Abstract
Accelerated cell aging, indexed in peripheral leukocytes by telomere length and in peripheral blood mononuclear cells (PBMCs) by telomerase activity, has been reported in several studies of major depressive disorder (MDD). However, the relevance of these peripheral measures for brain indices that are presumably more directly related to MDD pathophysiology is unknown. In this study, we explored the relationship between PBMC telomerase activity and leukocyte telomere length and magnetic resonance imaging-estimated hippocampal volume in un-medicated depressed individuals and healthy controls. We predicted that, to the extent peripheral and central telomerase activity are directly related, PBMC telomerase activity would be positively correlated with hippocampal volume, perhaps due to hippocampal telomerase-associated neurogenesis, neuroprotection or neurotrophic facilitation, and that this effect would be clearer in individuals with increased PBMC telomerase activity, as previously reported in un-medicated MDD. We did not have specific hypotheses regarding the relationship between leukocyte telomere length and hippocampal volume, due to conflicting reports in the published literature. We found, in 25 un-medicated MDD subjects, that PBMC telomerase activity was significantly positively correlated with hippocampal volume; this relationship was not observed in 18 healthy controls. Leukocyte telomere length was not significantly related to hippocampal volume in either group (19 unmedicated MDD subjects and 17 healthy controls). Although the nature of the relationship between peripheral telomerase activity and telomere length and the hippocampus is unclear, these preliminary data are consistent with the possibility that PBMC telomerase activity indexes, and may provide a novel window into, hippocampal neuroprotection and/or neurogenesis in MDD.
Keywords: Telomeres, Telomerase, Hippocampus, Leukocytes, Brain, Depression
1. Introduction
Telomeres are DNA-protein complexes that cap the ends of linear chromosomal DNA, protecting the genome from damage. When telomeres critically shorten, as can happen following repeated mitoses or exposure to oxidative stress or inflammation, cells become susceptible to senescence, apoptosis and chromosomal instability (Calado and Young, 2009; Sahin et al., 2011; Wolkowitz et al., 2011b; Price et al., 2013). However, telomerase, a ribonucleoprotein enzyme, can rebuild and restore telomere length, preventing or delaying cell senescence (Blackburn, 1999; Calado and Young, 2009). While undetectable in most normal somatic cells, telomerase remains active in certain replicating tissues, such as male germ cells and activated lymphocytes and in stem cells, including neural stem cells residing in the dentate gyrus of the hippocampus (HC) (Mattson and Klapper, 2001; Fu et al., 2002b; Cheng et al., 2007; Zhang et al., 2007; Wolkowitz et al., 2008; Jaskelioff et al., 2011; Zhou et al., 2011). Apart from its telomere-lengthening function, telomerase (or its catalytic enzyme component, telomerase reverse transcriptase; TERT) has certain non-canonical functions that are unrelated to telomere lengthening, including (in animal models) neurotrophic, cell survival-enhancing and “antidepressant-like” properties (Zhu et al., 2000; Mattson et al., 2001; Fu et al., 2002a; Fu et al., 2002b; Kang et al., 2004; Jaskelioff et al., 2011; Li et al., 2011; Niu and Yip, 2011; Zhou et al., 2011; Liu et al., 2012).
Several studies have now examined leukocyte telomere length and peripheral blood mononuclear cell (PBMC) telomerase activity in major depressive disorder (MDD). Most, but not all, have reported shortened leukocyte telomere length in MDD, at least in subsets of depressed individuals, such as those with more chronic or severe depression (Simon et al., 2006; Lung et al., 2007; Hartmann et al., 2010; Elvsashagen et al., 2011; Hoen et al., 2011; Wolkowitz et al., 2011a; Wikgren et al., 2012b; Verhoeven et al., 2014). In the only study to examine telomerase activity in MDD, we previously reported significantly elevated PBMC telomerase activity in un-medicated subjects with MDD compared to controls (Wolkowitz et al., 2011b; Wolkowitz et al., 2012), and we speculated this might represent a compensatory response to actual or incipient cell damage or telomere shortening, as also suggested in studies of increased PBMC telomerase activity in depressed caregivers (Damjanovic et al., 2007) as well as in men with high hostility ratings (Brydon et al., 2012) and in men with elevated levels of allostatic load and poor psychosocial resources (Zalli et al., 2014). These suggestions of a compensatory mechanism are consistent with preclinical data showing that telomerase activity is up-regulated in certain situations of cell or tissue damage (Fu et al., 2002a; Kang et al., 2004; Li et al., 2011; Qu et al., 2011; Zhao et al., 2012), increased glucocorticoid levels (Beery et al., 2012) (but see (Choi et al., 2008)), and oxidative stress (Saretzki, 2009; Maeda et al., 2013) and inflammation (Balaji et al., 2010; Gizard et al., 2011; Rentoukas et al., 2012). A salutary role of telomerase activation in MDD is also suggested by preliminary data that depressed subjects who responded best to antidepressant treatment were those who showed the greatest increase in PBMC telomerase activity during treatment (Wolkowitz et al., 2011b; Wolkowitz et al., 2012). These findings are consistent with preclinical findings that the selective serotonin reuptake inhibitor (SSRI) fluoxetine, increases HC telomerase in tandem with reducing “depression-like” behaviors in mice (Zhou et al., 2011). However, the relationship of PBMC telomerase activity to HC telomerase activity, neurogenesis or neurotrophic activity is unknown.
A major limitation in interpreting telomere length and telomerase activity studies in MDD and other mental illnesses is that the relationship of these peripheral cell aging markers to corresponding central markers is unknown (Eitan et al., 2014). In this study, we hypothesized that, to the extent PBMC telomerase activity indexes HC telomerase activity, it should be positively correlated with HC volume, perhaps due to the neurotrophic or neurogenesis-enhancing effects demonstrated in animals. We further hypothesized that positive correlations between PBMC telomerase activity and HC volume would be clearest in individuals with increased PBMC telomerase activity, as previously reported in un-medicated individuals with MDD (Wolkowitz et al., 2012). We did not have specific hypotheses regarding the relationship between leukocyte telomere length and HC volume, due to conflicting reports in the published literature, with some reports of a positive (Grodstein et al., 2008; Jacobs et al., 2014; King et al., 2014) and one report of a negative relationship (Wikgren et al., 2012a) between these measures in non-psychiatric populations, at least in ApoE ε4 non-carriers (Jacobs et al., 2014; Wikgren et al., 2012a).
2. Methods
2.1. Subjects
Participants comprised 25 MDD subjects and 18 healthy controls who completed brain magnetic resonance imaging (MRI) scanning and venipuncture. All of these subjects had MRI and PBMC telomerase activity data available for analysis, but only 19 MDD and 17 healthy control subjects had leukocyte telomere length data available. Data on peripheral blood mononuclear cell telomerase activity and leukocyte telomere length, but not HC volume, were previously reported on these MDD subjects and healthy controls (Wolkowitz et al., 2011a; Wolkowitz et al., 2012). All subjects gave informed consent and were compensated for their participation. The protocol and consent form were approved by the University of California, San Francisco, Committee on Human Research. Depressed subjects were all outpatients; they and the controls were recruited by fliers, craigslist postings, newspaper advertisements and, in the case of depressed subjects, clinical referrals. For the depressed group, the MDD diagnosis was arrived at by the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 2002), which was clinically verified by a clinical interview with a Board-certified psychiatrist (O.M.W.), and all MDD subjects had a minimum Hamilton Depression Rating Scale (17-item version, HDRS (Hamilton, 1967)) score of 17. Depressed subjects with psychosis or bipolar histories were excluded, although comorbid anxiety disorders were allowed when the depressive diagnosis was considered to be primary, with the exception of current or recent post-traumatic stress disorder, which was exclusionary. Healthy controls were required to have no present or past history of any DSM-IV Axis I diagnosis, also ascertained by the SCID. Potential subjects were also excluded if they met SCID criteria for alcohol or substance abuse within 6 months of entering the study. Subjects in both groups were medically healthy (as verified by medical review of systems, physical examination and routine screening laboratory tests [e.g., combined blood count, electrolytes, renal and liver function tests and thyroid function tests]) and had not had any vaccinations within 6 weeks of entering the study. All subjects (MDD and control) were free of psychotropic medications, including antidepressants, antipsychotics and mood stabilizers, as well as hormone supplements, steroid-containing birth control or other interfering medications (e.g., statins) or vitamin supplements above the United States Recommended Daily Allowances, for a minimum of 6 weeks before entry into the study (with the exception of short-acting sedative-hypnotics, as needed, up to a maximum of three times per week, but none within 1 week of testing). Clinical history and ratings, MRI analyses and telomerase activity and TL assays were performed blind to each other.
2.2. Procedures
On the day of testing, subjects were admitted as outpatients to the University of California, San Francisco, Clinical and Translational Science Institute at 0800 h, having fasted (except water) since 2200 h the night before. On the morning of testing, all subjects were required to test negative on a urine toxicology screen (measuring the presence of drugs of abuse) and, in women of childbearing capacity, a urine pregnancy test. Then, subjects were seated or reclined at rest before blood collection, after which the HDRS was administered. For leukocyte telomere length determination, high molecular weight DNA was extracted from frozen whole blood, and the telomere length measurement was adapted from the published original method of Cawthon (Cawthon, 2002) by quantitative polymerase chain reaction, as described earlier (Wolkowitz et al., 2011a). The inter-assay coefficient of variation (CV) for telomere length measurement was 4%. Peripheral blood mononuclear cells were obtained from fresh whole blood by Ficoll separation, as described previously (Wolkowitz et al., 2012), and telomerase activity was assayed with the commercially available kit, TRAPeze (Chemicon, USA), also as described previously (Wolkowitz et al., 2012). Measurement of 24 resting PBMC samples on different days produced an inter-assay coefficient of variation (CV) of 6.8%. Samples for leukocyte telomere length and PBMC telomerase activity were assayed in two batches, each containing samples of both depressed and control subjects. To mitigate possible differences between assay batches, telomere length and telomerase activity data from each assay batch were converted to Z-scores, and the resulting Z-scores were combined across assay batches.
Subjects also underwent 4-Tesla MR brain imaging at the San Francisco Veterans Administration Medical Center. Brain imaging occurred an average of 3.0 days (SD= 12.8 days) before or after the venipuncture; the latency between blood draw and MRI did not differ between the MDD and control groups (t=0.01, p=0.99). Hippocampal volume was determined by FreeSurfer with manual correction, as described previously (Fischl et al., 2004). Hippocampal volume was normed to total intracranial volume (ICV) on T2 images.
2.3. MRI acquisition
All imaging was performed on a Bruker MedSpec 4 T system. The following sequences were acquired. For the measurement of total hippocampal volume, a volumetric T1-weighted gradient echo MRI (MPRAGE) (TR/TE/TI = 2,300/3/950 ms, 7° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution, and acquisition time, 5:17 min). For the determination of the intracranial volume (ICV), a T2-weighted turbospin echo sequence (TR/ TE 8,390/70 ms, 150° flip angle, 0.9 × 0.9 × 3-mm nominal resolution, 54 slices, and acquisition time 3:06 min).
2.4. Hippocampus volumetry
The volume of the total HC determined from the T1 image using the hippocampal masks provided by the FreeSurfer subcortical parcellation routine (Fischl et al., 2002). All maps were visually checked for accuracy by different, specially trained raters who were blinded to the diagnosis and were manually corrected by overlaying the label generated in FreeSurfer onto the T1 image. This procedure generated a map of comparable accuracy as obtained by a manual marking scheme (ICC for manual correction of the Freesurfer labels: 0.9). The volumes from the left and right hemisphere were combined, that is, added to provide a single measure. Intra-cranial volume was determined from the T2-weighted image, which was skull-stripped using the BET program (FMRIB Image Analysis Group, Oxford University, www.fmrib.ox.ac.uk/fsl). To correct for volume differences due to different head sizes, all volumes were normalized to the ICV using the following formula: normalized volume = raw volume × 1,000 ccm/ICV ccm. Accordingly, normed HC volume was our a priori measure of HC volume for statistical analysis, but we also report non-normed (raw) HC volume as well as ICV.
2.5. Statistics
Telomere length, telomerase activity and HC volume data were first analyzed for normality, and non-normally distributed variables were Ln-transformed into normality. Next, bivariate correlations were conducted between the variables of interest and age, sex, body-mass index (BMI), lifetime and current tobacco use and alcohol use, and the variables with significant bivariate correlations were entered as covariates in the ensuing analyses. Accordingly, age and sex were entered as covariates in all analyses. Alpha was set at 0.05 for two-tailed tests. Our planned analyses were Analyses of Variance for between-group comparisons and partial correlations for PBMC telomerase activity or leukocyte telomere length vs. normed HC volume, controlling for age and sex.
In exploratory analyses, we assessed whether controlling for plasma interleukin-6 (IL-6, a marker of inflammation), overnight urinary free cortisol and plasma F2-isoprostanes (a marker of oxidative stress) altered our findings, since these could conceivably affect both peripheral cell aging markers and HC volume. In a final exploratory analysis, we examined whether our results differed in males vs. females.
3. Results
There were no significant differences in demographics between the MDD subjects and controls (Table 1). Normed hippocampal volume did not significantly differ in the MDD subjects vs. the controls (F=0.00, NS) (Table 1), nor did leukocyte telomere length (F=0.48, NS), as reported previously (Wolkowitz et al., 2011a). There were also no significant differences in raw HC volume or ICV between groups (F=0.34, NS, and F=0.87, NS, respectively). However, PBMC telomerase activity was significantly higher in the MDD subjects than in the controls (F=5.26, p=0.025) (Table 1), as reported previously (Wolkowitz et al., 2012).
Table 1.
Demographics, hippocampal volume and telomerase activity and telomere length
| Controls Telomerase cohort |
MDD Telomerase cohort |
Statistical test Telomerase cohort |
Controls Telomere cohort |
MDD Telomere cohort |
Statistical test Telomere cohort |
|
|---|---|---|---|---|---|---|
| Number | 18 | 25 | -- | 17 | 19 | -- |
| Sex | 61% Female | 68% Female | t=0.46, NS | 59% female | 63% female | t=0.26, NS |
| Age (years) | 34.9 ± 9.6 | 37.8 ± 12.0 | t=0.84, NS | 37.9 ± 12.3 | 37.5 ± 11.9 | t=0.12, NS |
| Body mass index (kg/m)2 |
24.8 ± 4.0 | 25.0 ± 4.4 | t=0.21, NS | 24.9 ± 4.0 | 24.6 ± 4.1 | t=0.21, NS |
| Hamilton Depression Rating Scale-17 item |
N/A | 19.2 ± 0.6 (range: 17- 26) |
N/A | N/A | 19.4 ± 0.7 (range: 17-26) |
N/A |
| Raw hippocampal volume |
7.30 ± 0.97 | 7.50 ± 1.13 | F=0.34*, NS | 0.24 ± 0.98 | 7.38 ± 1.15 | F=0.52*, NS |
| Intracranial volume (ICV) |
1585.7 ± 197.6 | 1624.9 ± 128.3 | F=0.87*, NS | 1624.4 ± 190.2 | 1627.6 ± 108.8 | F=0.79*, NS |
| Total hippocampal volume, Normed to Intra-cranial volume (ICV) |
4.64 ± 0.60 | 4.60 ± 0.71 | F=0.00*, NS | 4.48 ± 0.55 | 4.52 ± 0.79 | F=0.02*, NS |
| PBMC Telomerase activity (units/10,000 cells); Z-scores |
Raw value: 7.03 ± 0.60 Z-score: −0.250 ± 1.04 |
Raw value: 7.83 ± 5.90 Z-score: 0.242 ± 0.897 |
Z-score: F=5.26*, p=0.025 |
-- | -- | -- |
| Leukocyte telomere length (base pairs); Z- scores |
-- | -- | -- | Raw value (T/S): 0.851 ± 0.090 Z-score: 0.062 ± 0.749 |
Raw value (T/S): 0.881 ± 0.175 Z-score: 0.006 ± 1.149 |
Z-score: F=0.48*, NS |
Analysis of variance, controlling for age and sex.
Values are means ± SD.
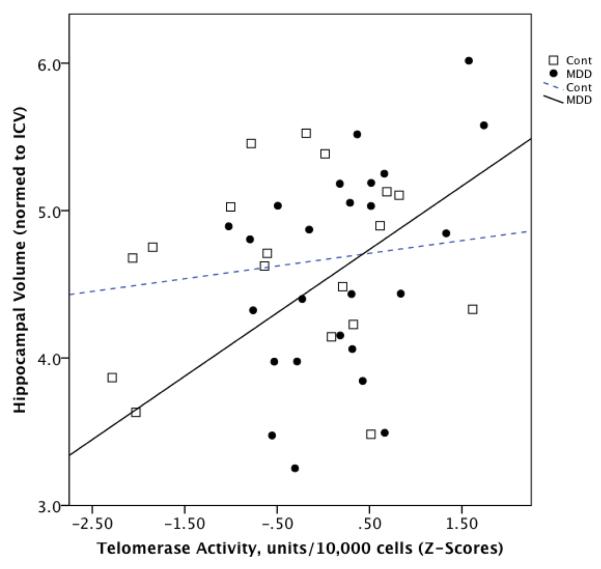
In the combined group of subjects, PBMC telomerase activity tended to be positively correlated with normed HC volume (r=0.27, p=0.081) (Fig. 1). Within the MDD group, PBMC telomerase activity was significantly positively correlated with normed HC volume (r=0.44, p=0.034) (Fig. 1), but this relationship was not seen in the controls (r=0.12, NS) (Fig. 1). Controlling for the number of days between venipuncture and MR imaging did not alter these results.
Fig. 1.
Relationship between peripheral blood mononuclear cell (PBMC) telomerase activity (Z-scores) and total hippocampal volume (normed to intracranial volume) in individuals with major depressive disorder (“MDD”) (black circles, solid line) and healthy controls (“Cont”) (open squares, dotted line). Statistical results are provided in the text.
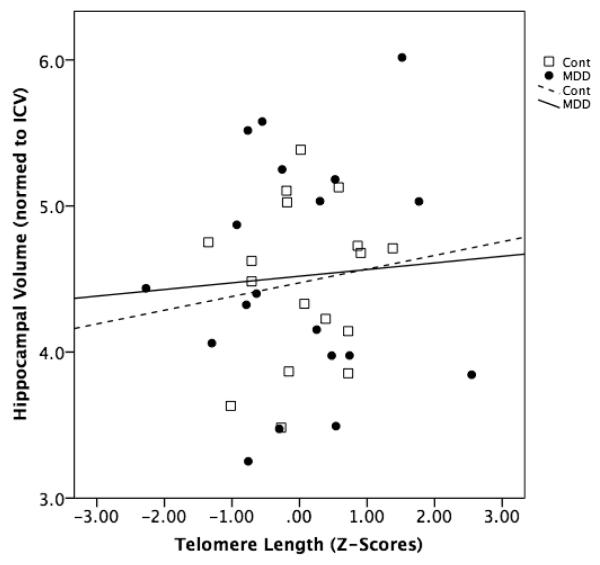
In the combined group of subjects, leukocyte telomere length was not significantly correlated with normed HC volume (r=0.02, NS) (Fig. 2). Within the individual MDD and control groups, leukocyte telomere length was also not significantly correlated with normed HC volume (r=0.05, NS, and r=0.35, p=0.15), respectively (Fig. 2). Controlling for the number of days between venipuncture and MR imaging did not alter these results.
Fig. 2.
Relationship between leukocyte telomere length (Z-scores) and total hippocampal volume (normed to intracranial volume) in individuals with major depressive disorder (“MDD”) (black circles, solid line) and healthy controls (“Cont”) (open squares, dotted line). Statistical results are provided in the text.
In exploratory analyses, we controlled for variables that could potentially affect cell aging markers and as well as HC volume. Controlling for plasma IL-6, overnight urinary free cortisol and plasma F2-isoprostanes, singly as well as together, did not appreciably affect our results and did not alter any of the reported significance tests.
4. Discussion
We found that PBMC telomerase activity is significantly and positively correlated with HC volume in unmedicated individuals with MDD, but not in healthy controls. On the contrary, we found no significant relationship between leukocyte telomere length and HC volume in either group. To our knowledge, this is the first study to examine either of these relationships in MDD. These findings add to a growing body of evidence that aspects of the telomere/ telomerase maintenance system are different in MDD vs. controls (Simon et al., 2006; Lung et al., 2007; Hartmann et al., 2010; Hoen et al., 2011; Elvsashagen et al., 2011; Wolkowitz et al., 2011a; Wikgren et al., 2012b; Wolkowitz et al., 2012; Verhoeven et al., 2014), and that PBMC telomerase activity is related to brain variables relevant to MDD (Honig et al., 2006; Martin-Ruiz et al., 2006; Canela et al., 2007; Grodstein et al., 2008; Mather et al., 2010; Valdes et al., 2010; Devore et al., 2011; Yaffe et al., 2011; Der et al., 2012; Wikgren et al., 2012a; Ma et al., 2013; Jacobs et al., 2014). The explanation of these relationships, however, is not clear, since the relationship between peripheral cell aging markers and HC telomerase activity (which could potentially increase HC volume, as described below) is not known.
We are not aware of any earlier studies directly comparing telomerase activity in peripheral blood to that in the brain, although several studies have assessed relationships between peripheral telomerase activity and aspects of brain structure or function. For example, PBMC telomerase activity was positively correlated with white and gray matter volume in the right dorsolateral prefrontal cortex (DLPFC) on MRI scanning in abstinent heroin users (Cheng et al., 2013). Healthy controls in the same study, however, showed no significant correlations. The authors suggested that, in the abstinent heroin users but not in healthy controls, “accelerated aging” was directly linked at the cellular and brain systems levels (Cheng et al., 2013). Although that study’s psychiatric population was different than our own, the results are similar in that healthy controls failed to show the telomerase activity- brain structure relationships that were seen in the psychiatric subjects.
A recent clinical study reported a significant positive correlation between leukocyte telomere length and HC volume in healthy control females, but only in those who where non-carriers of the ApoE ε4 genotype. They found no significant relationship between PBMC telomerase activity and HC volume in their subjects, regardless of ApoE e4 genotype, but they did find (in the ApoE ε4 non-carrier women) a significant negative correlation between HC volume and the PBMC telomerase activity/ leukocyte telomere length ratio, which they suggested is a more sensitive index of the telomerase compensatory response to neural damage (Jacobs et al., 2014). Their data contrast with ours, in which we found no significant correlations between telomere length, telomerase activity and HC volume in healthy controls, although our subjects were generally younger (mean age of 35-38 years vs. 58 years), were of men and women, and were not characterized by ApoE ε4 genotype. Most importantly, that study specifically excluded subjects with MDD (Jacobs et al., 2014). The only other studies, to our knowledge, that have examined the relationship between leukocyte telomere length and HC volume yielded conflicting results. King et al. (King et al., 2014), in a population-based study, reported a significant positive correlation between leukocyte telomere length and HC volume (regardless of ApoE ε4 genotype); this positive relationship was significantly greater in older individuals (over the age of 50) than in younger individuals. Another study similarly found a positive correlation between leukocyte telomere length and HC volume in an elderly (mean age 80 years) mixed population composed of non-demented women (Grodstein et al., 2008). In contrast, Wikgren et al., in a group of cognitively intact subjects (mean age 65.8 years), reported a significant negative correlation between leukocyte telomere length and HC volume in ApoE ε4 non-carrier men and women (and no relationship among ApoE ε4 carriers) (Wikgren et al., 2012a).
In the absence of adequate human or animal studies, it is difficult to fully explain possible relationships between peripheral central cell aging markers and HC volume. There are at least two mechanisms by which PBMC telomerase activity could bear a mechanistic relationship to HC volume. It is unlikely that PBMC telomerase activity directly affects the HC (or vice versa). Nonetheless, it is possible that PBMC telomerase activity directly indexes or parallels HC telomerase activity. For example, PBMC telomerase activity and HC volume could be jointly, but independently, regulated by similar mediators, such as cortisol levels, inflammation and oxidative stress (Wolkowitz et al., 2008; Zhou et al., 2011). Further, since senescent leukocytes (e.g., CD8+CD28- T cells) with shortened telomeres and diminished telomerase activity hyper-secrete pro-inflammatory cytokines (Effros, 1997), such inflammatory mediators might, themselves, adversely impact HC volume (Arisi, 2014). Our analyses of covariance, controlling for inflammatory cytokines as well as for cortisol and oxidative stress, did not support these as major mediators, but our analyses were limited by our small sample size and by the fact that only selected inflammatory, steroid and oxidative stress markers were assessed.
To the extent PBMC telomerase activity does index HC telomerase activity in MDD, the positive correlation could be a “window” into HC neurogenesis-enhancing, gliogenesis-enhancing or neuroprotective effects of telomerase in the HC (Eitan et al., 2014). Telomerase (or TERT) has several non-canonical functions apart from telomere lengthening that aid in cellular protection and survival, such as anti-apoptosis, anti-oxidant, anti-excitotoxicity, neurotrophic and neurogenesis-enhancing effects (Zhu et al., 2000; Mattson et al., 2001; Fu et al., 2002a; Fu et al., 2002b; Kang et al., 2004; Jaskelioff et al., 2011; Li et al., 2011; Niu and Yip, 2011; Zhou et al., 2011). Telomerase also mediates neurotrophic and cell survival-enhancing effects of brain-derived neurotrophic factor (BDNF) in immature and early post-mitotic neurons (Fu et al., 2002b; Niu and Yip, 2011), thereby facilitating neurogenesis. Telomerase (or TERT) is substantially expressed in neuronal stem cells and in neuronal progenitor cells and early post-mitotic neurons (Jaskelioff et al., 2011; Zhou et al., 2011; Eitan et al., 2014) such as those found in the subgranular layer of the dentate gyrus in the HC and in the subventricular zone, parts of the brain with neurogenesis capability (Hermann et al., 2006; Lee et al., 2010; Eitan et al., 2014) Telomerase activity is also up-regulated in parts of the brain responding to tissue injuries such as hypoxia/ ischemia (Li et al., 2011) and seizures (Fu et al., 2002a), indicating the potential for affecting brain cell resilience, recovery and viability.
Two mouse studies are particularly relevant to considering telomerase activity and HC volume preservation and well as putative associations of HC telomerase activity to MDD. Telomerase-deficient mice display short telomeres in neural stem cells, increased DNA damage signaling and marked degenerative changes, including decreased neural stem cell proliferation and neurogenesis in the subventricular zone, as well as decreased overall brain weight (Jaskelioff et al., 2011). These changes are reversed upon reactivation of telomerase for as little as four weeks (Jaskelioff et al., 2011). Another study specifically implicated HC telomerase in “depression-like” behaviors in adult mice (Zhou et al., 2011). In mice, chronic mild stress (CMS) significantly decreased TERT and telomerase expression in the HC. These stress-induced changes were reversed by administration of the SSRI fluoxetine. Inhibition of telomerase resulted in “depression-like” behaviors and decreased HC neurogenesis, whereas over-expressing HC telomerase increased HC neurogenesis and cell survival and produced “antidepressant-like” effects. However, important differences in telomere/ telomerase biology exist between mice and humans (Calado and Dumitriu, 2013), so extrapolation of these mouse findings to humans must be very cautious.
A notable finding in the present study is that PBMC telomerase activity was correlated with HC volume only in subjects with MDD but not in healthy controls, although this could represent a Type II error due to the slightly smaller number of control subjects. Two prior studies, however, also found no significant correlations between PBMC telomerase activity and HC volume in non-MDD controls (Cheng et al., 2013; Jacobs et al., 2014). The reasons for different results in MDD subjects vs. controls are unclear, though it is possible that significant correlations are only be apparent under “stressed” or stimulated conditions that result in telomerase activation. Specifically, telomerase activation, as seen in PBMC’s in depressed individuals (Wolkowitz et al., 2012), may parallel compensatory neurotrophic/ neurogenesis-enhancing effects in the HC only under pathologic cellular conditions that increase telomerase (Zhu et al., 2000; Fu et al., 2002a; Kang et al., 2004; Li et al., 2011; Zhao et al., 2012). Healthy controls, on the other hand, would not be expected to have compensatory up-regulation of telomerase activity and neurogenesis in the HC. This explanation is similar to findings in mice that telomerase deficiency has no effect on olfactory epithelium under basal homeostatic conditions but has marked inhibitory effects of olfactory epithelium regeneration following injury (Watabe-Rudolph et al., 2011).
Among the strengths of the present study are our use of well-characterized, physically healthy, unmedicated MDD subjects and controls. The latter point is especially important, as we have reported that antidepressant medication can alter PBMC telomerase activity (Wolkowitz et al., 2012). Other strengths are our simultaneous sampling of telomere length and telomerase activity and our use of very high resolution MRI (4 Tesla). Limitations of the study include our small sample size and our use of single time point blood sampling, since telomerase activity can change rapidly in response to stress and other factors (Epel et al., 2010). Finally, in the present case, it is not possible to specify the cellular changes responsible for the correlations observed, as HC volume changes can be caused by changes in neuronal size, dendritic length and branching, glial number and volume, extracellular fluid changes and gliogenesis (Eitan et al., 2014), as well as by neurogenesis (Czeh and Lucassen, 2007). The most obvious limitation in our study is our inability to directly assess telomere length, telomerase activity or neurogenesis in the HC, so the relationship between cell aging markers in the periphery and the corresponding markers in the HC is unknown. Future studies should assess PBMC telomerase activity across several time points and should include larger sample sizes. In addition, future studies should assess additional regional brain volumes outside of the HC (e.g., DLPFC) (Cheng et al., 2013; King et al., 2014), and include assessment of correlations between peripheral cell aging markers and additional imaging modalities (e.g., functional MRI, diffusion tensor imaging and magnetic resonance spectroscopic imaging). Finally, future studies, in animals and in deceased humans, are needed to directly assess correlations between peripheral telomerase activity and telomere length and corresponding measures in the HC and other brain regions.
In conclusion, these preliminary data suggest that telomerase activity, measured in peripheral immune cells, is informative about HC volume in un-medicated individuals with MDD, but not in healthy controls, whereas leukocyte telomere length is not significantly correlated with HC volume in either group. We suggest that peripheral telomerase activity is up-regulated in MDD, relative to matched healthy controls, and that this is a response to actual or incipient cellular or telomere endangerment. To the extent telomerase activity in the HC is similarly up-regulated in individuals with MDD, resulting cellular protection or neurogenesis could account for direct correlations with HC volume.
Highlights.
Major depression is characterized by accelerated cell aging in peripheral immune cells
Peripheral blood telomerase activity is directly correlated with hippocampus volume in depressed subjects but not in healthy controls
Peripheral telomere length is not significantly correlated with hippocampus volume
Larger scale studies are warranted to determine whether peripheral cell aging indexes hippocampal volume in depression
Acknowledgments
The research reported was supported by NIMH grant 1 R01 MH083784 (Co-PI’s: O.M.W., E.S.E., S.H.M.), the UCSF Academic Senate and private donations from the O’Shaughnessy Foundation and the Tinberg Family. This project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Financial conflict disclosure E.H.B., E.S.E. and J.L. were co-founders of a company, Telome Health (now Telomere Diagnostics). J.L. is now Director of Research of Telomere Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arisi GM. Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy & Behavior. 2014;38C:43–47. doi: 10.1016/j.yebeh.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Balaji TM, Vettriselvi V, Paul SF, Rao SR. Evaluation of telomerase expression in chronic periodontitis. Indian Journal of Dental Research. 2010;21:185–188. doi: 10.4103/0970-9290.66632. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. Chronic stress elevates telomerase activity in rats. Biology Letters. 2012;8:1063–1066. doi: 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. The telomere and telomerase: how do they interact? Mt. Sinai Journal of Medicine. 1999;66:292–300. [PubMed] [Google Scholar]
- Brydon L, Lin J, Butcher L, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Hostility and cellular aging in men from the Whitehall II cohort. Biological Psychiatry. 2012;71:767–773. doi: 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Dumitriu B. Telomere dynamics in mice and humans. Seminars in Hematology. 2013;50:165–174. doi: 10.1053/j.seminhematol.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS. Telomere diseases. New England Journal of Medicine. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Shin-ya K, Wan R, Tang SC, Miura T, Tang H, Khatri R, Gleichman M, Ouyang X, Liu D, Park HR, Chiang JY, Mattson MP. Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. Journal of Neuroscience. 2007;27:3722–3733. doi: 10.1523/JNEUROSCI.0590-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GL, Zeng H, Leung MK, Zhang HJ, Lau BW, Liu YP, Liu GX, Sham PC, Chan CC, So KF, Lee TM. Heroin abuse accelerates biological aging: a novel insight from telomerase and brain imaging interaction. Translational Psychiatry. 2013;3:e260. doi: 10.1038/tp.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain, Behavior and Immunity. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European Archives of Psychiatry and Clinical Neuroscience. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. Journal of Immunology. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der G, Batty GD, Benzeval M, Deary IJ, Green MJ, McGlynn L, McIntyre A, Robertson T, Shiels PG. Is telomere length a biomarker for aging: cross-sectional evidence from the west of Scotland? PloS One. 2012;7:e45166. doi: 10.1371/journal.pone.0045166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Prescott J, De Vivo I, Grodstein F. Relative telomere length and cognitive decline in the Nurses’ Health Study. Neuroscience Letters. 2011;492:15–18. doi: 10.1016/j.neulet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Developmental and Comparative Immunology. 1997;21:471–478. doi: 10.1016/s0145-305x(97)00027-x. [DOI] [PubMed] [Google Scholar]
- Eitan E, Hutchison ER, Mattson MP. Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends in Neurosciences. 2014;37:256–263. doi: 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, Malt UF, Blasco MA, Boye B. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. Journal of Affective Disorders. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain, Behavior and Immunology. 2010;24:531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl. 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fu W, Lee J, Guo Z, Mattson MP. Seizures and tissue injury induce telomerase in hippocampal microglial cells. Experimental Neurology. 2002a;178:294–300. doi: 10.1006/exnr.2002.8030. [DOI] [PubMed] [Google Scholar]
- Fu W, Lu C, Mattson MP. Telomerase mediates the cell survival-promoting actions of brain-derived neurotrophic factor and secreted amyloid precursor protein in developing hippocampal neurons. Journal of Neuroscience. 2002b;22:10710–10719. doi: 10.1523/JNEUROSCI.22-24-10710.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizard F, Heywood EB, Findeisen HM, Zhao Y, Jones KL, Cudejko C, Post GR, Staels B, Bruemmer D. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:245–252. doi: 10.1161/ATVBAHA.110.219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, De Vivo I. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PloS One. 2008;3:e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depression and Anxiety. 2010;27:1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- Hermann A, Maisel M, Liebau S, Gerlach M, Kleger A, Schwarz J, Kim KS, Antoniadis G, Lerche H, Storch A. Mesodermal cell types induce neurogenesis from adult human hippocampal progenitor cells. Journal of Neurochemistry. 2006;98:629–640. doi: 10.1111/j.1471-4159.2006.03916.x. [DOI] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, Blackburn E, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosomatic Medicine. 2011;73:541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Annals of Neurology. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Epel ES, Lin J, Blackburn EH, Rasgon NL. Relationship between leukocyte telomere length, telomerase activity and hippocampal volume in early aging. JAMA Neurology. 2014;71(7):921–923. doi: 10.1001/jamaneurol.2014.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, Horner JW, Maratos-Flier E, Depinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Choi YS, Hong SB, Kim KW, Woo RS, Won SJ, Kim EJ, Jeon HK, Jo SY, Kim TK, Bachoo R, Reynolds IJ, Gwag BJ, Lee HW. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. Journal of Neuroscience. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KS, Kozlitina J, Rosenberg RN, Peshock RM, McColl RW, Garcia CK. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurology. 2014;71:1247–1254. doi: 10.1001/jamaneurol.2014.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jo YS, Sung YH, Hwang IK, Kim H, Kim SY, Yi SS, Choi JS, Sun W, Seong JK, Lee HW. Telomerase deficiency affects normal brain functions in mice. Neurochemical Research. 2010;35:211–218. doi: 10.1007/s11064-009-0044-3. [DOI] [PubMed] [Google Scholar]
- Li J, Tang B, Qu Y, Mu D. Telomerase reverse transcriptase: a novel neuroprotective mechanism involved in neonatal hypoxic-ischemic brain injury. International Journal of Developmental Neuroscience. 2011;29:867–872. doi: 10.1016/j.ijdevneu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Liu M, Hu Y, Zhu L, Chen C, Zhang Y, Sun W, Zhou Q. Overexpression of the mTERT gene by adenoviral vectors promotes the proliferation of neuronal stem cells in vitro and stimulates neurogenesis in the hippocampus of mice. Journal of Biomedical Research. 2012;26:381–388. doi: 10.7555/JBR.26.20110078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Chen NC, Shu BC. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatric Genetics. 2007;17:195–199. doi: 10.1097/YPG.0b013e32808374f6. [DOI] [PubMed] [Google Scholar]
- Ma SL, Lau ES, Suen EW, Lam LC, Leung PC, Woo J, Tang NL. Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age and Ageing. 2013;42:450–455. doi: 10.1093/ageing/aft036. [DOI] [PubMed] [Google Scholar]
- Maeda T, Guan JZ, Koyanagi M, Makino N. Telomerase activity and telomere length distribution in vascular endothelial cells in a short-term culture under the presence of hydrogen peroxide. Geriatrics and Gerontology International. 2013;13:774–782. doi: 10.1111/j.1447-0594.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Annals of Neurology. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Anstey KJ, Milburn PJ, Easteal S, Christensen H. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: a population study. BMC Geriatrics. 2010;10:62. doi: 10.1186/1471-2318-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Fu W, Zhang P. Emerging roles for telomerase in regulating cell differentiation and survival: a neuroscientist’s perspective. Mechanisms of Ageing and Development. 2001;122:659–671. doi: 10.1016/s0047-6374(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Klapper W. Emerging roles for telomerase in neuronal development and apoptosis. Journal of Neuroscience Research. 2001;63:1–9. doi: 10.1002/1097-4547(20010101)63:1<1::AID-JNR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Niu C, Yip HK. Neuroprotective signaling mechanisms of telomerase are regulated by brain-derived neurotrophic factor in rat spinal cord motor neurons. Journal of Neuropathology and Experimental Neurology. 2011;70:634–652. doi: 10.1097/NEN.0b013e318222b97b. [DOI] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biological Psychiatry. 2013;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Duan Z, Zhao F, Wei D, Zhang J, Tang B, Li J, Yang C, Mu D. Telomerase reverse transcriptase upregulation attenuates astrocyte proliferation and promotes neuronal survival in the hypoxic-ischemic rat brain. Stroke. 2011;42:3542–3550. doi: 10.1161/STROKEAHA.111.626325. [DOI] [PubMed] [Google Scholar]
- Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, Kokkinou S, Haliassos A, Mamalaki A, Kouretas D, Tsitsimpikou C. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PloS One. 2012;7:e35739. doi: 10.1371/journal.pone.0035739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saretzki G. Telomerase, mitochondria and oxidative stress. Experimental Gerontology. 2009;44:485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, Aviv A, Cherkas LF. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiology of Aging. 2010;31:986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Molecular Psychiatry. 2014;19(8):895–901. doi: 10.1038/mp.2013.151. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Karlsson T, Lind J, Nilbrink T, Hultdin J, Sleegers K, Van Broeckhoven C, Roos G, Nilsson LG, Nyberg L, Adolfsson R, Norrback KF. Longer leukocyte telomere length is associated with smaller hippocampal volume among non-demented APOE epsilon3/epsilon3 subjects. PloS One. 2012a;7:e34292. doi: 10.1371/journal.pone.0034292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biological Psychiatry. 2012b;71:294–300. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: do stress and depression accelerate cell aging? World Journal of Biological Psychiatry. 2008;9:2–5. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PloS One. 2011a;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Molecular Psychiatry. 2012;17:164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues in Clinical Neuroscience. 2011b;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, Kuller L, Li R, Ayonayon HN, Rubin SM, Cummings SR, Health ABCS. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiology of Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4519–4524. doi: 10.1073/pnas.1322145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Dilley C, Mattson MP. DNA damage responses in neural cells: focus on the telomere. Neuroscience. 2007;145(4):1439–1448. doi: 10.1016/j.neuroscience.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Qu Y, Xiong T, Duan Z, Ye Q, Mu D. The neuroprotective role of TERT via an antiapoptotic mechanism in neonatal rats after hypoxia-ischemia brain injury. Neuroscience Letters. 2012;515:39–43. doi: 10.1016/j.neulet.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X, Luo CX, Wu HY, Zhang J, Zhu DY. Hippocampal telomerase is involved in the modulation of depressive behaviors. Journal of Neuroscience. 2011;31:12258–12269. doi: 10.1523/JNEUROSCI.0805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. Journal of Neurochemistry. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]