Summary
A large body of research has linked hypothalamic–pituitary–adrenal (HPA) axis function and alcohol consumption, including work suggesting that flatter diurnal cortisol slopes are associated with greater alcohol use. A lack of longitudinal studies and a focus on adult and alcoholic populations leaves unclear whether such associations are also present in younger, non-clinical populations and whether flatter diurnal slopes are a consequence of or preexisting risk factor for alcohol use; however, theory suggests such associations may be mutually reinforcing. In a longitudinal, community sample of 200 (55% female) adolescents, the current study demonstrates that flatter diurnal cortisol slope at age 11 predicts higher levels of alcohol use from ages 15-18, and that heavier alcohol use in turn predicts further flattening of diurnal cortisol rhythm at age 18.5. This is the first study to demonstrate a longitudinal chain of associations between diurnal cortisol slope and alcohol use. Findings support contemporary theoretical models of the neurobiological processes underlying alcohol use and can inform future research on risk factors for and consequences of underage drinking.
Keywords: Diurnal cortisol, HPA axis, Alcohol use, Adolescence, Longitudinal
1. Introduction
Although underage alcohol use is fairly common, it can have serious short- and long-term consequences. These include increased risk of accidental injury, risky sexual behavior, and lower educational attainment (U.S. Department of Health and Human Services, 2007; Masten et al., 2008), all of which increase the risk of morbidity and mortality. As such, it is essential to understand underlying neurobiological processes associated with underage drinking. Theory (Schepis et al., 2011) suggests that alcohol use and hypothalamic–pituitary–adrenal (HPA) axis activity are mutually reinforcing over time, but this has yet to be tested empirically, highlighting the need for longitudinal studies examining whether a longitudinal chain of associations links preadolescent cortisol functioning, alcohol use across adolescence, and later cortisol functioning.
As outlined by Schepis et al. (2011), different forms of HPA-axis dysfunction may act as risk factors for later alcohol use and abuse, and chronic alcohol consumption in turn may contribute to further disturbances in HPA-axis functioning (e.g., flattened diurnal cortisol slopes due to hypocortisolism). This theoretical work rests on existing preclinical (e.g., Allen et al., 2011) and human (e.g., Gianoulakis et al., 2003; Boschloo et al., 2011) studies. However, human research in this area has largely focused on cross-sectional associations in adults, often alcoholics, with cortisol response to acute stressors or to experimental administration of alcohol predominating (e.g., Adinoff et al., 2005). Nevertheless, basal cortisol levels and diurnal patterns have also been examined (e.g., Gianoulakis et al., 2003; Boschloo et al., 2011). Such work is integral to understanding health and behavior because deviations from the expected diurnal rhythm can reflect impaired integrity of day-to-day HPA-axis functioning, which is often indicative of more persistent health problems. Flatter diurnal cortisol slopes have been associated with greater alcohol consumption (Badrick et al., 2008), echoing links between flattened cortisol slopes and greater mental health symptoms in adolescence (Shirtcliff and Essex, 2008; Ruttle et al., 2011), but findings are inconsistent. For example, one cross-sectional study of adolescent alcohol-use onset found no association with diurnal cortisol (Evans et al., 2012), and one prospective study yielded null associations between morning, afternoon, and evening cortisol levels at ages 10-12 and alcohol use concurrently and at ages 13-14 (Huizink et al., 2009). However, both studies measured alcohol use very early in adolescence. Given that a variety of mental and physical health conditions (e.g., externalizing and internalizing symptoms, burnout, chronic fatigue) have been associated with hypocortisolism (Fries et al., 2005; Ruttle et al., 2011), it may be that more persistent drinking patterns, rather than acute drinking or early experimentation reflecting behavior at a single time point, are associated with flattened diurnal-cortisol patterns (Gianoulakis et al., 2003).
The present study takes an important first step to addressing the gaps in the literature by testing for a longitudinal chain of effects of HPA-axis function and alcohol consumption in a community sample of adolescents. We hypothesized that flatter diurnal cortisol slope at age 11 would relate to higher levels of alcohol use across ages 15-18, which would in turn relate to further flattening of diurnal cortisol slope at age 18.5. We also examined whether this latter association is present net of age 11 cortisol slope to ensure that the observed finding does not simply reflect developmental changes in HPA-axis function, such as increased basal cortisol levels across adolescence (Gunnar et al., 2009), which may result in the flattening of diurnal cortisol slope. Given prior work (Evans et al., 2012), we also considered whether other key concurrent factors linked to cortisol activity and/or adolescent drinking behavior (i.e., pubertal development, externalizing symptoms, and internalizing symptoms) might explain these associations.
2. Methods
2.1 Participants
Participants are drawn from a longitudinal study of 560 families recruited during mother's pregnancy and followed through the target child's adolescence (Hyde et al., 1995). The sample is primarily Caucasian (93%); initial annual family income ranged from $7,500 to > $200,000 (median = $47,000). The current study focused on the subsample (n = 200) of participants who provided saliva samples at ages 11 and 18.5. Participants did not differ from non-participants on demographics, mental health symptoms, or alcohol use. All procedures were approved by University of Wisconsin Institutional Review Boards.
3. Measures
3.1 Alcohol use
At modal ages 15, 16, 17, and 18 – the spring of grades 9-12 – adolescents reported whether they had ever had an alcoholic drink and, if so, the typical number of drinks consumed per occasion in the past 30 days as in major studies (e.g., Johnston et al., 2012). Responses were scored as 0 (I don't drink alcohol), 0.5 (Less than a drink), 1 (1 drink), 2 (2 drinks), 3 (3 drinks) and 5 (5 drinks). At each grade, number of drinks consumed per occasion ranged from 0 to 5: grade 9 M = 0.84, SD = 1.34; grade 10 M = 1.29, SD = 1.68; grade 11 M = 1.62, SD = 1.74; grade 12 M = 1.97, SD = 1.86. Alcohol use was averaged over time to create a summary measure of drinking habits across high school.
3.2 Salivary cortisol
Diurnal cortisol was assessed in the summers following grade 7 (modal age 11) and grade 12 (modal age 18.5, approximately 6 months after the last alcohol use measure). Saliva was collected three times per day for three days at set target times: shortly after waking, between 3:00 and 7:00 PM, and before bed. Adolescents were instructed to record collection time, collect samples before eating, and freeze samples upon collection. The vast majority of participants were able to provide samples across all three days of sampling at both assessments (n missing one day: age 11 = 6, age 18.5 = 1; n missing two days: age 11 = 0, age 18.5 = 1). Samples were transported to the laboratory by researchers and kept frozen at -80C until assayed in duplicate using well-established, salivary enzyme immunoassay kits (Salimetrics, State College, PA). Intra-assay and inter-assay coefficients of variation were ≤ 5.1% and 8.2%, respectively, and the detection sensitivity limit was .003 μg/dL. Raw scores were log-transformed and extreme values were Winsorized to normalize distributions.
3.3 Pubertal status
A pubertal-status summary score averaged mother- and youth-reported responses at age 11 regarding Tanner staging (Morris and Udry, 1980) and other physical markers (Petersen et al., 1988).
3.4 Mental health symptoms
Externalizing symptoms – conduct problems, oppositional defiance, and overt and relational aggression – and internalizing symptoms – anxiety and depression – were measured at age 11 and ages 15, 16, 17, and 18 via mother, teacher (ages 11 and 15 only), and adolescent reports on adolescent versions of the well-validated MacArthur Health and Behavior Questionnaire. Multi-informant composites were computed at each age with principal components analysis, then composites were averaged across ages 15-18. For details, see Shirtcliff and Essex (2008).
4. Data Analytic Strategy
Diurnal cortisol slopes were estimated using hierarchical linear modeling (HLM, Bryk and Raudenbush, 1992), which utilized the multiple cortisol samples within an individual to produce measures of predicted change across the day (i.e., slope) at ages 11 and 18.5. Cortisol slope values were computed by fitting a two-level HLM to the data (collected 3 times per day across 3 days) that included within-individual variations in the intercept and slope (Level 1) and random effects representing between-individual differences (Level 2), not distinguishing between days. Time since waking was included as a Level 1 predictor to model individual differences in cortisol due to variations in waking and sampling times. HLM imputed missing data and produced Empirical Bayes (EB) estimates that were extracted for each individual, reflecting predicted average cortisol slope at ages 11 and 18.5. Given that EB estimates often display “shrunken variance” (Hox, 2002), these variables were standardized to increase variability such that, compared to the average cortisol slope, positive values indicate a flatter or more blunted slope and negative values indicate a steeper decreasing slope.
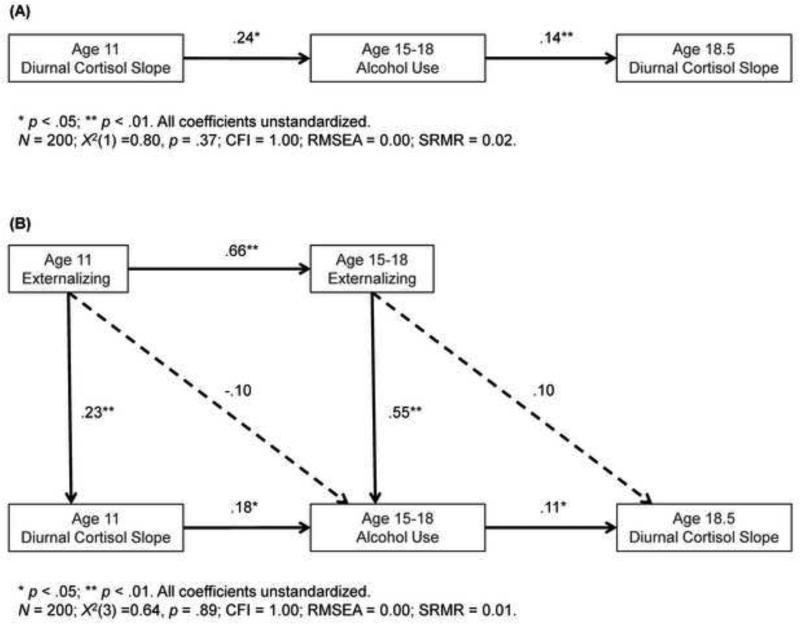
Remaining analyses were conducted via structural equation modeling (SEM) in Mplus version 5.2 (Muthén and Muthén, 1998-2012). Missing data were minimal (see Table 1); therefore, full information maximum likelihood estimation was used, retaining all 200 participants in analyses. The first model (Figure 1A) tested our primary hypothesis, that flatter diurnal cortisol slope at age 11 would predict heavier drinking from ages 15-18, which would predict flatter diurnal cortisol slope at age 18.5. Next we examined whether these associations were present net of age 11 cortisol slope by including a direct pathway from age 11 cortisol to age 18.5 cortisol. To consider possible concurrent factors through which these associations might operate, a model (Figure 1B) included covariates that were significantly associated at the bivariate level with either age 11 cortisol slope or age 15-18 alcohol use. Sex differences were tested using the multiple group option and would be indicated by significant improvement in model fit (per chi-square model comparisons) from a model with paths constrained versus freely estimated for boys and girls.
Table 1.
Descriptive Statistics and Pearson Correlations among Primary Variables and Potential Covariates
| Descriptive Statistics | Correlations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean | SD | Range | % Missing | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 1. Age 11 Diurnal Cortisol Slope | 0.00 | 1.00 | −3.46 – 2.55 | 0 | |||||||
| 2. Age 15-18 Alcohol Use | 1.43 | 1.36 | 0.00 – 5.00 | 0 | .18* | ||||||
| 3. Age 18.5 Diurnal Cortisol Slope | 0.00 | 1.00 | −3.58 – 2.75 | 0 | .09 | .18** | |||||
| 4. Age 11 Pubertal Status | 2.12 | 0.82 | 1.00 – 4.50 | 2.5 | −.05 | −.03 | .08 | ||||
| 5. Age 11 Externalizing | −0.07 | 0.94 | −1.40 – 3.06 | 6 | .21** | .22** | .10 | −.08 | |||
| 6. Age 11 Internalizing | −0.06 | 1.01 | −1.75 – 3.78 | 6 | .01 | −.03 | .08 | .03 | .49** | ||
| 7. Age 15-18 Externalizing | −0.00 | 0.93 | −1.42 – 3.83 | 1 | .15* | .35** | .14* | −.09 | .67** | .38** | |
| 8. Age 15-18 Internalizing | −0.01 | 0.92 | −1.58 – 3.22 | 1 | −.03 | .08 | .19* | .25** | .23** | .55** | .45** |
p ≤ .05
p ≤ .01
Figure 1.
Structural equation models depicting (A) the longitudinal associations between age 11 diurnal cortisol slope, age 15-18 alcohol consumption, and age 18.5 diurnal cortisol slope; and (B) longitudinal cortisol-alcohol associations while including significant covariates (see Data Analytic Strategy).
5. Results
Descriptive statistics and bivariate correlations of all variables are presented in Table 1.
SEM results (Figure 1A) support the hypothesized pattern of effects. Flatter diurnal cortisol slope at age 11 significantly predicted heavier alcohol use at ages 15-18, which in turn significantly predicted flatter cortisol slope at age 18.5. Including a direct pathway from age 11 to age 18.5 cortisol slope revealed that this pathway was non-significant (B = .06, p > .05) and its inclusion did not meaningfully alter the associations of primary interest (age 11 cortisol → adolescent drinking: B = .24, p < .05 versus B = .25, p < .05; adolescent drinking → age 18.5 cortisol: B = .14, p < .01 versus B = .13, p < .05). Further, a test of the indirect pathway of age 11 on age 18.5 cortisol slope through alcohol consumption revealed a non-significant effect (Indirect: B = 0.033, p = .067). As shown in Figure 1B, when including concurrent covariates significantly associated with age 11 cortisol slope or age 15-18 alcohol use (i.e., age 11 and age 15-18 externalizing), both associations between cortisol and alcohol use observed in the initial primary model remained significant. Examination of possible sex differences revealed no significant findings (χ2 (7) = 10.88, p > .05).
6. Discussion
Adolescence is the modal period of emergence of alcohol use, and a large literature has linked alcohol use and dependence to HPA-axis dysregulations. Nevertheless, while current theories suggest that altered adrenocortical activity and alcohol consumption are mutually reinforcing over time, this is the first study to empirically demonstrate a longitudinal chain of associations between diurnal cortisol slope and alcohol use in adolescence. Specifically, in a community sample of adolescents, flatter cortisol slope at age 11 predicted heavier drinking across ages 15-18, which in turn predicted flatter slopes at age 18.5. Importantly, the pattern of findings was largely unchanged after controlling for possible developmental flattening of the cortisol slope and known proximal predictors of diurnal cortisol rhythm and adolescent alcohol use, notably externalizing symptomatology. Additionally, the pattern of effects did not significantly vary by sex. Although the present model does not prove causation, this is the first study to empirically examine the links between cortisol and alcohol use as a chain of associations at any age, suggesting that the effect is a mutually reinforcing process rather than a unidirectional effect. Importantly, we examined this chain of associations in adolescence, a sensitive period in which the HPA-axis may be more susceptible to external influences than in other developmental epochs, such as childhood and adulthood. Moreover, it may be that associations between diurnal cortisol and alcohol are not immediately evident, but rather develop over time, suggesting the importance of longitudinal data and repeated measurements when assessing such processes in adolescents.
The finding that flattened cortisol slopes predicted greater alcohol consumption several years later may suggest that blunted HPA-axis activity may represent a risk factor for heavier later alcohol consumption. Blunted patterns of adrenocortical activity may be due to a variety of earlier experiences (see Gunnar & Vazquez, 2001) and possibly suggest persistent hypoarousal of the HPA axis. Given that acute alcohol consumption has been shown to produce increases in cortisol and corticosterone, adolescents with blunted HPA-axis activity may engage in behaviors that increase HPA-axis activity, such as the ingestion of alcohol, to normalize the diurnal rhythm and achieve equilibrium (for a review, see Schepis et al., 2011).
Although acute alcohol consumption and administration have been linked to increased basal and stress-reactive cortisol levels, habituation to alcohol administration may occur over time, resulting in further blunted adrenocortical activity (Schepis et al., 2011). This result is consistent with animal models showing dampening effects of chronic alcohol use on HPA-axis activity (Allen et al., 2011). While the present findings suggest that the associations between cortisol and alcohol were largely independent of mental health symptoms, they do not suggest that symptomatology and alcohol use are not intricately linked, particularly at clinical levels. Rather, the present findings reveal that increased alcohol consumption uniquely contributes to the flattening of the diurnal cortisol slope, possibly suggesting that interventions designed to address drinking behavior may modify HPA-axis functioning, which may in turn reduce the likelihood of the development or progression of various psychopathologies known to be associated with dysregulated HPA-axis function.
Limitations of this study include its fairly homogeneous sample; results may not generalize to more racially diverse or economically impoverished populations. Additionally, Wisconsin has one of the highest rates of adolescent alcohol use and binge drinking (Centers for Disease Control and Prevention, 2012); because effects of alcohol on the HPA axis are expected to occur through chronic drinking (Gianoulakis et al., 2003; Boschloo et al., 2011), it is possible that these effects may be less evident among samples with lower rates and levels of underage alcohol use. Future studies could also benefit from inclusion of explicit measures of binge and chronic drinking. Additionally, we cannot confirm that participants were alcohol naïve at age 11; however, chronic heavy drinking before age 11 is rare but becomes increasingly common in high school (Masten et al., 2008), so it is unlikely that many participants had consumed alcohol at high enough levels by age 11 to have already exerted a large, flattening effect on diurnal cortisol rhythms.
Although this study is the first to demonstrate a longitudinal chain of associations between diurnal cortisol slope and alcohol use across adolescence, we are likely tapping only part of a larger developmental process. Future research should expand on this research by examining a cross-lagged model, which would establish the direction of causality between diurnal cortisol slope and alcohol use. Further studies are also required to examine how this chain of associations may extend developmentally backward and forward, linking adolescent HPA-axis alterations and drinking to earlier stress/adversity in childhood and to later alcohol use/dependence in adulthood. Additionally, while preclinical work has identified various neural substrates that may be responsible for the interplay between alcohol consumption and HPA-axis activity (Allen et al., 2011), it is critical to determine whether the same neurobiological processes are responsible for the observed associations in humans.
Highlights.
We examine adolescent alcohol use in a longitudinal community sample.
Flatter age-11 diurnal cortisol slope was associated with drinking in high school.
Drinking in turn was associated with flatter age-18.5 diurnal cortisol slope.
This chain of associations persisted after controlling on likely confounders.
Acknowledgements
The authors wish to express their appreciation to the participating families, the staff of the Wisconsin Study of Families and Work, and Dr. Elizabeth Shirtcliff for providing consultation on the cortisol measures.
Funding
This research was supported by NIH grants R01-MH044340, P50-MH052354, P50-MH069315, P50-MH084051, R21-MH082705, and P20-DA017589; the HealthEmotions Research Institute at the University of Wisconsin–Madison; and the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development. Partial support for PLR was provided by the Canadian Institutes of Health Research Post-doctoral Fellowship and for JM by the Robert Wood Johnson Foundation Health & Society Scholars Program at the University of Wisconsin–Madison. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
J.M. Armstrong, & M.J. Essex designed the study and wrote the protocol. P.L. Ruttle, J. Maslowsky, & L. R. Burke managed the literature searches. P.L. Ruttle & J. Maslowsky undertook the statistical analysis, and P.L. Ruttle, J. Maslowsky, & J.M. Armstrong wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol Clin. Exp. Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain. Behav. Immun. 2011;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. The relationship between alcohol consumption and cortisol secretion in an aging cohort. J. Clin. Endocrinol. Metab. 2008;93:750–757. doi: 10.1210/jc.2007-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Licht CM, Vreeburg SA, Smit JH, van den Brink W, Veltman DJ, de Geus EJ, Beekman AT, Penninx BW. Heavy alcohol use, rather than alcohol dependence, is associated with dysregulation of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system. Drug Alcohol Depend. 2011;116:170–176. doi: 10.1016/j.drugalcdep.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods, Advanced Qualitative Techniques in the Social Sciences. Sage Publications; Thousand Oaks, CA.: 1992. [Google Scholar]
- Centers for Disease Control and Prevention [May 22, 2014];CDC-Youth Online: High School Youth Risk Behavior Survey 2011 Results. 2012 from http://nccd.cdc.gov/youthonline/App/Default.aspx.
- Evans BE, Greaves-Lord K, Euser AS, Franken IH, Huizink AC. The relation between hypothalamic-pituitary-adrenal (HPA) axis activity and age of onset of alcohol use. Addiction. 2012;107:312–322. doi: 10.1111/j.1360-0443.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol. Clin. Exp. Res. 2003;27:410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev. Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox J. Multilevel analysis techniques and applications. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US.: 2002. [Google Scholar]
- Huizink AC, Greaves-Lord K, Oldehinkel AJ, Ormel J, Verhulst FC. Hypothalamic-pituitary-adrenal axis and smoking and drinking onset among adolescents: the longitudinal cohort TRacking Adolescents' Individual Lives Survey (TRAILS). Addiction. 2009;104:1927–1936. doi: 10.1111/j.1360-0443.2009.02685.x. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women's mental health. Psychol. Women Q. 1995;19:257–285. [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor, MI.: 2012. [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: A developmental framework. Pediatrics. 2008;121:S235–S251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus User's Guide. 7th ed. Muthén & Muthén; Los Angeles: 1998-2012. [Google Scholar]
- Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm. Behav. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, Adinoff B. The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcohol. Clin. Exp. Res. 2011;35:595–605. doi: 10.1111/j.1530-0277.2010.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychobiol. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . The Surgeon General's Call to Action to Prevent and Reduce Underage Drinking: A Guide to Action for Communities. Office of the Surgeon General, U.S. Department of Health and Human Services; Washington, DC.: 2007. [PubMed] [Google Scholar]