Abstract
Individuals with body dysmorphic disorder (BDD) suffer from preoccupations with perceived defects in physical appearance, causing severe distress and disability. Although BDD affects 1-2% of the population, the neurobiology is not understood. Discrepant results in previous volumetric studies may be due to small sample sizes, and no study has investigated cortical thickness in BDD. The current study is the largest neuroimaging analysis of BDD. Participants included 49 medication-free, right-handed individuals with DSM-IV BDD and 44 healthy controls matched by age, sex, and education. Using high-resolution T1-weighted magnetic resonance imaging, we computed vertex-wise gray matter (GM) thickness on the cortical surface and GM volume using voxel-based morphometry. We also computed volumes in cortical and subcortical regions of interest. In addition to group comparisons, we investigated associations with symptom severity, insight, and anxiety within the BDD group. In BDD, greater anxiety was significantly associated with thinner GM in the left superior temporal cortex and greater GM volume in the right caudate nucleus. There were no significant differences in cortical thickness, GM volume, or volumes in regions of interest between BDD and control subjects. Subtle associations with clinical symptoms may characterize brain morphometric patterns in BDD, rather than large group differences in brain structure.
Keywords: Gray matter, Cortical thickness, Voxel-based morphometry, Volume, MRI, Anxiety
1. Introduction
Body dysmorphic disorder (BDD) is an under-studied psychiatric disorder, despite its relatively high prevalence (1-2%) (Mufaddel et al., 2013). Individuals with BDD are preoccupied with perceived defects in their physical appearance (American Psychiatric Association, 2013). These concerns are often obsessive, resulting in significant distress and disability. Due to similar symptoms, heredity, and comorbidity, BDD is conceptualized as an obsessive-compulsive related disorder (Phillips et al., 2010). BDD is also associated with depression and anxiety. In addition, those with BDD often have low insight into their psychiatric illness and exaggerate perceived “defects,” even though they are not noticeable or very slight to others (American Psychiatric Association, 2013).
There have only been a small number of neuropsychological and neuroimaging studies in BDD, and its neurobiology remains largely unknown. Of the four studies of brain morphometry in BDD (Rauch et al., 2003); Feusner et al., 2009; Atmaca et al., 2010; Buchanan et al., 2014), two found greater total white matter (WM) volume (Rauch et al., 2003; Atmaca et al., 2010) and two found smaller volumes in frontostriatal systems (anterior cingulate and the orbitofrontal cortices) (Atmaca et al., 2010; Buchanan et al., 2014). However, these studies had small sample sizes, and the results are discrepant. Moreover, these studies investigated whole brain or region of interest (ROI) volume measurements, but they did not assess gray matter (GM) thickness, which may be a more sensitive tool to uncover subtle or diffuse morphometric abnormalities.
Neuropsychological, psychophysical, and functional magnetic resonance imaging (fMRI) studies suggest that the pathophysiology of BDD involves abnormalities in executive functioning, visuospatial processing and memory, processing of emotional faces, and visual systems (for review, see Madsen et al., 2013). Several fMRI studies demonstrate an imbalance of global versus detailed processing when BDD patients view images of their own and others’ faces and of objects (Deckersbach et al., 2000; Feusner et al., 2007; Feusner et al., 2010; Feusner et al., 2011; Jefferies et al., 2012). Two studies found abnormal hypoactivity in primary and/or secondary visual processing systems, and one also found hyperactive frontostriatal systems for own-face stimuli. Abnormal neural activity plays a role in BDD, but whether there are structural abnormalities in these systems has not been established.
This is the first study to examine cortical thickness in individuals with BDD, and the largest to investigate brain morphometry. Differences in brain structure and function, specifically in visual processing regions of the brain, may underlie the dysfunctional preoccupation with details in physical appearance that are core BDD symptoms. We hypothesized that there would be regional differences (greater or lesser) in GM thickness and volumes between BDD patients and controls. Specifically, we expected between-group differences in frontostriatal and visual processing systems, where previous studies found abnormal volumes (Rauch et al., 2003; Atmaca et al., 2010; Buchanan et al., 2014) and/or functioning (Feusner et al., 2007; Feusner et al., 2010; Feusner et al., 2011). We also predicted significant associations between clinical symptoms and both cortical thickness and volumes. Knowledge of neuroanatomical abnormalities in BDD could contribute to mechanistic understandings of the pathophysiology.
2. Methods
2.1. Participants
The UCLA Institutional Review Board approved this study, and we obtained written informed consent from participants.
Ninety-three right-handed medication-free individuals, recruited from the community, participated. Each participant received a clinical evaluation by J.D.F., who has clinical expertise in BDD. Individuals who met criteria for BDD (Phillips et al., 1995) and who had a score of 20 on the BDD version of the Yale–Brown Obsessive– Compulsive Scale (BDD-YBOCS; Phillips et al., 1997) were eligible. We used the Mini International Neuropsychiatric Inventory to determine comorbid diagnoses (Sheehan et al., 1998). Severity of other psychiatric symptoms was measured using validated clinical scales as follows: the Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1959), the Brown Assessment of Beliefs scale (BABS, measuring insight about perceived defects and psychiatric illness; Eisen et al., 1998), and either the 17-item Hamilton Depression Rating Scale (HAMD-17, administered to n=26; Hamilton, 1960; Snaith, 1977) or the Montgomery-Åsberg Depression Rating Scale (MADRS, administered to n=44; Montgomery and Åsberg, 1979; Williams and Kobak, 2008). (Two depression scales were used because the dataset combined two similar protocols, one of which administered the MADRS and the other the HAMD-17.) Duration of illness data were available in n=36 BDD participants.
We excluded participants either currently taking psychoactive medications or having taken them within 8 weeks of the study, as well as participants currently in cognitive-behavioral therapy. Additional exclusion criteria included the following: lifetime neurological disorders, any current medical disorder affecting cerebral metabolism, recent substance abuse or dependence, or Axis I disorder comorbidity. Major depressive disorder (MDD), dysthymic disorder, generalized anxiety disorder (GAD), and social anxiety disorder (SAD) were allowed because anxiety and depression are common symptoms in BDD (American Psychiatric Association, 2013), and excluding these disorders would lead to a non-representative study group. However, BDD had to be the primary diagnosis.
2.2. Brain image acquisition
We acquired high-resolution T1-weighted 3D structural MRI brain scans on Siemens Allegra (n=29) or Trio (n=41) scanners, using a Magnetization Prepared Rapid Acquisition Gradient Echo (MP-RAGE) sequence. On the Allegra, images were obtained with a repetition time (TR) = 2.3 s, echo time (TE) = 2.93 ms, flip angle = 8°, field of view = 256 × 256, and voxel size = 1.3 × 1.3 × 1 mm3. On the Trio, images were obtained with a repetition time (TR) = 1.9 s, echo time (TE) = 2.26 ms, flip angle = 9°, field of view = 250 × 250, and voxel size = 1 × 1 × 1 mm3.
2.3. Brain tissue segmentation
Researchers were blind to diagnosis throughout data processing.
We used the FAST Automated Segmentation Tool in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST) (Zhang et al., 2001) to obtain segmentations from each participant's brain MRI scan. Subcortical and cortical GM and WM segmentations were manually edited by trained experts (A.Z., T.P., J.D.F.). Raters achieved 99% inter-rater reliability, as defined by number of overlapping voxels, on a training set of n=5.
2.4. Parcellation and cortical thickness analysis
We used FreeSurfer (v5.0.0, http://surfer.nmr.mgh.harvard.edu/) to obtain ROIs from the 2006 Desikan-Killiany atlas and 3D maps of cortical GM thickness. Technical details have been described previously (Fischl and Dale, 2000; Fischl et al., 2002). Briefly, the processing pipeline involves removal of non-brain tissue, intensity normalization, tessellation of the cortical GM/WM boundary, alignment of cortical anatomy, segmentation of total GM volume for left and right hemispheres, smoothing, and creation of 3D surfaces with GM thickness at each surface point in left and right hemispheres.
We excluded data from five participants due to scanner artifacts that prevented accurate cortical segmentations. We created separate cortical GM maps smoothed at 5, 10, 15, 20, and 25 mm full width at half-maximum (FWHM) because the spatial extent of between-groups differences is not known in BDD. Our primary hypothesis was that 15-mm smoothing would provide maximum sensitivity and specificity, based on other datasets (Zhao et al., 2013). As the appropriate smoothing kernel size is not yet known for this population, we tested other smoothing kernels post hoc to explore if between-group differences emerged with alternative image processing choices (discussed in greater detail in Section 4, especially Section 4.3).
2.5. Voxel-based morphometry
We also used voxel-based morphometry (VBM) in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM) (Smith et al., 2004), to investigate whole-brain differences in regional GM volume. Structural images were brain-extracted and tissue-segmented using FAST (Zhang et al., 2001). A non-biased average template was created from equal-sized groups of randomly selected BDD and control participants (n=44 each). Each participant's GM partial volume image was affine-registered to ICBM-152 standard space (Mazziotta et al., 2001), then averaged and flipped along the x-axis to create a symmetrical study-specific template. The native GM images were non-linearly re-registered to the template and modulated to correct for local expansion (or contraction), then smoothed with a 7-mm FWHM Gaussian kernel.
2.6. Statistical models
We tested a similar set of general linear models (GLMs) in each neuroimaging analysis separately as follows: (a) mean volume and GM thickness in ROIs; (b) voxel-wise VBM; and (c) vertex-wise cortical GM thickness. In each case, the brain morphometric measure was the dependent variable. To compare BDD patients versus healthy controls, we used group, gender, age, and scanner as independent covariates, with a significance threshold of alpha=0.05, corrected. To test associations between brain structure and clinical symptoms in BDD, we used clinical scores (BDD-YBOCS for symptom severity, BABS for insight, HAMA for anxiety), sex, age, and scanner as independent covariates, with a Bonferroni-corrected alpha=0.05/3=0.016 (Bland and Altman, 1995). To control for brain size, we normalized volumes for the ROI analyses, and used a covariate for intracranial volume (transformed with square root to bring all variables into a similar scale) for cortical models.
As an exploratory analysis in the VBM and cortical analyses, we used illness duration instead of age as a covariate (n=33). Age and illness duration are highly correlated, precluding their use within the same statistical model; however, the two variables are distinct in their clinical significance. Despite our limited ability to tease apart these two factors, this analysis is an important contribution to the whole picture.
We also included additional post hoc morphometric analyses after excluding BDD participants with any comorbidity (see Table 1 for detailed notes on comorbidity status). The non-comorbid BDD group comprised n=23 participants.
Table 1.
Demographics and psychometrics
| Healthy controls | BDD | ||
|---|---|---|---|
| N: | 44 | 49 | |
| Age | 25.34 ± 7.80 years | 26.43 ± 7.79 | p=0.50 |
| Sex | 30 Females 14 Males | 37 Females 12 Males | p=0.58 |
| Scanner: | 21 Trio 23 Allegra | 23 Trio 26 Allegra | p>0.99 |
| Illness duration (years): | -- | 12.42 ± 9.56 | -- |
| BDD-YBOCS | -- | 29.82 ± 5.51 | -- |
| BABS | -- | 15.15 ± 3.31 | -- |
| HAMA | 1.59 ± 1.54 | 12.39 ± 7.89 | p<0.01* |
| HAMD (26 BDD subjects, 23 HC subjects) | 1.35 ± 1.50 | 10.92 ± 6.53 | p<0.01* |
| MADRS (23 BDD subjects, 21 HC subjects) | 0.57 ± 0.93 | 17.87 ± 8.10 | p<0.01* |
| Comorbidity | -- | None (n=23) Agoraphobia (n=1) Dysthymia (n= 1) Dysthymia, GAD (n=2) GAD (n=5) MDD (n=9) GAD, MDD (n=6) GAD, MDD, social phobia (n=1) SAD (n=2) |
-- |
The demographic and psychometric information for the body dysmorphic disorder (BDD) and healthy control participants is listed (mean ± standard deviation), along with p-values for two-tailed unpaired t-tests for continuous variables and chi-squared tests for binary variables.
2.7. ROI analyses
ROIs included brain regions that have been previously found in the BDD literature to have abnormal volumes (frontal and striatal regions) (Rauch et al., 2003; Atmaca et al., 2010; Buchanan et al., 2014) and/or abnormal activity (frontal, striatal, and visual regions) (Feusner et al., 2007; Feusner et al., 2010; Feusner et al., 2011). We analyzed mean cortical thickness in the bilateral anterior cingulate (caudal + rostral anterior cingulate), bilateral medial orbitofrontal cortex, left inferior frontal gyrus (pars opercularis + pars orbitalis + pars triangularis), left lingual gyrus, bilateral precuneus, and bilateral lateral occipital cortex, obtained from FreeSurfer parcellations (Fischl et al., 2002). We also analyzed volumes for total GM, total WM, bilateral thalamus, bilateral anterior cingulate, bilateral medial orbitofrontal cortex, left inferior frontal gyrus, and caudate laterality ([left caudate – right caudate] / [0.5 * [left caudate + right caudate]]) (Rauch et al., 2003). To account for multiple comparisons, we performed an omnibus multivariate linear regression using PROC GLM in SAS® with all variables, including group. Bilateral volumes were used if we did not have a hemispheric hypothesis.
2.8. Whole-brain cortical surface analyses
We analyzed GM thickness at each vertex for left and right hemispheres using a general linear model (GLM) in FreeSurfer (v5.0.0) (Fischl and Dale, 2000). To control for multiple comparisons across the cortical surface and to preclude assumptions about the normality of our distributions, we performed 10,000 Monte Carlo simulations using an alpha=0.01 for between-group comparisons (0.05/2=0.025, Bonferroni-corrected for testing the left and right hemispheres, and the nearest existing FreeSurfer threshold was 0.01) and alpha=0.005 for clinical associations (0.05/(2*3)=0.008, Bonferroni-corrected for testing left and right hemispheres and the three clinical scores, and the nearest existing FreeSurfer threshold was 0.005) (Bland and Altman, 1995; Hagler et al., 2006). Resulting clusters were visualized at p<0.05. We did not correct for multiple comparisons performed across the range of smoothing kernels as those other than 15 mm were exploratory. As a post hoc alternative to the Monte Carlo simulations, we performed GLMs at each vertex and enforced a false discovery rate (FDR) correction of 5%.
2.9. VBM analyses
We analyzed GM volume at each voxel in the brain using a GLM in FSL. To control for multiple comparisons across the brain and to preclude assumptions about the normality of our distributions, we performed 10,000 permutations using a cluster threshold of p<0.05, corrected (Randomise in FSL, using threshold-free cluster enhancement) (Kennedy, 1995).
3. Results
3.1. Demographics and psychometrics
Table 1 presents demographic and psychometric information, including comorbidities within the BDD group, which are the following: None (n=23); Agoraphobia (n=1); Dysthymia (n=1); Dysthymia, GAD (n=2); GAD (n=5); MDD (n=9); GAD, MDD (n=6); GAD, MDD, social phobia (n=1); SAD (n=2).
Anxiety and depression scores were highly correlated (r=0.84 for MADRS and HAMA scores; r=0.85 for HAMD and HAMA scores). We chose to analyze HAMA scores because anxiety, especially related to physical appearance, is at the core of BDD symptomology, whereas depression is typically secondary (American Psychiatric Association, 2013). In addition, the same depression rating scale was not used across all participants.
3.2. ROI results
There were no significant between-group differences in volumes or cortical thickness for any ROI. Additionally, there were no significant associations between volumes or cortical thickness and clinical variables for any ROIs. There was a positive correlation between HAMA and total WM volume (p=0.01, R2 = 0.05), which did not survive Bonferroni correction for multiple comparisons (p = 0.05/4 = 0.0125).
3.3. Whole-brain cortical surface results
There were no significant differences between groups, although qualitatively there appears to be a pattern of greater mean thickness in several occipital, parietal, and frontal regions in the left hemisphere in the BDD group (Fig. 1). There were also no significant differences using the alternative FDR correction approach.
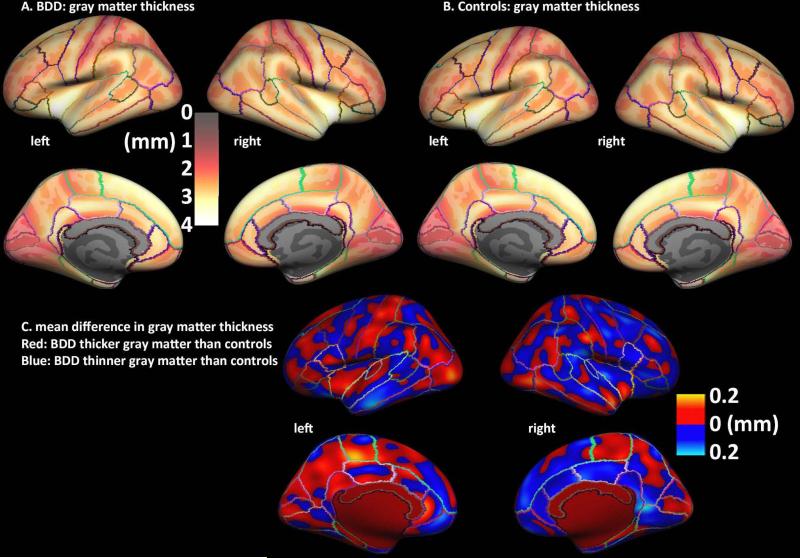
Fig. 1. Cortical thickness in body dysmorphic disorder (BDD) and healthy control participants.
A, B. Maps of average normalized gray matter thickness (mm) at each surface vertex for BDD (A) and healthy control groups (B). Lighter, yellow colors indicate thicker gray matter; darker, red colors indicate thinner gray matter.
C. Map of normalized differences in gray matter thickness for BDD vs. healthy controls. There were no significant differences between groups.
Qualitatively, both maps show the expected pattern of GM thickness in the adult brain (Fischl and Dale, 2000). The difference in mean GM thickness for BDD versus controls, which ranged from 0 to 0.2 mm in magnitude, is shown (Fig. 1C), without applying statistics, to provide a general impression of the pattern of differences in GM thickness.
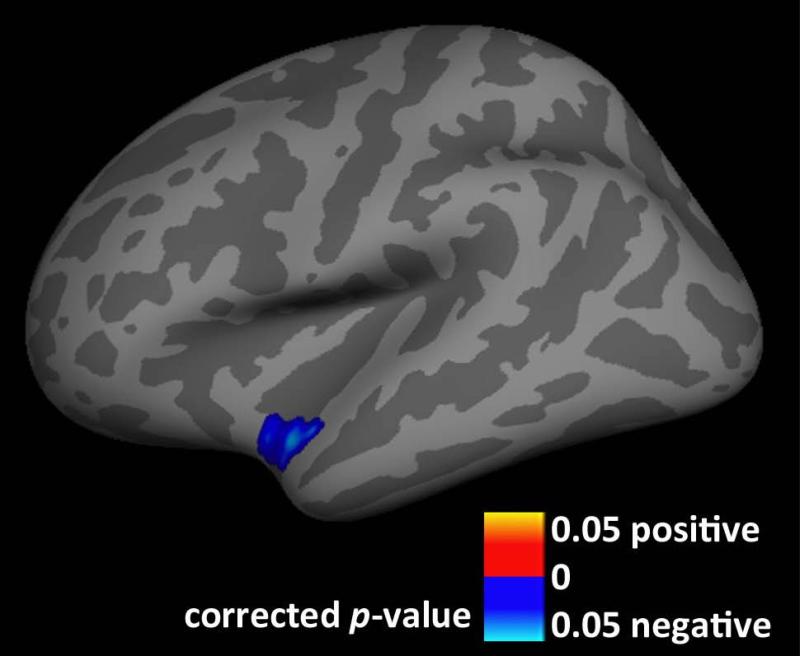
We found a significant negative association between cortical GM thickness and HAMA scores in BDD (Fig. 2). Worse anxiety scores were associated with thinner cortical GM in the left superior temporal cortex (5-mm FWHM smoothing kernel). There were no other significant associations with clinical variables.
Fig 2. Association between anxiety and gray matter (GM) thickness in body dysmorphic disorder (BDD).
In BDD, gray matter thickness in the left superior temporal cortex is negatively correlated with Hamilton Anxiety Rating Scale scores, cluster-wise p<0.05, after controlling for age, sex, and scanner. Worse anxiety is associated with thinner cortical gray matter in this area.
There were no significant associations between cortical GM thickness and clinical variables in BDD, when illness duration was included instead of age as a covariate.
3.4. VBM results
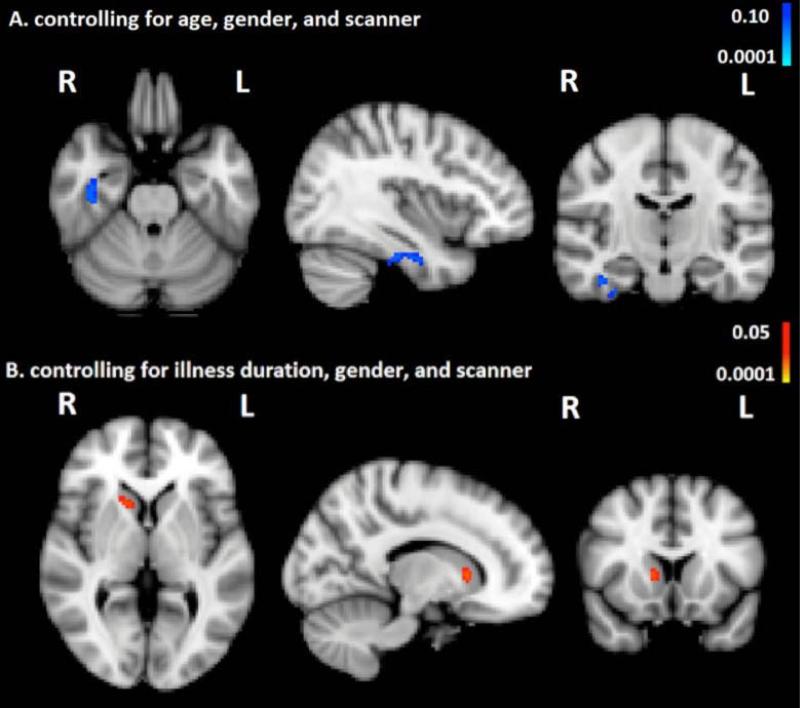
There were no significant differences between groups in regional brain volumes. As shown in Fig. 3A, there was a trend-level negative correlation between HAMA scores and GM volume in the right temporal fusiform cortex (p<0.10, corrected).
Fig. 3. Associations between anxiety and gray matter (GM) volume in body dysmorphic disorder (BDD).
A. BDD, there is a trend for gray matter volume in right temporal fusiform cortex to be negatively correlated with Hamilton Anxiety Rating Scale scores, p<0.1 corrected, after controlling for age, sex, and scanner. Worse anxiety is associated with lower gray matter volume in this region.
B. BDD, gray matter volume in the head of the right caudate is positively correlated with Hamilton Anxiety Rating Scale scores, p<0.05 corrected, after controlling for illness duration, sex, and scanner. Worse anxiety is associated with greater gray matter volume in this region.
As shown in Fig. 3B, there was a positive correlation between HAMA scores and GM volume in the dorsal head of the right caudate nucleus (p<0.05, corrected), in the exploratory analysis using the covariate of illness duration instead of age.
3.5. Results with non-comorbid BDD group
Results for the ROI, whole-brain cortical surface, and VBM neuroimaging analyses were unchanged after exclusion of BDD participants with any other psychiatric comorbidities.
4. Discussion
4.1. Between-group comparisons
We found no evidence of significant abnormalities in BDD for GM thickness or volume, using multiple different modalities of morphometric investigation. This suggests that BDD may not be characterized by prominent abnormalities in brain morphometry.
The absence of significant abnormalities in brain volumes agrees with one previous morphometric study in BDD (Feusner et al., 2009), but not with three other studies (Rauch et al., 2003; Atmaca et al., 2010; Buchanan et al., 2014). Three out of the four previous studies found no abnormalities in total GM (Rauch et al., 2003; Feusner et al., 2009; Atmaca et al., 2010), while one found lower total GM in BDD patients, compared with controls (Buchanan et al., 2014). Two studies found greater total WM volume in BDD (Atmaca et al., 2010; Rauch et al., 2003), although two other studies did not (Buchanan et al., 2014; Feusner et al., 2009). Contrary to our hypotheses, we did not find abnormalities in the anterior cingulate and the orbitofrontal cortices, despite findings in two previous studies of smaller volumes in these regions (Atmaca et al., 2010; Buchanan et al., 2014), nor did we find abnormalities in visual processing regions. We also did not replicate findings in a previous morphometric study performed by our group (Feusner et al., 2009) of correlations between BDD-YBOCS scores and volumes of the left IFG and right amygdala.
Discrepancies among these prior studies may be due to factors such as small sample size and unrepresentative sex distributions; one study enrolled only females (n=8 BDD, n=8 healthy controls) (Rauch et al., 2003), and another one enrolled only males (n=12 in each group) (Atmaca et al., 2010). The two other studies had a mixture of males and females, but also had small sample sizes: n=12 per group (Feusner et al., 2009) and n=20 in each group (Buchanan et al., 2014) . Another factor possibly accounting for discrepancies is that 18 of 20 BDD participants in one study were taking psychiatric medications (Buchanan et al., 2014), while in the other three studies all were unmedicated. Psychoactive medications can have confounding effects on brain structure and clinical state (Winkler et al., 2010). An additional factor is the use of volume normalization, which might have affected results. There is no clear consensus on this aspect of the methodology. Our current and past studies (Feusner et al., 2009) applied normalization with intracranial volume (ICV), while two previous studies applied normalization with whole brain volume (Atmaca et al., 2010; Buchanan et al., 2014), and another study did not use normalization (Rauch et al., 2003). If there were differences between groups in whole brain volume or ICV, then the results after normalization would yield only relative differences in brain regions. Theoretically it is possible for there to be no differences between groups in total brain volume but differences in ICV, or vice versa; if so, findings across studies that used different normalization techniques might produce discrepant results. Importantly, there is no evidence to suggest that ICV differs between BDD and healthy controls; the current study reports no significance difference in ICV, and one other (Buchanan et al., 2014) reported no significance difference in ICV or global brain volume.
In OCD, a disorder related to BDD, six studies have reported thinner cortical GM mainly in the anterior cingulate cortex and also in several frontal, temporal, and parietal regions (Shin et al., 2007; Nakamae et al., 2012; Venkatasubramanian et al., 2012; Kuhn et al., 2013; Fullana et al., 2014; Peng et al., 2014). Two studies have reported thicker cortical GM in three brain regions (right inferior frontal and posterior middle temporal gyri (Narayan et al., 2008) and right inferior parietal cortex (Fan et al., 2013). Cortical thickness in OCD was also associated with symptom severity and clinical outcome (Nakamae et al., 2012; Fullana et al., 2014). Given the similarities in phenomenology between BDD and OCD and previous findings of abnormal frontostriatal activity in BDD (Feusner et al., 2010), the observation in this study of no cortical thickness abnormalities nor of associations with clinical symptoms was unexpected. Sample size differences do not likely explain this, as four of the OCD studies had smaller sample sizes of approximately n=40-60 (Narayan et al., 2008; Nakamae et al., 2012; Fan et al., 2013; Peng et al., 2014), two of the OCD studies had similar sample sizes as that in our study (Shin et al., 2007; Venkatasubramanian et al., 2012), and two studies had larger sample sizes of nearly n=200 (Kuhn et al., 2013; Fullana et al., 2014). One possibility is that if there are any morphometric abnormalities in BDD, they may be more subtle or heterogeneous, and therefore more difficult to detect, than those in OCD. It should also be noted, however, that the findings in OCD are not entirely consistent across studies, and this inconsistency may also be due to heterogeneity within the disorder.
Compared with previous studies, we analyzed a much larger sample size (n=93), all participants were unmedicated, and we used both ROI-based and voxel-wise analyses of volumes, in addition to ROI-based and vertex-wise cortical thickness analyses. GM volume maps created with VBM provide an unbiased, data-driven exploratory analysis of GM volume across the whole brain, both in subcortical and cortical structures. GM thickness quantifies the thickness of the cortical ribbon along the GM surface, and, in some cases, it may be a more sensitive measure than overall GM volume for detecting subtle GM abnormalities (Winkler et al., 2010).
4.2. Associations with clinical variables
We found that severity of anxiety, a common symptom in BDD, is associated with brain structure in the left superior temporal cortex, right temporal fusiform cortex, and head of the caudate – regions that relate to BDD symptomology and pathophysiology.
These results complement previous BDD studies that found functional abnormalities in similar brain regions, and prior morphometric studies of related disorders such as OCD and anxiety (Feusner et al., 2010). Several prior studies report associations between anxiety and GM volumes; a VBM meta-analysis found increased caudate volumes in OCD (Radua et al., 2010). Although the current study did not observe enlarged caudate nuclei compared with findings in controls, the pathophysiology of OCD and BDD may nevertheless be associated with neuroanatomical findings in the caudate nucleus, perhaps mediated by anxiety. The association we found between anxiety and caudate structure was significant, when controlling for illness duration, yet there were no significant group differences in caudate size. A possible explanation is that anxiety may have differential and perhaps even opposite effects on caudate volume, compared with the effects of the other BDD-related symptoms that may accumulate over time.
In our study, anxiety was associated with GM structure in the caudate nucleus and superior temporal gyrus - both previously implicated in the pathophysiology of BDD (Feusner et al., 2007; Feusner et al., 2010) These two areas are anatomically connected (Yeterian and Pandya, 1998) and are a hub between the dorsal and ventral visual streams (Karnath, 2001), where disruption may cause altered perception of physical appearance in BDD (Feusner et al., 2009; Buchanan et al., 2013; Buchanan et al., 2014). In the caudate nucleus, we found that worse anxiety was associated with greater GM volume. The caudate is a major hub in frontostriatal circuits that, amongst other functions, mediate inhibitory control (Chamberlain et al., 2005; Menzies et al., 2008) and are disrupted in OCD. In OCD, the caudate is abnormally large and hyperactive, compared with findings in controls (Whiteside et al., 2004; Feusner et al., 2013) and, in BDD, the caudate is hyperactive when individuals view images of their own face compared with a familiar face (Feusner et al., 2010). Abnormal morphometry and activity in the caudate may relate to anxiety in BDD and OCD, and may be indirectly related to altered visual perception in BDD. We also found a negative association between anxiety and GM thickness in the superior temporal gyrus, which is hyperactive when individuals with BDD view images of faces (Feusner et al., 2007). The left superior temporal gyrus also connects to the amygdala, in which anxiety levels were found to modulate activity when BDD participants viewed images of faces (Bohon et al., 2012). In sum, previous research shows symptom-related abnormalities in structure, task activations, and functional connectivity for the areas where the current study found associations with anxiety in BDD.
We tested two sets of covariates of non-interest in our statistical models. One included age and the other included illness duration (in addition to sex, scanner, and intracranial volume). Each variable captures slightly different characteristics; however, they cannot be used in the same model because they are highly collinear (correlation in our BDD sample of r=0.88). Using age as a covariate of non-interest allows us to identify morphometric patterns that are independent of well-described general age-related effects (Hagler et al., 2006). Using illness duration as a covariate of non-interest allows us to identify morphometric patterns that are independent of the potentially cumulative effects of years of experiencing core BDD symptoms, and anxiety, depression, and social isolation that often accompany this disorder. The results using this covariate, namely, the association between right caudate GM volume and anxiety, may therefore be closer to a possible trait biomarker of a brain structure-clinical symptom relationship in BDD.
4.3. Limitations
There are several limitations to consider. Although we found no significant differences between groups, small morphometric abnormalities may exist in BDD for which the current study was underpowered to detect. For example, qualitative inspection of the raw difference maps (Fig. 1C) suggests that there may be thicker cortex in BDD in left hemisphere regions, particularly in the occipital and parietal cortices. A limitation is that participants were scanned on two different scanners, which may influence GM measures. However, the proportion of BDD and healthy control participants did not differ between scanners (p>0.99), and we included scanner type as a regressor of non-interest in our models. As is often the case in studies of disorders in which a ground truth has not yet been established, some of the parameters in our analysis were selected without prior knowledge. In particular, selection of smoothing kernel size for cortical thickness and VBM analyses is somewhat arbitrary. We chose a 7-mm smoothing kernel for VBM as is standard in the absence of prior evidence, and for cortical thickness we analyzed a range of kernel sizes. (Smoothing kernel size may be different in VBM and cortical GM thickness due to the differences in the nature of the data.) The prior morphometric studies in BDD that found group differences used only ROIs rather than unconstrained voxel-wise analyses (and findings were not consistent across studies) so there was no prior basis for selecting smoothing kernel size. Another limitation is that this is a cross-sectional study, so cause-and-effect cannot be determined for relationships among the degree of anxiety, and cortical thickness and volumetric measures.
4.4. Future directions
We do not yet understand the relationships between brain structure and a) predisposing traits that confer a predisposition to developing BDD and are stable across time; b) current clinical state; or c) abnormalities that accumulate over time as the illness progresses. A longitudinal neuroimaging study of BDD, with or without treatment, could address these important remaining questions by following the same cohort of individuals over time, as their BDD may progress or improve. For example, if successful treatment in BDD with improvement in anxiety results in brain structure changes, this would provide evidence that morphometric relationships may be anxiety symptom-related. If brain morphometry does not change after reduction of anxiety, then existing relationships in brain structure may represent predisposing traits towards having BDD with high anxiety. Alternative and more complex explanations are also possible. To assess clinical interventions, it would be useful to have reliable state biomarkers that provided information about whether treatments that reduce anxiety (or other symptoms in BDD) also result in changes in brain morphometry. Studying unaffected siblings, who may share some genetic and subclinical traits, would help distinguish predisposing traits from effects of having the illness. This could be useful for identifying those at risk for developing BDD.
4.5. Conclusions
In the largest brain-imaging study of BDD to date, we found no evidence of morphometric abnormalities at the group level in the brains of individuals with BDD. There were, however, associations between anxiety and brain morphometry in specific regions previously linked to BDD symptoms, anxiety, and OCD. Thus, while previous studies suggest abnormal functional brain activity in BDD, these findings may not be associated with anatomical abnormalities at the group level.
Highlights.
Brain structure was assessed in body dysmorphic disorder (BDD) and controls
We tested associations with clinical scores in BDD
No significant group differences were found
Worse anxiety in BDD is associated with localized gray matter differences
BDD may be characterized by subtle associations with clinical features, rather than widespread morphometric abnormalities
Table 2.
Gray matter volumes and thickness in regions of interest
| Healthy control (raw) | Healthy control (normalizeda) | BDD (raw) | BDD (normalized*) | F | p | |
|---|---|---|---|---|---|---|
| GM and WM Volumes | ||||||
| White matter | 383721 ± 52993 | 379279 ± 40636 | 0.08 | 0.78 | ||
| Gray matter | 392550 ± 47144 | 383569 ± 38843 | 1.01 | 0.32 | ||
| Thalamus (right) | 8517 ± 905 | 5541 ± 422 | 7916 ± 748 | 5496 ± 462 | 0.23 | 0.63 |
| Thalamus (left) | 8261 ± 835 | 5707 ± 377 | 8102 ± 750 | 5610 ± 436 | 1.29 | 0.26 |
| Anterior cingulate (right) | 4388 ± 922 | 2944 ± 555 | 4216 ±782 | 2895 ± 436 | 0.22 | 0.64 |
| Anterior cingulate (left) | 4730 ± 1118 | 3145 ± 527 | 4612 ± 891 | 3186 ± 542 | 0.14 | 0.71 |
| Medial orbital frontal cortex (right) | 5193 ± 748 | 3479 ± 395 | 5121 ± 639 | 3555 ± 404 | 0.83 | 0.36 |
| Medial orbital frontal cortex (left) | 5404 ± 1009 | 3623 ± 620 | 51922 ± 125183 | 3539 ± 560 | 0.47 | 0.49 |
| Left lnferior frontal gyrus | 11262 ± 2029 | 7526 ± 940 | 10570 ± 1639 | 7337 ± 1081 | 0.70 | 0.41 |
| Caudate laterality | −0.0035 ± 0.0357 | −0.0017 ± 0.0513 | 0.04 | 0.85 | ||
| GM thickness | ||||||
| Anterior cingulate (left) | 5.65 ± 0.6 | 3.82 ± 0.57 | 5.66 ± 0.62 | 3.96 ± 0.61 | 1.57 | 0.21 |
| Anterior cingulate (right) | 5.47 ± 0.46 | 3.71 ± 0.58 | 5.41 ± 0.47 | 3.77 ± 0.46 | 0.41 | 0.52 |
| Medial orbitofrontal corte (left) | 2.46 ± 0.15 | 1.67 ± 0.22 | 2.43 ± 0.18 | 1.70 ± 0.22 | 0.52 | 0.47 |
| Medial orbitofrontal cortex (right) | 2.44 ± 0.22 | 1.65 ± 0.25 | 2.46 ± 0.29 | 1.72 ± 0.27 | 1.83 | 0.18 |
| Left inferior frontal gyrus | 7.91 ± 0.59 | 1.79 ± 0.23 | 7.87 ± 0.63 | 1.83 ± 0.25 | 0.97 | 0.33 |
| Lingual gyrus (left) | 1.99 ± 0.11 | 1.35 ± 0.16 | 1.99 ± 0.13 | 1.39 ± 0.17 | 1.91 | 0.17 |
| Precuneus (left) | 2.44 ± 0.17 | 1.65 ± 0.22 | 2.46 ± 0.19 | 1.72 ± 0.23 | 2.20 | 0.14 |
| Precuneus (right) | 2.46 ± 0.17 | 1.67 ± 0.22 | 2.44 ± 0.21 | 1.70 ± 0.23 | 0.60 | 0.44 |
| Lateral occipital cortex (left) | 2.21 ± 0.14 | 1.49 ± 0.18 | 2.24 ± 0.17 | 1.56 ± 0.21 | 3.10 | 0.08 |
| Lateral occipital cortex (right) | 2.28 ± 0.17 | 1.54 ± 0.2 | 2.30 ± 0.19 | 1.61 ± 0.21 | 2.72 | 0.10 |
Values normalized to intracranial volumes: raw volume/total intracranial volume
106 Body dysmorphic disorder, BDD. Mean total volumes (mm3) and mean cortical thickness (mm) are listed for ROIs in healthy control and BDD participants, along with F and p-values from multivariate linear regressions of normalized volumes controlling for age, sex, and scanner.
Funding and acknowledgements
This research was supported by Government support under and awarded by a DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a (Dr. Sarah K. Madsen), NIH R01 grants MH097268, MH085667, MH089722, MH094343, and P41 EB015922 (Dr. Sarah Madsen and Dr. Paul M. Thompson), National Institute of Mental Health grants K23 MH079212, R01MH093535, and R01MH085900 (Dr. Jamie D. Feusner). This work was supported in part by a Consortium grant (U54 EB020403) from the NIH Institutes contributing to the Big Data to Knowledge (BD2K) Initiative, including the NIBIB and NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Author; Washington, DC.: 2013. [Google Scholar]
- Atmaca M, Bingol I, Aydin A, Yildirim H, Okur I, Yildirim MA, Mermi 0, Gurok MG. Brain morphology of patients with body dysmorphic disorder. Journal of Affective Disorders. 2010;123:258–263. doi: 10.1016/j.jad.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. British Medical Journal. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Hembacher E, Moller H, Moody TD, Feusner JD. Nonlinear relationships between anxiety and visual processing of own and others' faces in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2012;204:132–139. doi: 10.1016/j.pscychresns.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B, Rossell S, Maller JJ, Toh WL, Brennan S, Castle D. Regional brain volumes in body dysmorphic disorder compared to controls. Australian and New Zealand Journal of Psychiatry. 2014 doi: 10.1177/0004867413520253. [DOI] [PubMed] [Google Scholar]
- Buchanan BG, Rossell SL, Maller JJ, Toh WL, Brennan S, Castle DJ. Brain connectivity in body dysmorphic disorder compared with controls: a diffusion tensor imaging study. Psychological Medicine. 2013;43(12):2513–2521. doi: 10.1017/S0033291713000421. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian J. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Phillips KA, Wilhelm S, Buhlmann U, Rauch SL, Baer L, Jenike MA. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–681. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. American Journal of Psychiatry. 1998;155:102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C, Zhang T, Jiang K, Xiao Z, Liddle PF. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: a study of cortical thickness, folding and surface area. Psychological Medicine. 2013;43:1081–1091. doi: 10.1017/S0033291712001845. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Arienzo D, Li W, Zhan L, GadElkarim J, Thompson PM, Leow AD. White matter microstructure in body dysmorphic disorder and its clinical correlates. Psychiatry Research. Neuroimaging. 2013;211:132–140. doi: 10.1016/j.pscychresns.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychological Medicine. 2011;41:2385–2397. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64:1417–1426. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, McKinley M, Moller H, Bookheimer S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2009;172:161–167. doi: 10.1016/j.pscychresns.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Cardoner N, Alonso P, Subira M, Lopez-Sola C, Pujol J, Segalas C, Real E, Bossa M, Zacur E, Martinez-Zalacain I, Bulbena A, Menchon JM, Olmos S, Soriano-Mas C. Brain regions related to fear extinction in obsessive-compulsive disorder and its relation to exposure therapy outcome: a morphometric study. Psychological Medicine. 2014;44:845–856. doi: 10.1017/S0033291713001128. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fmri data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies K, Laws KR, Fineberg NA. Superior face recognition in body dysmorphic disorder. Journal of Obsessive-Compulsive and Related Disorders. 2012;1:175–179. [Google Scholar]
- Karnath HO. New insights into the functions of the superior temporal cortex. Nature Reviews Neuroscience. 2001;2:568–576. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- Kennedy PE. Randomization tests in econometrics. Journal of Business & Economic Statistics. 1995;13:85–94. [Google Scholar]
- Kuhn S, Kaufmann C, Simon D, Endrass T, Gallinat J, Kathmann N. Reduced thickness of anterior cingulate cortex in obsessive-compulsive disorder. Cortex. 2013;49:2178–2185. doi: 10.1016/j.cortex.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Madsen SK, Bohon C, Feusner JD. Visual processing in anorexia nervosa and body dysmorphic disorder: similarities, differences, and future research directions. Journal of Psychiatric Research. 2013;47:1483–1491. doi: 10.1016/j.jpsychires.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philosophical Transactions of the Royal Society B-Biological Sciences. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression rating scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mufaddel A, Osman OT, Almugaddam F, Jafferany M. A review of body dysmorphic disorder and its presentation in different clinical settings. The Primary Care Companion for CNS Disorders. 2013;15(4) doi: 10.4088/PCC.12r01464. pii: PCC.12r01464. doi: 10.4088/PCC.12r01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamae T, Narumoto J, Sakai Y, Nishida S, Yamada K, Kubota M, Miyata J, Fukui K. Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;37:90–95. doi: 10.1016/j.pnpbp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Narayan VM, Narr KL, Phillips OR, Thompson PM, Toga AW, Szeszko PR. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport. 2008;19:1551–1555. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Shi F, Shi C, Miao G, Yang Q, Gao W, Wolff JJ, Chan RC, Shen D. Structural and diffusion property alterations in unaffected siblings of patients with obsessive-compulsive disorder. PLoS One. 2014;9:e85663. doi: 10.1371/journal.pone.0085663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Atala KD, Pope HG., Jr. Diagnostic instruments for body dysmorphic disorder.. American Psychiatric Association 148th Annual Meeting; Miami, FL. 1995. p. 15. [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, Stein DJ, Rauch SL, Hollander E, Fallon BA, Barsky A, Fineberg N, Mataix-Cols D, Ferrao YA, Saxena S, Wilhelm S, Kelly MM, Clark LA, Pinto A, Bienvenu OJ, Farrow J, Leckman J. Should an obsessive-compulsive spectrum grouwgw disorders be included in DSM-V? Depression and Anxiety. 2010;27:528–555. doi: 10.1002/da.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Archives of General Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Phillips KA, Segal E, Makris N, Shin LM, Whalen PJ, Jenike MA, Caviness VS, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2003;122:13–19. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Shin YW, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, Kim IY, Kim SI, Kwon JS. Cortical thinning in obsessive compulsive disorder. Human Brain Mapping. 2007;28:1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang YY, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snaith RP. Hamilton Rating Scale for Depression. British Journal of Psychiatry. 1977;131:431–432. doi: 10.1192/bjp.131.4.431. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Zutshi A, Jindal S, Srikanth SG, Kovoor JM, Kumar JK, Janardhan Reddy YC. Comprehensive evaluation of cortical structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: a surface-based morphometry study. Journal of Psychiatric Research. 2012;46:1161–1168. doi: 10.1016/j.jpsychires.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2004;132:69–7. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). British Journal of Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? the importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticostriatal connections of the superior temporal region in rhesus monkeys. Journal of Comparative Neurology. 1998;399:384–402. [PubMed] [Google Scholar]
- Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhao L, Boucher M, Rosa-Neto P, Evans AC. Impact of scale space search on age- and gender-related changes in MRI-based cortical morphometry. Human Brain Mapping. 2013;34:2113–2128. doi: 10.1002/hbm.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]