 2008), and elimination of accumulated human islet amyloid polypeptide deposits (Kim et al., 2014; Rivera et al., 2014; Shigihara et al., 2014). Dysfunctional β-cell macroautophagy induced by deletion of a key autophagosomal initiation protein, autophagy-related 7 (Atg7), also leads to mitochondrial dysfunction (Jung et al., 2008). It is unclear whether mitochondrial defects following Atg7 loss of function are due to prevention of normal mitochondrial turnover or due to secondary defects (such as ER stress) indirectly leading to reduced cellular respiration.
4.3 Consequences of dysfunctional mitophagy in β-cells
Originally identified as key genetic components of Familial Parkinson’s disease, PINK1 and Parkin have been implicated in mediating mitochondrial turnover and cellular function in numerous cell types (Scarffe et al., 2014). Previously observed associations between T2DM and Parkinson’s disease (Sandyk, 1993; Sun et al., 2012) could be suggestive of common defects in mitophagy that may permeate both β-cells and dopaminergic neurons of the substantia nigra pars compacta. Furthermore, the PINK1 N521T variant and several Parkin SNPs associate with T2DM and reduced insulin secretion respectively, suggesting a direct role for PINK1 and Parkin in β-cell dysfunction and diabetes pathogenesis (Jin et al., 2014; Qu et al., 2011). However, it should be known that these genetic connections are from limited sample sizes and require validation in larger cohorts.
Studies on the importance of regulators of mitophagy to β-cell function have been limited to date but have provided interesting insights on the importance of mitochondrial turnover to insulin secretion. The β-cell specific role of Parkin has been controversial when extrapolated from studies in β-cell lines, with evidence of impaired insulin secretion when Parkin expression is transiently increased (Hofmeister-Brix et al., 2013) or reduced by RNAi (Jin et al., 2014). Parkin loss in vivo impairs insulin secretion, mitochondrial function, mitochondrial structure and mitophagy in pancreatic β-cells in the context of streptozotocin (STZ)-induced toxicity (Hoshino et al., 2014); however, β-cell survival was not assessed in this study. Interestingly, the effects of Parkin loss of function were limited to induction of β-cell damage following STZ treatment but were not observed in untreated mice. This suggests an induction of β-cell mitochondrial damage is necessary to elicit Parkin-dependent mitochondrial clearance, consistent with previous observations of mitochondrial depolarization preceding Parkin recruitment to selectively eliminate impaired (but not healthy) mitochondria (Narendra et al., 2008). Unfortunately, these studies do not address the importance of Parkin during physiologic mitophagy in the turnover of impaired or aging mitochondria in β-cells.
Recent profiling of PINK1 in β-cell function and glucose homeostasis indicates additional roles for PINK1 outside of mitophagy (Deas et al., 2014). PINK1 deficiency in islets or MIN6 cells led to reduced glucose-stimulated mitochondrial function and downstream calcium flux. Surprisingly, whole body PINK1 knockout mice displayed improved glucose clearance as well as basal and glucose-stimulated insulin secretion that were not due to effects on peripheral insulin sensitivity. It is possible that PINK1 deficient mice display enhanced insulin release and glucose control due to upregulation of several key β-cell specific factors, including glucokinase, NeuroD, Nkx6.1, and FoxA2 (Deas et al., 2014) or increases in β-cell replication and mass as PINK1 has been shown to regulate cell proliferation (O’Flanagan et al., 2014), but this remains to be determined. Similar to approaches utilized in Parkin deficient models, it is possible that induction of mitochondrial stress or damage is necessary to observe PINK1 relevance in control of diabetes and β-cell-related mitophagy.
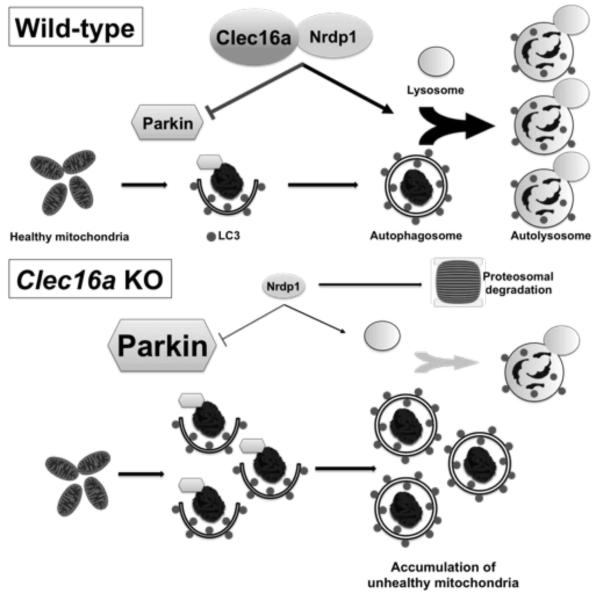
We recently identified a role for mitophagy in the maintenance of β-cell function in the study of the type 1 diabetes susceptibility gene C-type lectin domain family 16, member A (also known as Clec16a or KIAA0350) (Soleimanpour et al., 2014). While Clec16a does not actually possess a true C-type lectin domain, high-throughput proteomic analyses have identified novel interacting proteins that shed light into Clec16a function. Clec16a is an endolysosomal protein that interacts with the E3 ubiquitin ligase neuregulin receptor degradation protein-1 (Nrdp1). Nrdp1 targets both Parkin and Nrdp1 itself for proteosomal degradation (Qiu and Goldberg, 2002; Zhong et al., 2005). Clec16a stabilizes and prevents the proteosomal degradation of Nrdp1 limiting recruitment of Parkin to the mitochondrial surface and also promotes autophagosome-lysosome fusion during the terminal steps of mitophagy (Soleimanpour et al., 2014) (Figure 3). Nrdp1 interaction with, and stabilization by, the deubiquitination enzyme USP8 (Wu et al., 2004) could suggest a tripartite complex whereby Clec16a, USP8, and Nrdp1 coordinate together to regulate Parkin-mediated mitophagy. The importance of USP8 in removal of K6-linked polyubiquitin moieties from Parkin to allow for Parkin recruitment to defective mitochondria is supportive of this model (Durcan et al., 2014).
Figure 3. Clec16a regulates mitophagy.
Schematic model of Clec16a control of Parkin-mediated mitophagy and autophagosome-lysosome fusion, via its interaction with, and stabilization of, the E3 ubiquitin ligase Nrdp1. Loss of Clec16a-Nrdp1 leads to an accumulation of unhealthy mitochondria due to unrestrained Parkin activity that are trapped and cannot complete mitophagy as a result of impaired autophagosome-lysosome fusion.
Adapted (with permission) from Soleimanpour, et al. Cell, 2014.
Loss of Clec16a in pancreatic islets leads to an accumulation of unhealthy mitochondria due to dysfunctional mitophagy, ultimately leading to impaired glucose homeostasis and glucose-stimulated insulin secretion in mice. Importantly, loss of Clec16a in vivo leads to defects in physiologic mitophagy, illustrating the importance of mitochondrial turnover as a key regulatory process in β-cells. Additionally, diabetogenic CLEC16A polymorphisms lead to reduced islet CLEC16A expression, as well as reduced β-cell function and glucose control in human subjects (Dupuis et al., 2010; Soleimanpour et al., 2014). While of importance to β-cell mitophagy and type 1 diabetes pathogenesis, Clec16a may also play an important role in the pathogenesis of T2DM. Regulation of Clec16a expression by Pdx1 (Sachdeva et al., 2009) could serve as a potential connection between dysfunctional mitophagy and T2DM. The role of mitophagy (and its regulation by PINK1, Parkin, and Clec16a) in the pathogenesis of T2DM remains to be determined.
5. Mitochondria and oxidative stress: a unifying hypothesis
Among the major metabolic triggers of β-cell dysfunction in T2DM is glucose and lipid-induced toxicity. In the context of obesity, elevated circulating glucose and fatty acids increase insulin demand and contribute to β-cell oxidative stress. Mitochondrial oxidative stress leads to oxidation of numerous targets and culminates in impaired GSIS and β-cell apoptosis (Ma et al., 2012; MacDonald et al., 2013). The cellular responses to this stress may vary by experimental system or approach. Increased oxidative stress can activate UCP2, a mitochondrial inner membrane protein that uncouples membrane potential, possibly to reduce mitochondrial oxidant generation at the expense of ATP generation. Reduction in UCP2-dependent proton leak improves insulin resistance-driven β-cell dysfunction in vitro and in vivo (De Souza et al., 2007; Joseph et al., 2002; Zhang et al., 2001), although some background-dependent effects have been observed (reviewed in (Pi and Collins, 2010)). Indeed, the UCP2 β-cell specific knockout shows enhanced GSIS with elevated reactive oxygen species (ROS) levels, which is explained as increased metabolic flux to fuel β-cell function (Robson-Doucette et al., 2011). However, these mice also develop glucose intolerance related to intra-islet ROS signals that potentiate glucagon release. Additional studies are needed to identify whether mitochondrial-specific ROS, and possibly oxidative modifications, are enhanced in the mitochondria and whether cellular adaptation or mitochondrial biogenesis has occurred thus contributing to enhanced insulin release. Nonetheless, these studies suggest that adaptation to β-cell oxidative stress involving increased UCP2 expression could contribute to a decline in GSIS during obesity and insulin resistance.
The concept that an increase in levels of oxidant production could drive normal metabolic adaptation, however, are in conflict with other observations of oxidative stress induced β-cell failure. Antioxidant enzyme expression within β-cells is low compared to other tissues, suggesting they are more sensitive to oxidative stress induced dysfunction (Robertson and Harmon, 2006). For example, β-cell specific transgenic overexpression of the antioxidant enzyme glutathione peroxidase 1 (Gpx1) substantially improves β-cell mass, insulin secretion, and glucose homeostasis in db/db mice (Harmon et al., 2009). Similarly, β-cell specific transgenic overexpression of thioredoxin also improves glucose homeostasis and insulin content in db/db mice (Yamamoto et al., 2008). Overexpression of antioxidant enzymes is principally thought to improve β-cell function by preventing inactivation of key β-cell transcription factors, including Pdx1 and MafA (Guo et al., 2013). It is also possible that inactivation of Pdx1 by oxidative stress reduces the expression of key downstream mitochondrial targets necessary to maintain oxygen consumption and insulin secretion. Alternatively, the potential decrease in oxidant load in β-cell antioxidant transgenic mouse models may decrease regulated expression of UCP2, thus increasing mitochondrial membrane potential and driving increased GSIS (reviewed in (Lei and Vatamaniuk, 2011)). Islet oxidative stress could also be ameliorated by inhibition of proinflammatory enzymes that suppress antioxidant expression, such as 12-lipooxygenase, which has been shown to suppress Gpx1 and superoxide dismutase 1 expression as well as preventing nuclear localization of Nrf2 (Tersey et al., 2014). The many pathways that respond to mitochondrial function, including acetyl-CoA availability, NAD+/NADH levels, oxidant load, and ATP availability all bear on nuclear expression posture, and each could contribute ultimately to the demise the essential maintenance expression pathways (Pdx1, MafA, and others).
The dichotomy of responses to oxidative stress in pancreatic β-cells could be best explained by the capacity of β-cell mitochondria to appropriately adapt to cellular stressors, including glucolipotoxicity. Hormesis, an adaptive response to cellular stress, refers to the capacity of cells to withstand and respond to sublethal toxin exposure by upregulating repair and defense mechanisms (Kolb and Eizirik, 2012). The generation and responsiveness to ROS in β-cells primarily involves the mitochondria and could therefore be suggestive of mitochondrial hormesis as a key defense mechanism, which may be dysfunctional in the progression to β-cell failure and T2DM (Li et al., 2012a). Future studies directed at β-cell mitohormesis (perhaps mediated by UCP2) may be instructive in evaluating the importance of this hypothesis in β-cell mitochondrial dysfunction. In addition, the key targets causing ROS-mediated lethality are not known, which may inform preventative therapies.
6. Challenges
Developing a complete image of factors that render β-cells incapable of glycemic control remains a major challenge. Currently, 1 in 3 patients require insulin replacement therapy for T2DM. Can we identify triggers of mitochondrial dysfunction or vulnerability to mitochondrial dysfunction to preserve β-cell mass in pre-diabetic or diabetic patients? The potential for improved β-cell function through reduction of toxic mitochondrial ROS or reducing UCP2-dependent proton leak may prove to be useful approaches for future mitochondrial targeted therapies (Mahadevan et al., 2013; Zhang et al., 2006). Alternatively, approaches to improve peripheral insulin sensitivity and reduce lipotoxicity may prove to be important mitochondrial-directed measures to preserve β-cell function by reducing energy demands on the β-cell. As reviewed here, there exists in the literature a plethora of data supporting a model in which mitochondrial metabolism and mass are key determinants of beta cell function (Figure 1). Thus, it is likely that approaches to improve mitochondrial momentum through maintenance of mitochondrial mass and velocity could improve insulin secretion and glucose control. Many new insights are needed to understand the factors involved, and then to propose solutions to restore mitochondrial metabolism in the context of aging and the metabolic syndrome.
Acknowledgements
The authors wish to acknowledge funding support from the National Institutes of Health (K08-DK089117) and the Brehm family. The authors also wish to thank Dr. Kathryn Claiborn for useful discussions in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48(2):282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277(13):11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochimica et biophysica acta. 2012;1819(9-10):921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Chandran S, Yap F, Hussain K. Molecular mechanisms of protein induced hyperinsulinaemic hypoglycaemia. World journal of diabetes. 2014;5(5):666–677. doi: 10.4239/wjd.v5.i5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YM, Shin HD, Park BL, Kim JH, Park KS, Kim SY, Lee HK. Association between polymorphisms in the nuclear respiratory factor 1 gene and type 2 diabetes mellitus in the Korean population. Diabetologia. 2005;48(10):2033–2038. doi: 10.1007/s00125-005-1855-7. [DOI] [PubMed] [Google Scholar]
- Copeland WC. Defects in mitochondrial DNA replication and human disease. Critical reviews in biochemistry and molecular biology. 2012;47(1):64–74. doi: 10.3109/10409238.2011.632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree LM, Patel SK, Pyle A, Lynn S, Turnbull DM, Chinnery PF, Walker M. Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia. 2008;51(8):1440–1443. doi: 10.1007/s00125-008-1054-4. [DOI] [PubMed] [Google Scholar]
- Damas J, Samuels DC, Carneiro J, Amorim A, Pereira F. Mitochondrial DNA rearrangements in health and disease--a comprehensive study. Hum Mutat. 2014;35(1):1–14. doi: 10.1002/humu.22452. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Stoppiglia LF, Pauli JR, Ropelle E, Rocco SA, Marin RM, Franchini KG, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA. Inhibition of UCP2 expression reverses diet-induced diabetes mellitus by effects on both insulin secretion and action. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21(4):1153–1163. doi: 10.1096/fj.06-7148com. [DOI] [PubMed] [Google Scholar]
- Deas E, Piipari K, Machhada A, Li A, Gutierrez-del-Arroyo A, Withers DJ, Wood NW, Abramov AY. PINK1 deficiency in beta-cells increases basal insulin secretion and improves glucose tolerance in mice. Open biology. 2014;4:140051. doi: 10.1098/rsob.140051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon G, Nolan J, McClenaghan N, Flatt PR, Newsholme P. A comparative study of amino acid consumption by rat islet cells and the clonal beta-cell line BRIN-BD11 - the functional significance of L-alanine. The Journal of endocrinology. 2003;179(3):447–454. doi: 10.1677/joe.0.1790447. [DOI] [PubMed] [Google Scholar]
- Dlaskova A, Spacek T, Santorova J, Plecita-Hlavata L, Berkova Z, Saudek F, Lessard M, Bewersdorf J, Jezek P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochimica et biophysica acta. 2010;1797(6-7):1327–1341. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J, Collins HW, Li C, Stanley CA, Wilson DF, Grimsby J, Sarabu R, Naji A, Matschinsky FM. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2012;302(1):E87–E102. doi: 10.1152/ajpendo.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan TM, Tang MY, Perusse JR, Dashti EA, Aguileta MA, McLelland GL, Gros P, Shaler TA, Faubert D, Coulombe B, Fon EA. USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO J. 2014;33(21):2473–2491. doi: 10.15252/embj.201489729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8(4):325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83(2):254–260. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans SS, Floyd JC, Jr., Knopf RF, Conn FW. Effect of amino acids and proteins on insulin secretion in man. Recent progress in hormone research. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15(15):1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Ford EL, Tran H, Wice BM, Crosby SD, Dorn GW, 2nd, Polonsky KS. Loss of Nix in Pdx1-deficient mice prevents apoptotic and necrotic beta cell death and diabetes. J Clin Invest. 2010;120(11):4031–4039. doi: 10.1172/JCI44011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19(6):2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZY, Li G, Najafi H, Wolf BA, Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes. 1999;48(8):1535–1542. doi: 10.2337/diabetes.48.8.1535. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Brun T, Sarret EJ, Ishihara H, Schaad O, Descombes P, Wollheim CB. Oligonucleotide microarray analysis reveals PDX1 as an essential regulator of mitochondrial metabolism in rat islets. J Biol Chem. 2004;279(30):31121–31130. doi: 10.1074/jbc.M405030200. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Wiederkehr A, Baquie M, Dai C, Powers AC, Kerr-Conte J, Pattou F, MacDonald RJ, Ferrer J, Wollheim CB. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 2009;10(2):110–118. doi: 10.1016/j.cmet.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19(24):4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gillberg L, Jacobsen S, Ribel-Madsen R, Gjesing AP, Boesgaard TW, Ling C, Pedersen O, Hansen T, Vaag A. Does DNA methylation of PPARGC1A influence insulin action in first degree relatives of patients with type 2 diabetes? PloS one. 2013;8(3):e58384. doi: 10.1371/journal.pone.0058384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat. 2003;22(5):353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- Gugneja S, Scarpulla RC. Serine phosphorylation within a concise amino-terminal domain in nuclear respiratory factor 1 enhances DNA binding. J Biol Chem. 1997;272(30):18732–18739. doi: 10.1074/jbc.272.30.18732. [DOI] [PubMed] [Google Scholar]
- Gugneja S, Virbasius JV, Scarpulla RC. Four structurally distinct, non-DNA-binding subunits of human nuclear respiratory factor 2 share a conserved transcriptional activation domain. Mol Cell Biol. 1995;15(1):102–111. doi: 10.1128/mcb.15.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123(8):3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hani EH, Stoffers DA, Chevre JC, Durand E, Stanojevic V, Dina C, Habener JF, Froguel P. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest. 1999;104(9):R41–48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150(11):4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa M, Zhou YT, Ravazzola M, Baetens D, Orci L, Unger RH. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci U S A. 1999;96(20):11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister-Brix A, Kollmann K, Langer S, Schultz J, Lenzen S, Baltrusch S. Identification of the ubiquitin-like domain of midnolin as a new glucokinase interaction partner. J Biol Chem. 2013;288(50):35824–35839. doi: 10.1074/jbc.M113.526632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Ariyoshi M, Okawa Y, Kaimoto S, Uchihashi M, Fukai K, Iwai-Kanai E, Ikeda K, Ueyama T, Ogata T, Matoba S. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic beta-cell function in diabetes. Proc Natl Acad Sci U S A. 2014;111(8):3116–3121. doi: 10.1073/pnas.1318951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BY, Kelly A, Thornton PS, Greenberg CR, Dilling LA, Stanley CA. Protein-sensitive and fasting hypoglycemia in children with the hyperinsulinism/hyperammonemia syndrome. J Pediatr. 2001;138(3):383–389. doi: 10.1067/mpd.2001.111818. [DOI] [PubMed] [Google Scholar]
- Hussain K, Clayton PT, Krywawych S, Chatziandreou I, Mills P, Ginbey DW, Geboers AJ, Berger R, van den Berg IE, Eaton S. Hyperinsulinism of infancy associated with a novel splice site mutation in the SCHAD gene. J Pediatr. 2005;146(5):706–708. doi: 10.1016/j.jpeds.2005.01.032. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278(38):36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Jin HS, Kim J, Lee SJ, Kim K, Go MJ, Lee JY, Lee HJ, Song J, Jeon BT, Roh GS, Kim SJ, Kim BY, Hong KW, Yoo YH, Oh B, Kang Y, Jeong SY. The PARK2 gene is involved in the maintenance of pancreatic beta-cell functions related to insulin production and secretion. Mol Cell Endocrinol. 2014;382(1):178–189. doi: 10.1016/j.mce.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125(Pt 4):795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51(11):3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8(4):318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Kelly A, Ng D, Ferry RJ, Jr., Grimberg A, Koo-McCoy S, Thornton PS, Stanley CA. Acute insulin responses to leucine in children with the hyperinsulinism/hyperammonemia syndrome. J Clin Endocrinol Metab. 2001;86(8):3724–3728. doi: 10.1210/jcem.86.8.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Khoo C, Yang J, Weinrott SA, Kaestner KH, Naji A, Schug J, Stoffers DA. Research resource: the pdx1 cistrome of pancreatic islets. Mol Endocrinol. 2012;26(3):521–533. doi: 10.1210/me.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey RG, Choi CS, Lee HY, Cabrera O, Pongratz RL, Zhao X, Birkenfeld AL, Li C, Berggren PO, Stanley C, Shulman GI. Mitochondrial GTP insensitivity contributes to hypoglycemia in hyperinsulinemia hyperammonemia by inhibiting glucagon release. Diabetes. 2014;63(12):4218–4229. doi: 10.2337/db14-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cheon H, Jeong YT, Quan W, Kim KH, Cho JM, Lim YM, Oh SH, Jin SM, Kim JH, Lee MK, Kim S, Komatsu M, Kang SW, Lee MS. Amyloidogenic peptide oligomer accumulation in autophagy-deficient beta cells induces diabetes. J Clin Invest. 2014;124(8):3311–3324. doi: 10.1172/JCI69625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeck T, Olsson AH, Nitert MD, Sharoyko VV, Ladenvall C, Kotova O, Reiling E, Ronn T, Parikh H, Taneera J, Eriksson JG, Metodiev MD, Larsson NG, Balhuizen A, Luthman H, Stancakova A, Kuusisto J, Laakso M, Poulsen P, Vaag A, Groop L, Lyssenko V, Mulder H, Ling C. A common variant in TFB1M is associated with reduced insulin secretion and increased future risk of type 2 diabetes. Cell Metab. 2011;13(1):80–91. doi: 10.1016/j.cmet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Kolb H, Eizirik DL. Resistance to type 2 diabetes mellitus: a matter of hormesis? Nature reviews. Endocrinology. 2012;8(3):183–192. doi: 10.1038/nrendo.2011.158. [DOI] [PubMed] [Google Scholar]
- Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189(4):671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS. Role of islet beta cell autophagy in the pathogenesis of diabetes. Trends in endocrinology and metabolism: TEM. 2014;25(12):620–627. doi: 10.1016/j.tem.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Lei XG, Vatamaniuk MZ. Two tales of antioxidant enzymes on beta cells and diabetes. Antioxidants & redox signaling. 2011;14(3):489–503. doi: 10.1089/ars.2010.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Palladino A, Narayan S, Russell LK, Sayed S, Xiong G, Chen J, Stokes D, Butt YM, Jones PM, Collins HW, Cohen NA, Cohen AS, Nissim I, Smith TJ, Strauss AW, Matschinsky FM, Bennett MJ, Stanley CA. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285(41):31806–31818. doi: 10.1074/jbc.M110.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li M, Chen P, Narayan S, Matschinsky FM, Bennett MJ, Stanley CA, Smith TJ. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J Biol Chem. 2011a;286(39):34164–34174. doi: 10.1074/jbc.M111.268599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, Zhang T, Daikhin Y, Stokes D, Yudkoff M, Bennett MJ, Stanley CA, Matschinsky FM, Naji A. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by gamma-Hydroxybutyrate and Glycine. J Biol Chem. 2013;288(6):3938–3951. doi: 10.1074/jbc.M112.385682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Matter A, Kelly A, Petty TJ, Najafi H, MacMullen C, Daikhin Y, Nissim I, Lazarow A, Kwagh J, Collins HW, Hsu BY, Yudkoff M, Matschinsky FM, Stanley CA. Effects of a GTP-insensitive mutation of glutamate dehydrogenase on insulin secretion in transgenic mice. J Biol Chem. 2006;281(22):15064–15072. doi: 10.1074/jbc.M600994200. [DOI] [PubMed] [Google Scholar]
- Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem. 2003;278(5):2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- Li M, Li C, Allen A, Stanley CA, Smith TJ. The structure and allosteric regulation of glutamate dehydrogenase. Neurochem Int. 2011b;59(4):445–455. doi: 10.1016/j.neuint.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Stojanovski S, Maechler P. Mitochondrial hormesis in pancreatic beta cells: does uncoupling protein 2 play a role? Oxidative medicine and cellular longevity. 2012a;2012:740849. doi: 10.1155/2012/740849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhu S, Wu X, Zhu X, Li J, Pan L, Xin Z, Niu F, Wu J, Liu Y. Association of polymorphisms in mitofusin-2 gene with type 2 diabetes in Han Chinese. Journal of biomedicine & biotechnology. 2012b;2012:205752. doi: 10.1155/2012/205752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51(4):615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Niu N, Zhu X, Du T, Wang X, Chen D, Wu X, Gu HF, Liu Y. Genetic variation and association analyses of the nuclear respiratory factor 1 (nRF1) gene in Chinese patients with type 2 diabetes. Diabetes. 2008;57(3):777–782. doi: 10.2337/db07-0008. [DOI] [PubMed] [Google Scholar]
- Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59(2):448–459. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Experimental diabetes research. 2012;2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MJ, Langberg EC, Tibell A, Sabat G, Kendrick MA, Szweda LI, Ostenson CG. Identification of ATP synthase as a lipid peroxide protein adduct in pancreatic islets from humans with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(4):E727–731. doi: 10.1210/jc.2012-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan J, Parazzoli S, Oseid E, Hertzel AV, Bernlohr DA, Vallerie SN, Liu CQ, Lopez M, Harmon JS, Robertson RP. Ebselen treatment prevents islet apoptosis, maintains intranuclear Pdx-1 and MafA levels, and preserves beta-cell mass and function in ZDF rats. Diabetes. 2013;62(10):3582–3588. doi: 10.2337/db13-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, Morgan GP, Rhodes CJ. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21(9):2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- Matschinsky F, Liang Y, Kesavan P, Wang L, Froguel P, Velho G, Cohen D, Permutt MA, Tanizawa Y, Jetton TL, et al. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest. 1993;92(5):2092–2098. doi: 10.1172/JCI116809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45(2):223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- Metodiev MD, Lesko N, Park CB, Camara Y, Shi Y, Wibom R, Hultenby K, Gustafsson CM, Larsson NG. Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 2009;9(4):386–397. doi: 10.1016/j.cmet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Michiorri S, Gelmetti V, Giarda E, Lombardi F, Romano F, Marongiu R, Nerini-Molteni S, Sale P, Vago R, Arena G, Torosantucci L, Cassina L, Russo MA, Dallapiccola B, Valente EM, Casari G. The Parkinson-associated protein PINK1 interacts with Beclin1 and promotes autophagy. Cell death and differentiation. 2010;17(6):962–974. doi: 10.1038/cdd.2009.200. [DOI] [PubMed] [Google Scholar]
- Misaka T, Miyashita T, Kubo Y. Primary structure of a dynamin-related mouse mitochondrial GTPase and its distribution in brain, subcellular localization, and effect on mitochondrial morphology. J Biol Chem. 2002;277(18):15834–15842. doi: 10.1074/jbc.M109260200. [DOI] [PubMed] [Google Scholar]
- Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58(10):2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, Njolstad PR, Jellum E, Sovik O. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53(1):221–227. doi: 10.2337/diabetes.53.1.221. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol. 2009;297(1-2):34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Abou-Sleiman PM, Saurin AT, Harvey K, Gandhi S, Deas E, Eaton S, Payne Smith MD, Venner K, Matilla A, Healy DG, Gilks WP, Lees AJ, Holton J, Revesz T, Parker PJ, Harvey RJ, Wood NW, Latchman DS. Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem. 2006;98(1):156–169. doi: 10.1111/j.1471-4159.2006.03845.x. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans. 2007;35(Pt 5):1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- Nicolino M, Claiborn KC, Senee V, Boland A, Stoffers DA, Julier C. A novel hypomorphic PDX1 mutation responsible for permanent neonatal diabetes with subclinical exocrine deficiency. Diabetes. 2010;59(3):733–740. doi: 10.2337/db09-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flanagan CH, Morais VA, Wurst W, De Strooper B, O’Neill C. The Parkinson’s gene PINK1 regulates cell cycle progression and promotes cancer-associated phenotypes. Oncogene. 2014 doi: 10.1038/onc.2014.81. 0. [DOI] [PubMed] [Google Scholar]
- Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P, Demaurex N, Wollheim CB. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283(48):33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010;12(Suppl 2):141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- Poulton J, O’Rahilly S, Morten KJ, Clark A. Mitochondrial DNA, diabetes and pancreatic pathology in Kearns-Sayre syndrome. Diabetologia. 1995;38(7):868–871. doi: 10.1007/s001250050366. [DOI] [PubMed] [Google Scholar]
- Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci U S A. 2002;99(23):14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Sun L, Yang Z, Han R. Variation in the PTEN-induced putative kinase 1 gene associated with the increase risk of type 2 diabetes in northern Chinese. Journal of genetics. 2011;90(1):125–128. doi: 10.1007/s12041-011-0020-y. [DOI] [PubMed] [Google Scholar]
- Quan W, Hur KY, Lim Y, Oh SH, Lee JC, Kim KH, Kim GH, Kim SW, Kim HL, Lee MK, Kim KW, Kim J, Komatsu M, Lee MS. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012;55(2):392–403. doi: 10.1007/s00125-011-2350-y. [DOI] [PubMed] [Google Scholar]
- Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell. 2012;148(4):716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124(8):3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free radical biology & medicine. 2006;41(2):177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhattacharjee A, Prentice KJ, Sereda SB, Shirihai OS, Wheeler MB. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60(11):2710–2719. doi: 10.2337/db11-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG, Stoffers DA. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A. 2009;106(45):19090–19095. doi: 10.1073/pnas.0904849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. The International journal of neuroscience. 1993;69(1-4):125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13(3):251–262. doi: 10.1385/ENDO:13:3:251. [DOI] [PubMed] [Google Scholar]
- Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends in neurosciences. 2014;37(6):315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104(49):19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288(5787):187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- Sharoyko VV, Abels M, Sun J, Nicholas LM, Mollet IG, Stamenkovic JA, Gohring I, Malmgren S, Storm P, Fadista J, Spegel P, Metodiev MD, Larsson NG, Eliasson L, Wierup N, Mulder H. Loss of TFB1M results in mitochondrial dysfunction that leads to impaired insulin secretion and diabetes. Hum Mol Genet. 2014;23(21):5733–5749. doi: 10.1093/hmg/ddu288. [DOI] [PubMed] [Google Scholar]
- Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, Miyatsuka T, Hiddinga HJ, Sakagashira S, Koike M, Uchiyama Y, Yoshimori T, Eberhardt NL, Fujitani Y, Watada H. Human IAPP-induced pancreatic beta cell toxicity and its regulation by autophagy. J Clin Invest. 2014;124(8):3634–3644. doi: 10.1172/JCI69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Kohler M, Graff C, Oldfors A, Magnuson MA, Berggren PO, Larsson NG. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26(3):336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, Spruce LA, Kushner JA, Groop L, Seeholzer SH, Kaufman BA, Hakonarson H, Stoffers DA. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell. 2014;157(7):1577–1590. doi: 10.1016/j.cell.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, Perlman K, Rich BH, Zammarchi E, Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338(19):1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284(39):26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Roden M. ESCI Award 2006. Mitochondrial function and endocrine diseases. European journal of clinical investigation. 2007;37(4):236–248. doi: 10.1111/j.1365-2362.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- Stiles L, Shirihai OS. Mitochondrial dynamics and morphology in beta-cells. Best practice & research. Clinical endocrinology & metabolism. 2012;26(6):725–738. doi: 10.1016/j.beem.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17(2):138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35(5):1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supale S, Thorel F, Merkwirth C, Gjinovci A, Herrera PL, Scorrano L, Meda P, Langer T, Maechler P. Loss of prohibitin induces mitochondrial damages altering beta-cell function and survival and is responsible for gradual diabetes development. Diabetes. 2013;62(10):3488–3499. doi: 10.2337/db13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, Mirmira RG. 12-lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Mol Cell Biol. 2014;34(19):3735–3745. doi: 10.1128/MCB.00157-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn DR. Mitochondrial disorders: prevalence, myths and advances. Journal of inherited metabolic disease. 2004;27(3):349–362. doi: 10.1023/B:BOLI.0000031098.41409.55. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtat B, Riveline JP, Zhang P, Singh-Estivalet A, Armanet M, Venteclef N, Besseiche A, Kelly DP, Tronche F, Ferre P, Gautier JF, Breant B, Blondeau B. Fetal PGC-1alpha overexpression programs adult pancreatic beta-cell dysfunction. Diabetes. 2013;62(4):1206–1216. doi: 10.2337/db12-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1(5):368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harbor perspectives in biology. 2013;5(6) doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Humbeeck C, Cornelissen T, Hofkens H, Mandemakers W, Gevaert K, De Strooper B, Vandenberghe W. Parkin interacts with Ambra1 to induce mitophagy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(28):10249–10261. doi: 10.1523/JNEUROSCI.1917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassy JL, Hivert MF, Porneala B, Dauriz M, Florez JC, Dupuis J, Siscovick DS, Fornage M, Rasmussen-Torvik LJ, Bouchard C, Meigs JB. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes. 2014;63(6):2172–2182. doi: 10.2337/db13-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, 2nd, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988a;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Zheng XX, Lott MT, Shoffner JM, Hodge JA, Kelley RI, Epstein CM, Hopkins LC. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988b;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Silva JP, Gustafsson CM, Rustin P, Larsson NG. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci U S A. 2001;98(7):4038–4043. doi: 10.1073/pnas.061038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RG, Schaefer AM, McFarland R, Taylor RW, Walker M, Turnbull DM. Prevalence and progression of diabetes in mitochondrial disease. Diabetologia. 2007;50(10):2085–2089. doi: 10.1007/s00125-007-0779-9. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147(6):2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Maechler P. Beta-cell mitochondria and insulin secretion: messenger role of nucleotides and metabolites. Diabetes. 2002;51(Suppl 1):S37–42. doi: 10.2337/diabetes.51.2007.s37. [DOI] [PubMed] [Google Scholar]
- Wu X, Yen L, Irwin L, Sweeney C, Carraway KL., 3rd Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol Cell Biol. 2004;24(17):7748–7757. doi: 10.1128/MCB.24.17.7748-7757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamato E, Toyoda S, Tashiro F, Ikegami H, Yodoi J, Miyazaki J. Transgenic expression of antioxidant protein thioredoxin in pancreatic beta cells prevents progression of type 2 diabetes mellitus. Antioxidants & redox signaling. 2008;10(1):43–49. doi: 10.1089/ars.2007.1586. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev Cell. 2003a;5(1):73–83. doi: 10.1016/s1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003b;23(15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent D, Najafi H, Odili S, Buettger C, Weik-Collins H, Li C, Doliba N, Grimsby J, Matschinsky FM. Glucokinase and glucose homeostasis: proven concepts and new ideas. Biochem Soc Trans. 2005;33(Pt 1):306–310. doi: 10.1042/BST0330306. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA, Jr., Lowell BB. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3(6):417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ney PA. NIX induces mitochondrial autophagy in reticulocytes. Autophagy. 2008;4(3):354–356. doi: 10.4161/auto.5552. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, Clerc P, Polster BM, Aja SM, Pletnikov MV, Kensler TW, Shirihai OS, Iijima M, Hussain MA, Sesaki H. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22(13):2235–2245. doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Fu J, Xue P, Zhao R, Dong J, Liu D, Yamamoto M, Tong Q, Teng W, Qu W, Zhang Q, Andersen ME, Pi J. CNC-bZIP protein Nrf1-dependent regulation of glucose-stimulated insulin secretion. Antioxidants & redox signaling. 2015 doi: 10.1089/ars.2014.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Tan Y, Zhou A, Yu Q, Zhou J. RING finger ubiquitin-protein isopeptide ligase Nrdp1/FLRF regulates parkin stability and activity. J Biol Chem. 2005;280(10):9425–9430. doi: 10.1074/jbc.M408955200. [DOI] [PubMed] [Google Scholar]