Summary
While South Asians have high rates of obesity and kidney disease, little is known about the effect of regional body composition on kidney function. We investigated the association between body composition measures and cystatin C-based estimated glomerular filtration rate (eGFRcysC) in 150 immigrant South Asians. The inverse association between overall adiposity and eGFRcysC was attenuated by C-reactive protein (CRP), while the association of ectopic fat was completely attenuated by metabolic covariates and CRP. In immigrant South Asians, the associations between overall adiposity and ectopic fat with decreased kidney function are largely explained by metabolic alterations and inflammation.
Keywords: Body composition, Ectopic fat, Cystatin C, South Asian
Chronic kidney disease (CKD) is of great concern in South Asians (SAs), a population with a high prevalence of diabetes, hypertension and kidney disease progression [1,2]. Obesity is a risk factor for CKD, but less is known about the role of regional body composition [3]. This is of interest in SAs where body mass index (BMI) underestimates visceral fat [4]. SAs are prone to falsely normal values of creatinine (Cr) in early CKD given their lower lean mass [5]. Therefore, cystatin C (cysC) may be useful in improving detection of CKD [6]. The effect of higher levels of adiposity on kidney function in SAs remains unknown. We hypothesized that in immigrant SAs, higher overall and regional adiposity is associated with worse kidney function, measured by cystatin C-based estimated glomerular filtration rate (eGFRcysC).
Using data from the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study, we performed a cross-sectional analysis of 150 immigrant SAs recruited from the San Francisco Bay Area. Sampling strategy, recruitment procedures, and detailed study methods have been previously described [7]. Eligible participants were: ages 45-84, self-identified as Asian Indian, and had no clinical cardiovascular disease.
Details on data collection, clinical diagnoses, and laboratory measurements including glucose, lipids, Cr, and urine microalbumin have been described [7]. GFR was estimated using the CKD-EPI equation (female- Cr ≤ 0.7: 144 × (Cr/0.7)−0.329 × 0.993age; Cr>0.7: 144 × (Cr/0.7) −1.209 × 0.993age; male - Cr≤0.9: 141 × (Cr/0.9) −0.411 × 0.993age; Cr>0.9: 141 × (Cr/0.9) −1209 × 0.993age) [8]. C-reactive protein (CRP) was measured using a particle enhanced immunonepholometric assay (Dade Behring/Siemens). CysC was measured using the N Latex cysC kit (Dade-Behring/Siemens) on a BN II Nephelometer at the University of Vermont. To achieve harmonisation across reagent lots, measured cysC values were adjusted upwards by 17%. The CKD-EPI cysC equation (Cr ≤ 0.8: 133 × (cystatin C/0.8) −0.499 × 0.996age (×0.932 if female); Cr>0.8: 133 × (cystatin C/0.8) −1.328 × 0.996age (×0.932 if female)) was used to calculate eGFRcysC [8]. Details on body composition measurements (BMI, waist circumference, total fat mass, subcutaneous and visceral fat area, and hepatic fat) have been described [7].
Baseline characteristics were compared across tertiles of eGFRcysC using the chi-squared test, ANOVA or Kruskall–Wallis. Based on biologic plausibility, multivariate models were specified to assess the relationship between body composition and eGFRcysC. Using linear regression, we sequentially adjusted for covariates, and STATA (version 13.1, College Station, TX) was used.
CysC measurements were available in 149 participants. Table 1 shows baseline characteristics of participants by tertile of eGFRcysC. Age, systolic blood pressure, diabetes prevalence, CRP and BMI were inversely associated with eGFRcysC. Cr-based eGFR was associated with eGFRcysC.
Table 1.
Baseline characteristicsa of MASALA study participants by tertile of cystatin C-based estimated GFR, 2006–2007.
| Characteristic | Tertile of cystatin C-based estimated GFR | |||
|---|---|---|---|---|
|
|
||||
| Tertile 1, n = 50 (41.65–87.97 ml/min/1.73m2) | Tertile 2, n = 50 (88.34–101.14ml/min/1.73m2) | Tertile 3, n = 49 (101.53–131.43 ml/min/1.73m2) | P-value | |
| Age (years) | 62 ± 9 | 57 ± 7 | 53 ± 6 | <0.001 |
| Male sex | 26 (52) | 23 (46) | 26 (53) | 0.75 |
| Alcohol (≥1 drink/week) | 19 (38) | 27 (54) | 28 (57) | 0.12 |
| Ever smoker | 11 (22) | 6 (12) | 8 (16) | 0.41 |
| Physical activity (METS/week) | 1339 (525, 2760) | 1501 (683, 3150) | 1155 (683, 2250) | 0.23 |
| Systolic blood pressure (mmHg) | 129 ± 16 | 124 ± 17 | 120 ± 17 | 0.04 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 73 ± 12 | 73 ± 12 | 0.88 |
| Hypertension | 25 (50) | 22 (44) | 16 (33) | 0.21 |
| Fasting glucose (mg/dL) | 97.6 (87.1, 115) | 94 (86.4, 102) | 92.5 (86.4, 111) | 0.81 |
| 2-h post-challenge glucose (mg/dL) | 163 (137, 227) | 133 (104, 174) | 140 (108, 204) | 0.03 |
| Diabetes | 14 (29) | 11 (22) | 15 (31) | 0.05 |
| Total cholesterol (mg/dL) | 184 ± 38 | 194 ± 34 | 187 ± 28 | 0.30 |
| LDL-cholesterol (mg/dL) | 108 ± 33 | 117 ± 31 | 112 ± 25 | 0.27 |
| HDL-cholesterol (mg/dL) | 48 ± 13 | 50 ± 14 | 49 ± 14 | 0.72 |
| Triglycerides (mg/dL) | 122 (87, 178) | 121 (89, 159) | 109 (91, 156) | 0.78 |
| C-reactive protein (μg/ml) | 1.90 (0.87, 4.45) | 1.24 (0.75, 2.6) | 1.04 (0.47, 1.61) | 0.005 |
| Measures of renal function | ||||
| Creatinine–estimated GFR (ml/min/1.73m2) | 75 ± 14 | 79 ± 12 | 93 ± 15 | <0.001 |
| Spot urine albumin to Cr ratio (μg/mg) | 8.0 (4.6, 17.4) | 6.3 (4, 9.9) | 5.1 (4.0, 8.6) | 0.07 |
| Body composition | ||||
| Body mass index (kg/m2) | 27.6 ± 5.1 | 25.4 ± 5.1 | 25.5 ± 3.2 | 0.03 |
| Waist circumference (cm) | 99.0 ± 13.5 | 95.4 ± 13.4 | 94.3 ± 9.7 | 0.14 |
| Waist-hip ratio | 0.95 ± 0.07 | 0.94 ± 0.07 | 0.95 ± 0.07 | 0.62 |
| Total fat (kg) | 26.6 ± 9.5 | 24.3 ± 8.7 | 22.9 ± 5.0 | 0.07 |
| Subcutaneous fat area (cm2) | 249 (202, 353) | 233 (170, 290) | 220 (172, 262) | 0.11 |
| Visceral fat area (cm2) | 146 ± 63 | 124 ± 57 | 131 ± 46 | 0.14 |
| Liver-minus-spleen (HU) | 11 (5.5, 16.5) | 13 (5, 17.8) | 13.5 (6, 19) | 0.57 |
| Liver-to-spleen attenuation ratio | 1.21 ± 0.21 | 1.21 ± 0.27 | 1.24 ± 0.22 | 0.73 |
| Fatty liver | 7 (15) | 11 (23) | 6 (14) | 0.49 |
Values represent n(%) for chi-square analyses, mean ± SD for analysis of variance (ANOVA) and median (25th percentile, 75th percentile) for Kruskall–Wallis test. P-values resulted using the chi-square test, ANOVA or Kruskall–Wallis test as appropriate.
Next, we conducted multivariate analyses with stepwise adjustments to investigate the association between body composition and eGFRcysC (Fig. 1). Inverse associations of BMI and subcutaneous fat with eGFRcysC were attenuated by CRP. Associations of waist circumference, visceral fat, and hepatic fat with decreased kidney function were attenuated by metabolic covariates and CRP.
Figure 1.
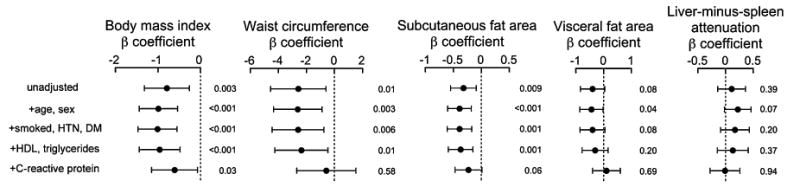
Forest plot of sequentially adjusted associations between body composition measures and cystatin C-based eGFR. Model 1 is unadjusted; model 2 is adjusted for age and sex; model 3 is adjusted for age, sex, history of ever smoking, hypertension and diabetes; model 4 is adjusted for age, sex, history of ever smoking, hypertension, diabetes, HDL and log triglycerides; and model 5 is adjusted for age, sex, history of ever smoking, hypertension, diabetes, HDL, log triglycerides and log CRP. The β-coefficient and 95% confidence interval for sequentially adjusted associations between body composition measures and cystatin C-based eGFR are represented here as a forest plot.
These findings add to the literature on adiposity and cystatin C. The Framingham Offspring Study found that visceral and subcutaneous fat are independently associated with cysC, while the Cardiovascular Health Study did not find such an association [3,9]. Furthermore, cysC is secreted by adipose tissue and is elevated in obese individuals, including SAs, independent of eGFR [10,11]. Therefore, the adiposity and cysC story is complex.
The roles of various body composition measures in kidney disease remain unclear. Visceral fat is metabolically active and therefore distinct from subcutaneous fat. Metabolic dysregulation and inflammation play a role in kidney disease [3]. One study found that in Caucasians and African Americans, the association between overall adiposity and central obesity with eGFR was attenuated by hypertension, diabetes, and CRP [3]. Studies that did not adjust for inflammation found a significant association between visceral and subcutaneous fat and kidney function [9,12]. Our data are consistent with these findings. However, findings from another study suggest that subcutaneous fat does not contribute to the inflammation [13]. And a study in SAs found a significant association between central obesity and albuminuria, even after adjusting for CRP [14].
This is the first study in immigrant SAs to investigate the association of several ectopic fat measures with kidney function using eGFRcysC. However, our small sample size and relatively homogeneous population limit the generalizability of this study. In conclusion, the association between overall and visceral adiposity with kidney function is largely mediated by metabolic dysregulation and inflammation in immigrant SAs. Ongoing larger prospective cohort studies will better evaluate the effect of body composition on kidney function in SAs compared to other ethnicities [15].
Acknowledgments
Sources of support: The MASALA study was supported by the NIH [grant no. K23 HL080026-01] and by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Dr. Shah was supported by the T32 Training Grant Number 5T32DK007418 and the Wilsey Family Foundation. Dr. Kanaya was supported by K24HL112827 and RO1HL093009.
Abbreviations
- MASALA
Metabolic Syndrome and Atherosclerosis in South Asians Living in America
- eGFR
estimated glomerular filtration rate
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- Cr
creatinine
- HU
Hounsfield unit
- cysC
cystatin C
- CRP
C-reactive protein
- CKD
chronic kidney disease
- SAs
South Asians
- BMI
body mass index
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
Contributor Information
Arti D. Shah, Email: Arti.Shah@ucsf.edu, Division of Endocrinology and Metabolism, University of California, San Francisco, United States.
Heidi Schmidt, Division of General Internal Medicine, Department of Medicine, University of California, San Francisco, United States.
Saunak Sen, Department of Epidemiology and Biostatistics, University of California, San Francisco, United States.
Michael G. Shlipak, Department of Epidemiology and Biostatistics, University of California, San Francisco, United States Division of General Internal Medicine, San Francisco Veterans Affairs Medical Center, United States.
AlkaM. Kanaya, Division of General Internal Medicine, Department of Medicine, University of California, San Francisco, United States Department of Epidemiology and Biostatistics, University of California, San Francisco, United States.
References
- 1.Agarwal SK, Srivastava RK. Chronic kidney disease in India: challenges and solutions. Nephron Clin Pract. 2009;111(3):c197, 203. doi: 10.1159/000199460. discussion c203. [DOI] [PubMed] [Google Scholar]
- 2.Dreyer G, Hull S, Mathur R, Chesser A, Yaqoob MM. Progression of chronic kidney disease in a multi-ethnic community cohort of patients with diabetes mellitus. Diabet Med. 2013 Aug;30(8):956–63. doi: 10.1111/dme.12197. [DOI] [PubMed] [Google Scholar]
- 3.de Boer IH, Katz R, Fried LF, Ix JH, Luchsinger J, Sarnak MJ, et al. Obesity and change in estimated GFR among older adults. Am J Kidney Dis. 2009 Dec;54(6):1043–51. doi: 10.1053/j.ajkd.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007 Aug;86(2):353–9. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 5.Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, Mente A, et al. Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: The Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE) PLoS ONE. 2011;6(7):e22112. doi: 10.1371/journal.pone.0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teo BW, Sabanayagam C, Liao J, Toh QC, Saw S, Wong TY, et al. Comparison of CKD-EPI cystatin C and creatinine glomerular filtration rate estimation equations in Asian Indians. Int J Nephrol. 2014;2014:746497. doi: 10.1155/2014/746497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaya AM, Wassel CL, Mathur D, Stewart A, Herrington D, Budoff MJ, et al. Prevalence and correlates of diabetes in South Asian Indians in the United States: findings from the Metabolic Syndrome and Atherosclerosis in South Asians Living in America study and the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2010 Apr;8(2):157–64. doi: 10.1089/met.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012 Jul;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JA, Hwang SJ, Sarnak MJ, Hoffmann U, Massaro JM, Levy D, et al. Association of visceral and subcutaneous adiposity with kidney function. Clin J Am Soc Nephrol. 2008 Nov;3(6):1786–91. doi: 10.2215/CJN.02490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naour N, Fellahi S, Renucci JF, Poitou C, Rouault C, Bas-devant A, et al. Obesity. 12. Vol. 17. Silver Spring; 2009. Dec, Potential contribution of adipose tissue to elevated serum cystatin C in human obesity; pp. 2121–6. [DOI] [PubMed] [Google Scholar]
- 11.Sabanayagam C, Wong TY, Liao J, Sethi S, Teo BW. Body mass index and preclinical kidney disease in Indian adults aged 40 years and above without chronic kidney disease. Clin Exp Nephrol. 2014 doi: 10.1007/s10157-014-0945-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Kim SR, Yoo JH, Song HC, Lee SS, Yoo SJ, Kim YD, et al. Relationship of visceral and subcutaneous adiposity with renal function in people with type 2 diabetes mellitus. Nephrol Dial Transplant. 2011 Nov;26(11):3550–5. doi: 10.1093/ndt/gfq634. [DOI] [PubMed] [Google Scholar]
- 13.Spoto B, Leonardis D, Parlongo RM, Pizzini P, Pisano A, Cutrupi S, et al. Plasma cytokines, glomerular filtration rate and adipose tissue cytokines gene expression in chronic kidney disease (CKD) patients. Nutr Metab Cardiovasc Dis. 2012 Nov;22(11):981–8. doi: 10.1016/j.numecd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Chandie Shaw PK, Berger SP, Mallat M, Frolich M, Dekker FW, Rabelink TJ. Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care. 2007 Jul;30(7):1840–4. doi: 10.2337/dc07-0028. [DOI] [PubMed] [Google Scholar]
- 15.Kanaya AM, Kandula N, Herrington D, Budoff MJ, Hulley S, Vittinghoff E, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol. 2013 Dec;36(12):713–20. doi: 10.1002/clc.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]


